Keywords
telemedicine, heart failure, reduced ejection fraction, mortality, hospitalization
Telemedicine has improved adherence to heart failure (HF) treatment, however it has not yet been tailored specifically to address HF with reduced ejection fraction (HFrEF). Our objective is to undertake a comprehensive systematic review and meta-analysis of existing research studies that focus on telemedicine in HFrEF.
We conducted an extensive literature review encompassing trials which included outpatients with HFrEF who underwent telemedicine compared with usual care. We exclude any studies without ejection fraction data. Three bibliographic databases from PubMed, ScienceDirect, and Cochrane Library were utilized in our search from January 1999 to May 2023. The endpoints of interest included all-cause mortality, cardiovascular-related mortality, all-cause hospitalization, and HF-related hospitalization. The Cochrane risk-of-bias (RoB) and the risk of bias in non-randomized studies – of interventions (ROBINS-I) were used for non-randomized or observational studies. To quantitatively analyze the collective findings, a pooled odds ratio (OR) was computed for each outcome.
Out of the initial pool of 4,947 articles, we narrowed down our analysis to 27 studies, Results showed that telemedicine significantly reduced all-cause mortality (OR: 0.65; 95% CI 0.54 – 0.78; p<0.00001), cardiovascular-related mortality (OR 0.68, 95% CI 0.58 – 0.80, p < 0.00001), and HF-related hospitalization based on number of events (OR 0.77, 95% CI 0.64 – 0.94, p = 0.009) as well as number of patients (OR 0.78, 95% CI 0.69 – 0.87, p < 0.0001).
Telemedicine was shown significantly beneficial in decreasing mortality and hospitalization in HFrEF patients. Future research should focus on standardizing effective telemedicine practices due to the existing variability in methods and clinical situation of the patients.
PROSPERO: CRD42023471222 registerd on October 21, 2023
telemedicine, heart failure, reduced ejection fraction, mortality, hospitalization
We added the Clinical Impact and Future Perspective part before conclusion, added some explanation in the strengths and limitations part, and emphasized more in the conclusion.
See the authors' detailed response to the review by Nisha Nambiar
See the authors' detailed response to the review by Vito Damay
HF has been known for its major public health concern with substantial morbidity and mortality. Its 1-year mortality rate is 7.2% and 31.9% of 1-year hospitalization rate.1 In the US, approximately 1 million hospitalizations of HF, are caused by HFrEF.2 The OPTIMIZE-HF study enrolling 20,118 patients with HFrEF, reported a higher in-hospital mortality in HFrEF (3.9%) than in HF with preserved ejection fractions (HFpEF) patients (2.9%).3 In a prospective longitudinal study conducted in multiple centers in New Zealand and Singapore, 17% of patients (343 individuals) died during the two-year follow-up period. After accounting for factors such as age, sex, and clinical risk factors, it was observed that patients with HFpEF had a reduced risk of death in comparison to those with HFrEF (HR 0.62, 95% CI 0.46–0.85, p = 0.003).4 In a cohort of 4880 individuals from China, a 5-year follow-up until the end of December 2019 revealed a decrease in ejection fraction category was identified as an independent factor associated with an elevated 5-year mortality risk. Specifically, individuals with HFrEF had a mortality rate of 25.2%, while those with HFpEF exhibited a lower rate at 13.4% (adjusted hazard ratio (aHR) 1.85, CI 95% 1.45-2.35, p <0.001).5
Despite the proven efficacy of guideline-directed medical therapy (GDMT) in reducing morbidity and mortality, a significant number of eligible patients diagnosed with HFrEF do not receive one or more of the recommended medications. This shortfall is frequently attributed to suboptimal initiation and titration practices in the outpatient setting. After discharge, the continuation of progress and the safety of initiating and adjusting GDMT in the hospital relies on the successful transition of care to the outpatient setting.6
The COVID-19 pandemic had brought about a positive outcome in the form of the widespread adoption of telemedicine. This approach, involving the remote delivery of optimal diagnostic and therapeutic services, has proven particularly beneficial for patients with HF during the pandemic. The effectiveness of telemedicine is enhanced through intensive monitoring and more frequent transmission of patient data. This not only improves patient outcomes but also minimizes the risk of virus exposure for healthcare workers.7 The challenge of achieving GDMT can be alleviated by incorporating telemedicine which has proven some beneficial effects. A systematic review conducted by Yun et al. revealed a 19% reduction in all-cause mortality through telemedicine.8 Specifically, several studies have confirmed the enduring benefits of telemedicine for individuals with HFrEF, particularly in terms of reducing mortality and hospitalization rates. A RCT conducted over a 120-month period demonstrated a significant and prolonged decrease in all-cause mortality, underscoring the lasting positive effects of telemedicine.9 In the most recent systematic review and meta-analysis by Scholte et al., a noteworthy 16% decrease in all-cause mortality was observed. Additionally, there were significant reductions of 19% in first HF hospitalization and 15% in total HF hospitalization.10 It is important to note, however, that none of these studies distinguished HF based on ejection fraction.
We aim to dig deeper to answer the question of whether it is truly beneficial for patients with reduced ejection fraction, who clearly show higher frailty with a higher risk of adverse clinical outcomes.11
We conducted an extensive and systematic literature review based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, encompassing trials spanning from January 1999 to May 2023.12 Three bibliographic databases from PubMed, ScienceDirect, and Cochrane Library were utilized in our search. Our study was registered in PROSPERO: CRD42023471222 on October 21, 2023. The search terms were “heart failure”, “HF”, “reduced”, “ejection fraction”, “HFrEF”, “telemonitoring”, “telemedicine”, “telemedical”, “telecardiology”. The PRISMA flow diagram is displayed in Figure 1 in data availability section.
The inclusion criteria are trials/studies which include HFrEF patients or studies with mentioned ejection fraction of ≤40%, with consistent outcome of all-cause mortality, cardiovascular-related mortality, all-cause hospitalization, or HF-related hospitalization. We included any form of telemedicine or telemonitoring such as home telemonitoring (HTM) or telephone support (TS). Any forms of abstracts without full-text articles, case reports, expert opinions, conference presentations, preclinical studies, or whether the articles were not available in English, were excluded from our study.
All articles retrieved through the systematic search underwent initial screening of their titles and abstracts by two authors and were subsequently grouped into a single folder. The same authors then conducted a thorough examination of the full texts to determine whether the articles met the eligibility criteria or not.
We included all-cause mortality as our primary outcome, where HF-related mortality, all-cause hospitalization, and HF-related hospitalization as our secondary outcomes. All-cause hospitalization and HF-related hospitalization were both extracted based on number of events (per patient-year) and number of patients. Patient-year was calculated as follows: the total number of patients who participated in the study is multiplied by the total years of follow-up. Studies involving more than two telemedicine interventions were divided into separate studies within the forest plot and meta-analysis. In the table outlining baseline characteristics, we incorporated information on NYHA classification, ejection fraction, follow-up duration in months, study design, participant age, monitoring methods, telemedicine types, and usual care modalities. These data were extracted from the supplementary tables and figures provided in the articles. Two independent investigators each collected the data from the retrieved article. In cases where data interpretation was inconclusive, we sought the input of our third author and reached a consensus. Articles that satisfied the eligibility criteria but lacked the raw data necessary for meta-analysis were excluded from the study. Table 1 presents the fundamental feature of each study, while Table 2 provides a summary of the results and subgroup analysis.
| No | First Author, Year | NYHA | EF (%) | Time of follow-up (Months) | Methods of monitoring | Design | Age | Time of follow-up (Months) | Methods of monitoring | Types of telemonitoring | Types of usual care |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Angermann, 2012 (INH)24 | I-IV | ≤40 | 1.5 | HeartNetCare: (1) in-hospital face-to-face contact; (2) telephone-based structured monitoring using a standardized 19-item questionnaire; (3) uptitration of heart failure medication in cooperation with GPs, where possible, and teaching of patients regarding adjustment of diuretics; (4) needs-adjusted specialist care, which nurses coordinated with patients’ physician(s); (5) Measures for appropriate education and supervision of interveners to ensure high intervention quality. | RCT | 68.6 ± 12.2 | 1.5 | Non Invasive | TSM | In-clinic follow up |
| 2 | Angermann, 2023 (E-INH)9 | II-III | ≤40 | 60-120 | HeartNetCare: (1) in-hospital face-to-face contact; (2) telephone-based structured monitoring using a standardized 19-item questionnaire; (3) uptitration of heart failure medication in cooperation with GPs, where possible, and teaching of patients regarding adjustment of diuretics; (4) needs-adjusted specialist care, which nurses coordinated with patients’ physician(s); (5) Measures for appropriate education and supervision of interveners to ensure high intervention quality. | RCT | 70 (60-77) | 60, 120 | Non Invasive | TSM | In-clinic follow up |
| 3 | Antonicelli, 200825 | II-IV | <40 | 12 | Subjects of the control group were contacted monthly by telephone to collect data on new hospital admissions, cardiovascular complications and death. | RCT | 78 ± 8.5 | 12 | Non Invasive | Combination | In-clinic follow up and contact once a month from the team |
| 4 | Bohm, 201617 | II-III | ≤35 | 18 | Intrathoracic fluid index threshold crossing (FTC). | RCT | 66.3 ± 10.4 | 18 | Invasive | HTM | In-clinic follow up |
| 5 | Chiu, 2021 (REMOTE-CIED)18 | II-III | ≤35 | 12 | Clinical nursing specialist-led telephone consultations 48 – 72 h after initial discharge and subsequently at 2-weekly intervals. | RCT | 65 (59–73) | 12 | Invasive | HTM | In-clinic follow up |
| 6 | Cleland, 2005 (TEN-HMS)26 | I-IV | <40 | 15 | Low-profile, electronic, weighing scales, an automated sphygmomanometer, and a single-lead electrocardiogram using wrist-band electrodes. | RCT | 67.2 ± 11.6 | 15 | Non Invasive | Combination | In-clinic follow up |
| 7 | DeWalt, 200627 | II-IV | <40 | 12 | The program coordinator made scheduled follow-up phone calls (days 3, 7, 14, 21, 28, 56) and monthly during months 3–6. | RCT | 62.5 ± 10.1 | 12 | Non Invasive | TS | In-clinic follow up and brochure |
| 8 | Domingues, 201128 | not clear | ≤45 | 3 | Systematic telephone contacts for a three-month investigational period (intervention group - IG) vs out-patient visit with telephone contacts. | RCT | 63 ± 13 | 3 | Non Invasive | TS | In-clinic follow up |
| 9 | Gattis,1999 (PHARM)29 | I-IV | <45 | 6 | Telephone follow-up at 2, 12, and 24 weeks after the initial clinic visit to identify problems with drug therapy | RCT | NA | 6 | Non Invasive | TS | In-clinic follow up |
| 10 | Giordano, 200930 | II-IV | <40 | 12 | Home-based telemanagement by medical/nursing interventions made over the telephone, with the possibility to transmit an ECG trace to a workstation. | RCT | 57 ± 10 | 12 | Non Invasive | Combination | In-clinic follow up |
| 11 | Goldberg, 2003 (WHARF)31 | III-IV | ≤35 | 6 | Electronic scale placed in patients’ homes and an individualized symptom response system (DayLink monitor) linked via a standard phone line using a toll-free telephone number to a computerized database monitored by trained cardiac nurses. | RCT | 59.1 ± 15.3 | 6 | Non Invasive | HTM | In-clinic follow up and feature to contact their physicians |
| 12 | Hansen, 2018 (InContact)19 | I-III | ≤35 | 12 | Implanted Cardioverter Defibrillators SysTems, quarterly automated follow-up only (telemetry group) were compared to those receiving personal physician contact. | RCT | 63.8 ± 11.1 | 12 | Invasive | Combination | In-clinic follow up or TS |
| 13 | Hindricks, 2014 (IN-TIME)20 | II-III | ≤35 | 12 | Lumax dual-chamber ICD or CRT-D, Monitoring function. At a set time every day (typically 0300 h) or on detection of tachyarrhythmia, the devices transmitted cumulative and last-saved diagnostic. | RCT | 65.5 ± 9.4 | 12 | Invasive | Combination | In-clinic follow up |
| 14 | Koberich, 201532 | II-IV | ≤40 | 3 | Consecutive telephone follow-up over three months. | RCT | 61.7 ± 12.0 | 3 | Non Invasive | TS | In-clinic follow up |
| 15 | Koehler, 201134 | II-III | ≤35 | 12 | RTM using portable devices for ECG, blood pressure, and body weight measurements and sent to telemedical centre. | RCT | 66.9 ± 10.6 | 12 | Non Invasive | HTM | In-clinic follow up |
| 16 | Krum, 2013 (CHAT)35 | II-IV | <40 | 12 | Usual care (UC) or UC and telephone support intervention (UC+I). | RCT | 73.0 ± 10.5 | 12 | Non Invasive | TS | In-clinic follow up |
| 17 | Kurek, 2017 (COMMIT-HF)14 | not clear | ≤35 | 36 | Implanted ICD/CRTD, The RM group included the 121 patients with varying manufacturer. | OBS | NA | 36 | Invasive | HTM | In-clinic follow up |
| 18 | Landolina, 2012 (EVOLVO)21 | I-III | ≤35 | 16 | Data transmission through ICD, without patients intervention. | RCT | NA | 16 | Invasive | HTM | Audible notification of ICD and in-clinic follow up |
| 19 | Mo, 2021 (HHH)15 | II-IV | ≤40 | 12 | Standard care plus telehealth intervention in which participants were called by registered nurses. | OBS | 53.1 ± 11.4 | 12 | Non Invasive | TS | In-clinic follow up |
| 20 | Mortara, 200933 | I-IV | ≤40 | 12 | Monthly telephone contact + weekly vital signs transmission + monthly 24h recording of cardiorespiratory activity. | RCT | 60 ± 12 | 12 | Non Invasive | Combination | In-clinic follow up |
| 21 | Nunes-ferreria, 202016 | I-III | ≤40 | 12 | Prospective TM programme, prospective HF protocol follow-up programme (PFP) with no TM facilities, and retrospective propensity-matched usual care (UC). | OBS | 65.9 ± 11.9 | 12 | Non Invasive | Combination | Protocol based or in-clinic follow up |
| 22 | Sardu, 2016 (TELECART)22 | II-III | <35 | 12 | CRT-D as telemonitoring device and transmitted data were reviewed by independent investigators. | RCT | 72.2 ± 7.2 | 12 | Invasive | Combination | In-clinic follow up |
| 23 | Soran, 200836 | II-III | ≤40 | 6 | Electronic scale and an individualized symptom response system (DayLink monitor) linked via a standard phone line to a computerized database. | RCT | 76 ± 7 | 6 | Non Invasive | HTM | In-clinic follow up |
| 24 | Tajstra, 2020 (RESULT)23 | II-IV | ≤35 | 12 | Available ICD or CRT manufactured by four companies, equipped with remote monitoring systems. | RCT | NA | 12 | Invasive | Combination | In-clinic follow up |
| 25 | Villani, 201437 | III-IV | ≤40 | 12 | Telemonitoring technology comprised a patient front- end, a medical front-end and a web-based system for assisting with clinical decisions. | RCT | 72 ± 3 | 12 | Non Invasive | HTM | In-clinic follow up |
| 26 | Völler, 2022 (CardioBBEAT)38 | II-IV | ≤40 | 12 | Standard of Care (SoC) enriched by RTM connecting them wirelessly to the participating care providers using the telemedicine platform Motiva and transferred to the hospital. | RCT | 63.0 ± 11.5 | 12 | Non Invasive | Combination | In-clinic follow up |
| 27 | Wita, 202239 | II-III | not clear | 24 | Self-monitoring using a telemonitoring set: a blood pressure (BP) monitor, a 3-lead electrocardiogram (ECG) recorder, a weighing machine, and a personal tablet. | RCT | 66.1 ± 10.5 | 24 | Non Invasive | HTM | In-clinic follow up |
| Subgroup analyses | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-invasive | Invasive | |||||||||
| OR, 95% CI | P-value | I2 | OR, 95% CI | P-value | I2 | OR, 95% CI | P-value | I2 | ||
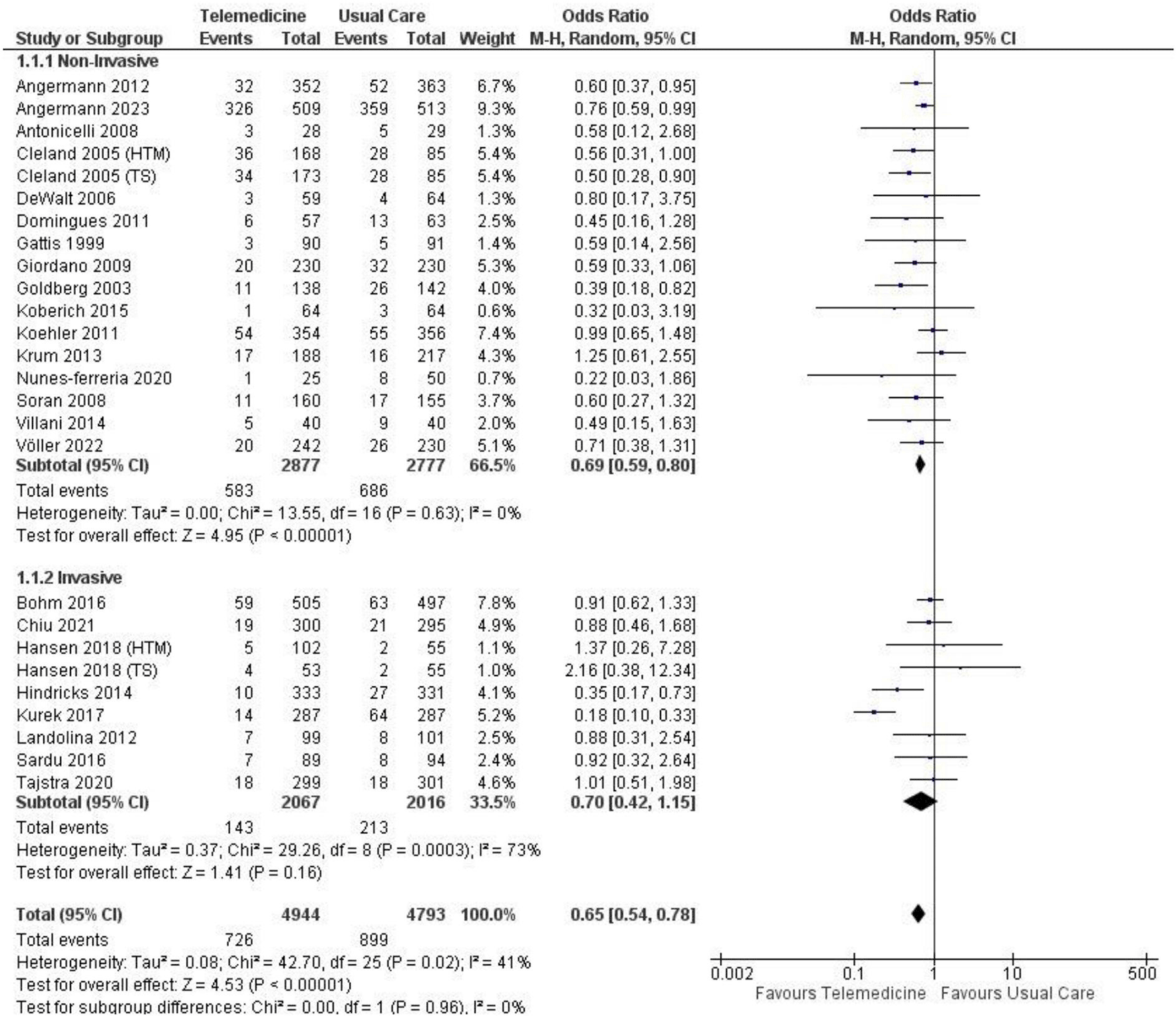
| All-cause mortality | 0.69, 0.59 – 0.80 | <0.00001 | 0% | 0.70, 0.42 – 1.15 | 0.16 | 73% | 0.65, 0.54 – 0.78 | <0.00001 | 41% | |
| Sensitivity analyses | <6 months | 0.56, 0.37 – 0.85 | 0.007 | 0% | (not enough studies) | |||||
| 6-11 months | 0.49, 0.29 – 0.81 | 0.006 | 0% | |||||||
| ≥12 months | 0.74, 0.62 – 0.87 | 0.0003 | 0% | |||||||
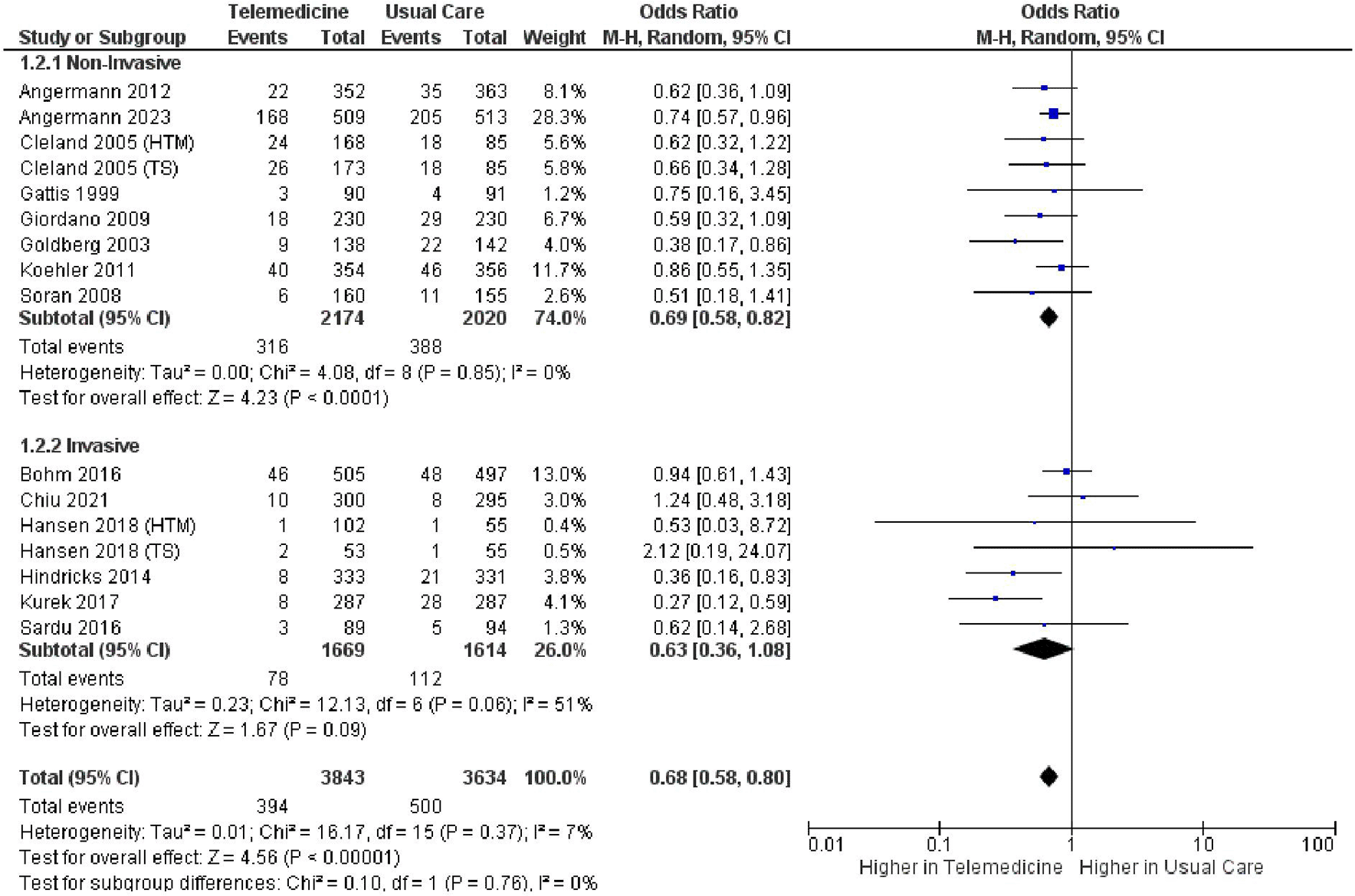
| Cardiovascular-related mortality | 0.69, 0.58 – 0.82 | <0.0001 | 0% | 0.63, 0.36 – 1.08 | 0.09 | 51% | 0.68, 0.58 – 0.80 | <0.00001 | 7% | |
| Sensitivity analyses | <6 months | 0.60, 0.37 – 0.97* | 0.04 | 0% | (not enough studies) | |||||
| 6-11 months | 0.44, 0.22 – 0.91* | 0.03 | 0% | |||||||
| ≥12 months | 0.73, 0.60 – 0.88 | 0.001 | 0% | |||||||
| All-cause hospitalization | number of events | 0.68, 0.48 – 0.94 | 0.02 | 79% | (not enough studies) | 0.73, 0.54 – 1.00 | 0.05 | 78% | ||
| number of patients | 0.83, 0.66 – 1.04 | 0.11 | 68% | 0.85, 0.69 – 1.03 | 0.1 | 66% | ||||
| Sensitivity analyses (number of patients) | <6 months | 1.11, 0.83 – 1.48* | 0.47 | 0% | (not enough studies) | |||||
| 6-11 months | 1.19, 0.76 – 1.86* | 0.44 | NA | |||||||
| ≥12 months | 0.76, 0.57 – 1.00 | 0.05 | 72% | |||||||
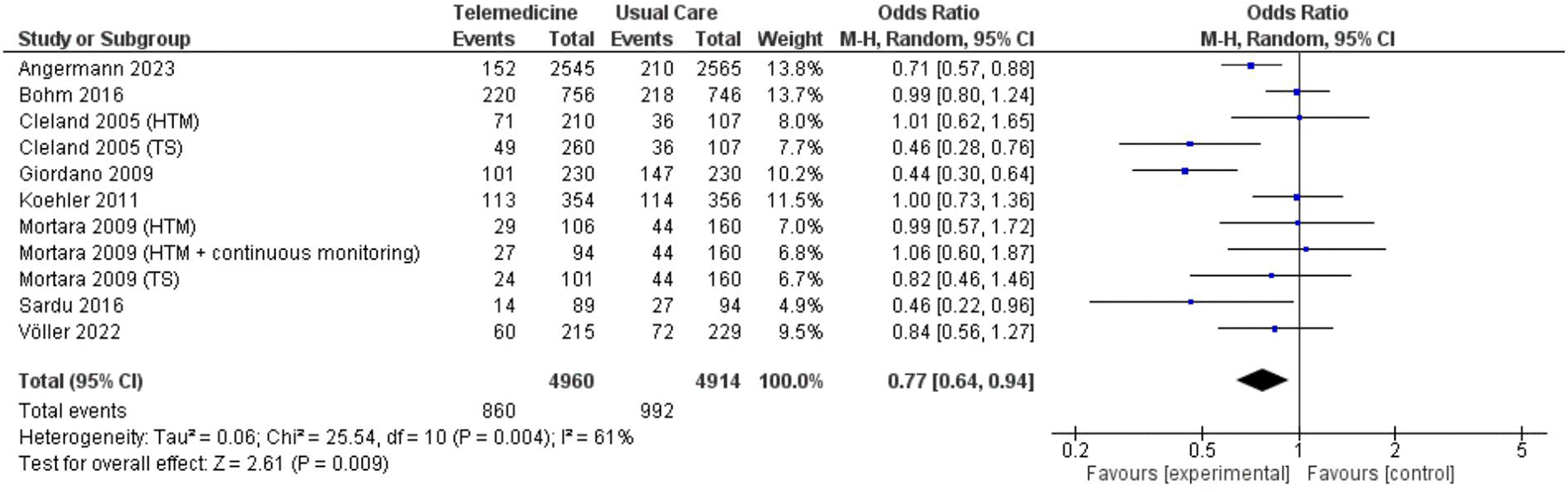
| HF-related hospitalization | number of events | 0.77, 0.62 – 0.95 | 0.01 | 58% | (not enough studies) | 0.77, 0.64 – 0.94 | 0.009 | 61% | ||
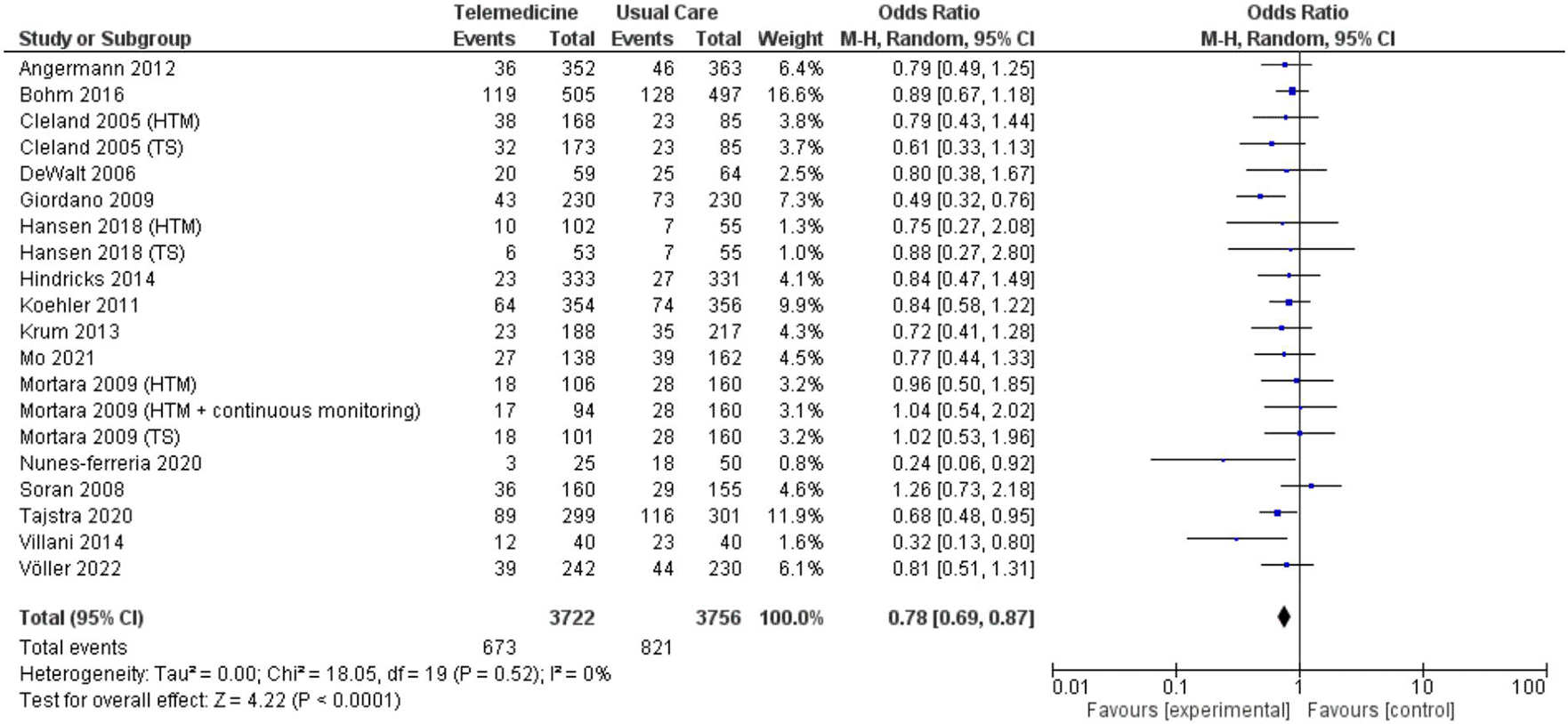
| number of patients | 0.77, 0.65 – 0.90 | 0.001 | 15% | 0.80, 0.65 – 0.97 | 0.03 | 0% | 0.78, 0.69 – 0.87 | <0.0001 | 0% | |
| Sensitivity analyses (number of patients) | <6 months | 0.79, 0.49 – 1.25* | 0.31 | NA | (not enough studies) | |||||
| 6-11 months | 1.26, 0.73 – 2.18* | 0.41 | NA | |||||||
| ≥12 months | 0.73, 0.62 – 0.86 | 0.0002 | 7% | |||||||
We employed Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) as the recommended tool for evaluating the risk of bias in randomized trials. Two authors independently evaluated the articles for risk-of-bias, and if any author encountered confusion, a third author was consulted to establish a consensus.
We employed a forest plot to effectively present the outcomes of our primary endpoints. Specifically, we will use forest plots for binary endpoints, accompanied by 95% confidence intervals (CI). To assess heterogeneity among the studies, we will utilize the I2 statistic. I2 values ranging from 0% to 25% will be labeled as “low,” 25% to 50% as “moderate,” and >50% as “high”.13 Subgroup analysis of non-invasive and invasive group was conducted to explore possible causes of heterogeneity. Any p-values below 0.05 will be considered statistically significant. All statistical analyses will be carried out using Review Manager (RevMan). The odds ratio was calculated to evaluate the risk of mortality and hospitalization in the available studies. Mortality was calculated based on the total number of patients, while hospitalization was calculated based on the numbef of events of total patient-year and number of patients. Sensitivity analyses were carried out based on different follow-up durations (< 6 months, 6-11 months, and ≥ 12 months) to assess the strength of the study results.
We conducted an extensive literature review encompassing trials spanning from 1999 to the present. Out of the initial pool of 4,947 articles, we narrowed down our analysis to 53 studies, in which our two main authors each reviewed the full-text articles. We excluded 16 articles because of the numerous reasons mentioned in our PRISMA figure ( Figure 1). Finally, 27 studies were included in qualitative and quantitative synthesis. Among all of the studies which compared usual care and telemedicine, we found 24 articles reported all-cause mortality and 14 articles reported cardiovascular-related mortality. For all-cause hospitalization, there are 5 studies which reported all-cause hospitalization based on number of events while 13 studies reported with total number of patients. In HF-related hospitalization, 8 studies reported HF-related hospitalization based on number of events, and 16 studies reported with total number of patients. We also conducted subgroup analysis of non-invasive and invasive approach, as well as sensitivity analyses with the aim of attaining a lower level of heterogeneity. Table 2 provides a summary of the included studies and the outcomes associated with subgroup analyses (non-invasive and invasive approache) as well as the sensitivity analyses ( Table 2).
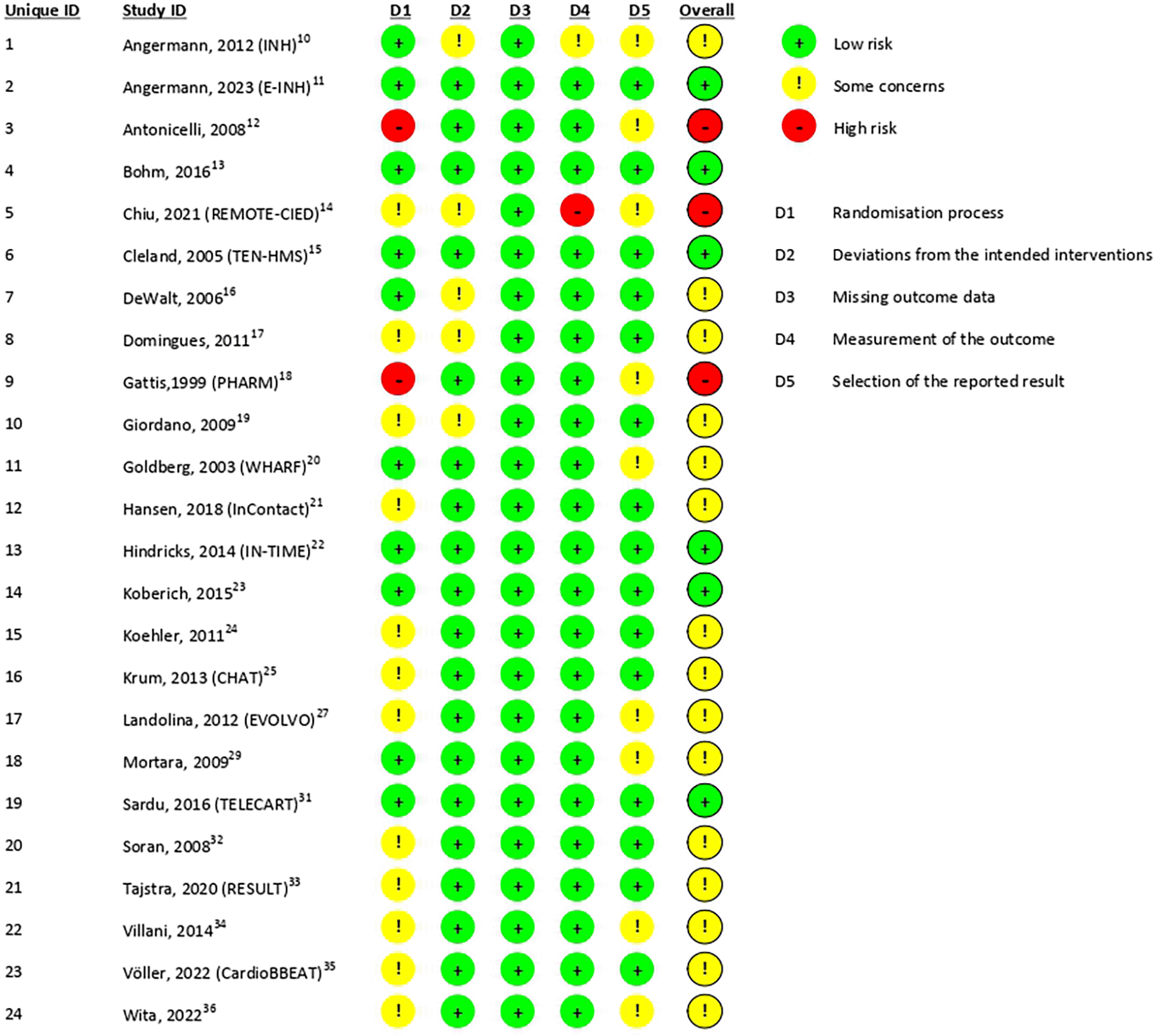
We evaluated the quality of our 24 RCT studies using the Cochrane RoB 2 tool ( Figure 2), while the 3 observational studies were assessed using the ROBINS-I tool ( Figure 3). For RCT studies, the overall bias with the RoB 2 tool was distributed as follows: 37.5% at low risk, 50% with some concerns, and 12.5% at high risk ( Figure 4). High-risk studies were primarily attributed to poorly described randomization processes. The overall quality of the observational studies by ROBINS-I tool was assessed as moderate risk, primarily within domain 1 bias (bias due to confounding). This was because the studies did not explicitly mention confounding factors or adjustments made for them.
The general characteristics of the population, type of interventions and control group were included in Table 1 along with the primary and secondary outcomes. The lowest time of follow-up was 1.5 months and the longest time of follow-up was 120 months.9,14 Of the 27 studies included, there are 3 studies which were not randomized.15–17 8 studies used implanted devices either it is implantable-cardiac-device (ICD) or cardiac-resynchronization-therapy device (CRT-D).15,18–24 The other 19 studies used non-invasive devices.9,14,16,17,25–39 While most of the usual care/control groups were categorized for in-clinic follow-up only, there were 3 studies which allowed for the patients in the control group to occasionally contact their physician/nurse.25,31,33 All of the noninvasive interventions monitored the patients using standard telephone/telemonitored/telehealth features interactively. Most reported outcomes included all-cause mortality, cardiovascular-related mortality, all-cause hospitalization, and HF-related hospitalization.
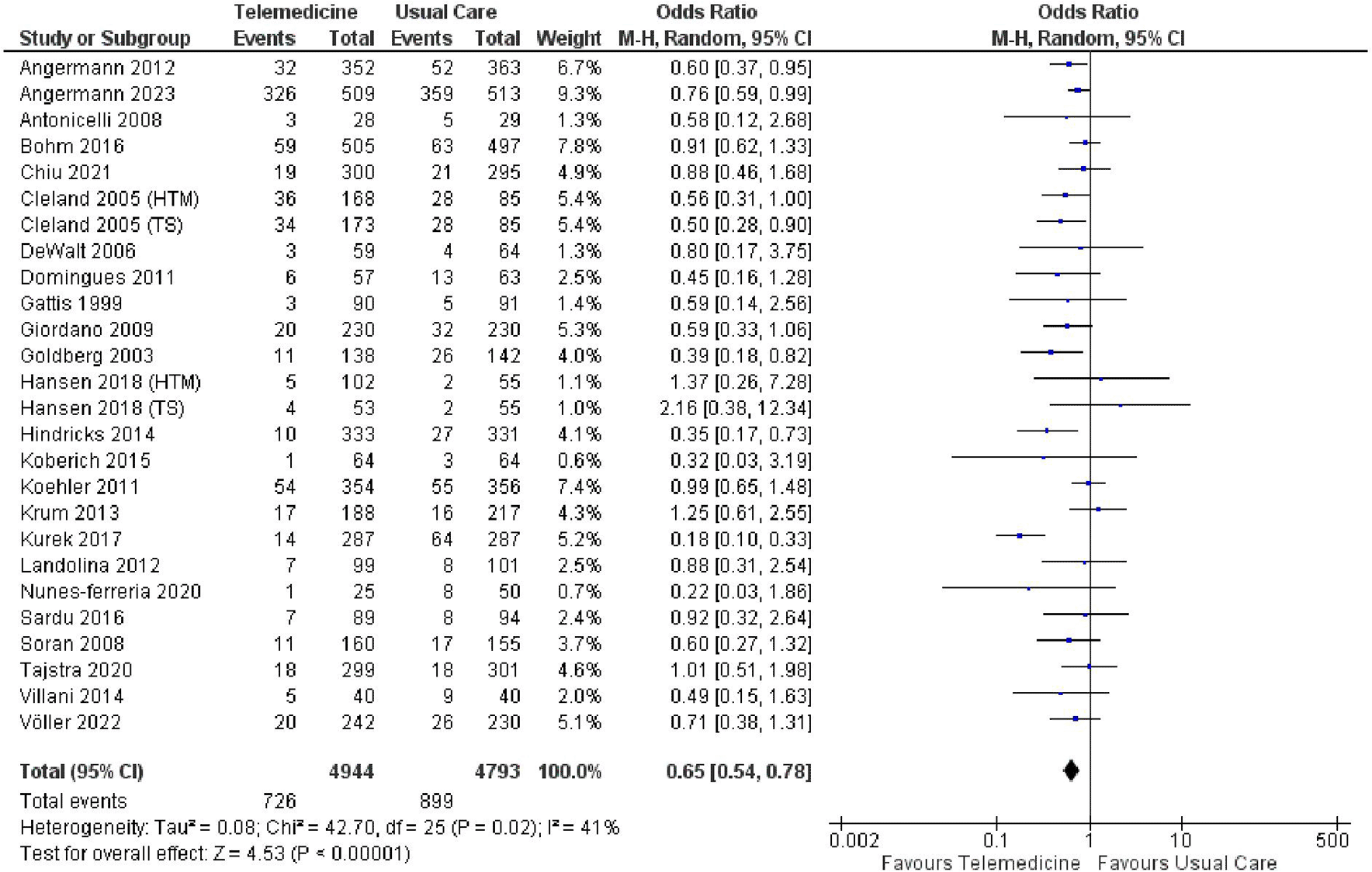
Twenty-four studies reported all-cause mortality, involving a total of 9,737 participants, while 14 studies presented data on cardiovascular-related mortality, involving a total of 7,477 participants (studies with more than 2 intervention arms were divided). The meta-analysis revealed a noteworthy decrease in the risk of all-cause mortality (OR 0.65, 95% CI 0.54 – 0.78, p < 0.00001, I2 = 41%) ( Figure 5) and a significant reduction in the risk of cardiovascular-related mortality (OR 0.68, 95% CI 0.58 – 0.80, p < 0.00001, I2 = 7%) ( Figure 6).
(HTM=home telemonitoring; TS=telephone support).
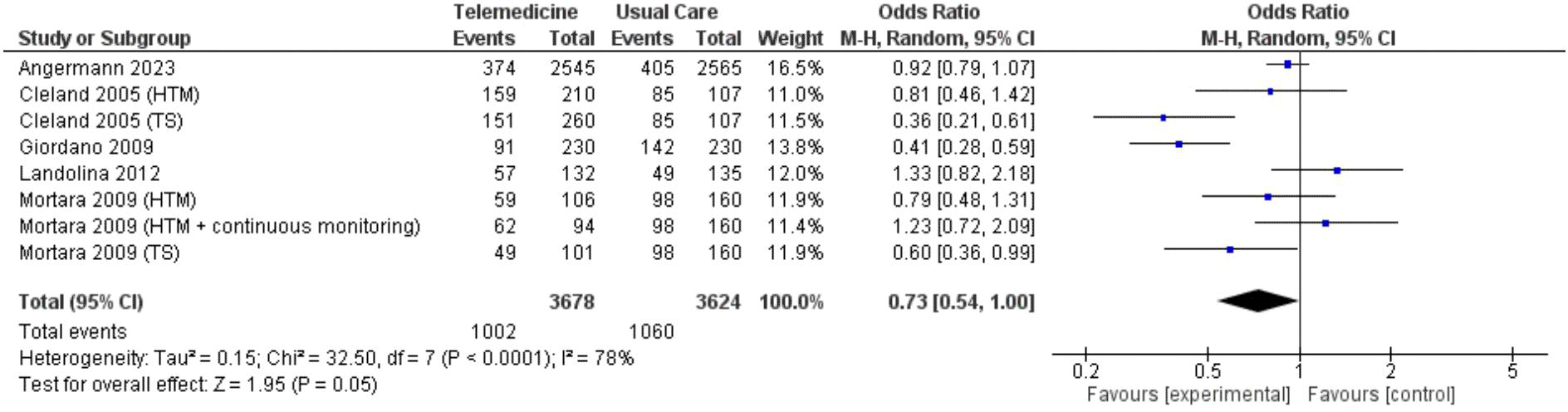
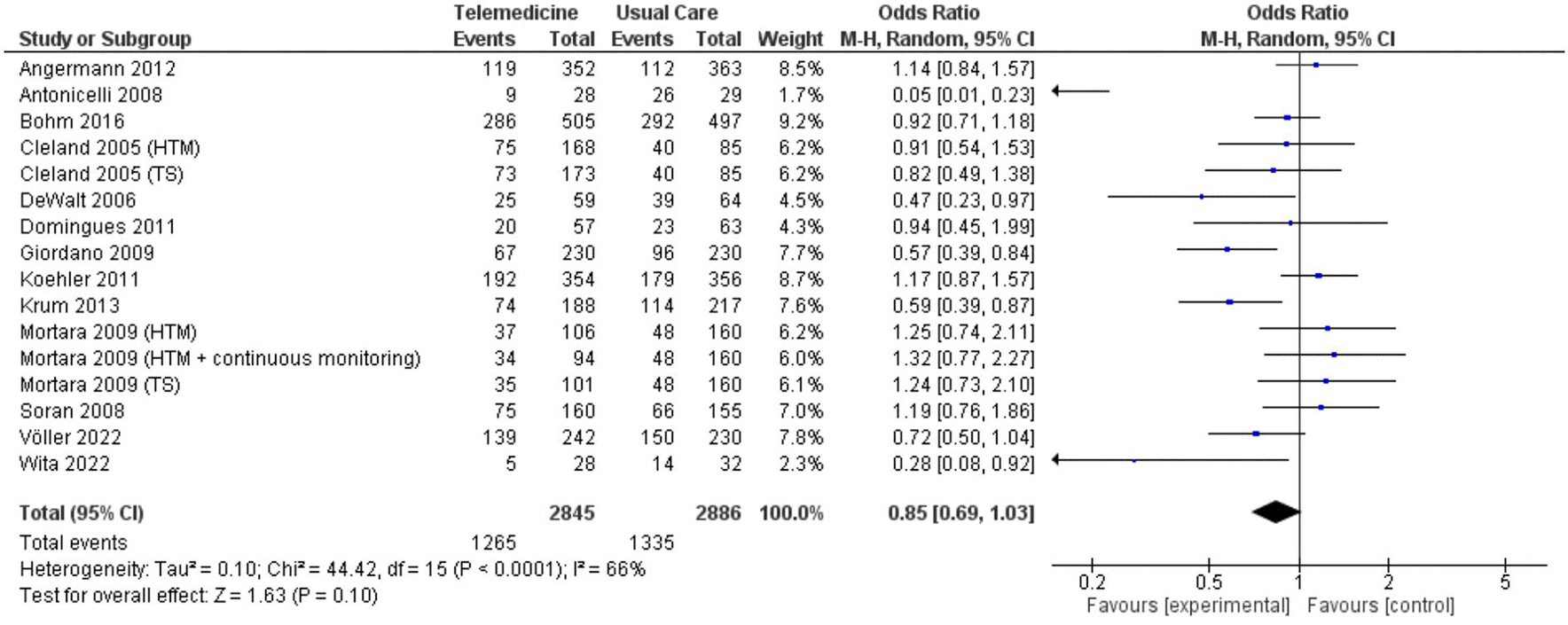
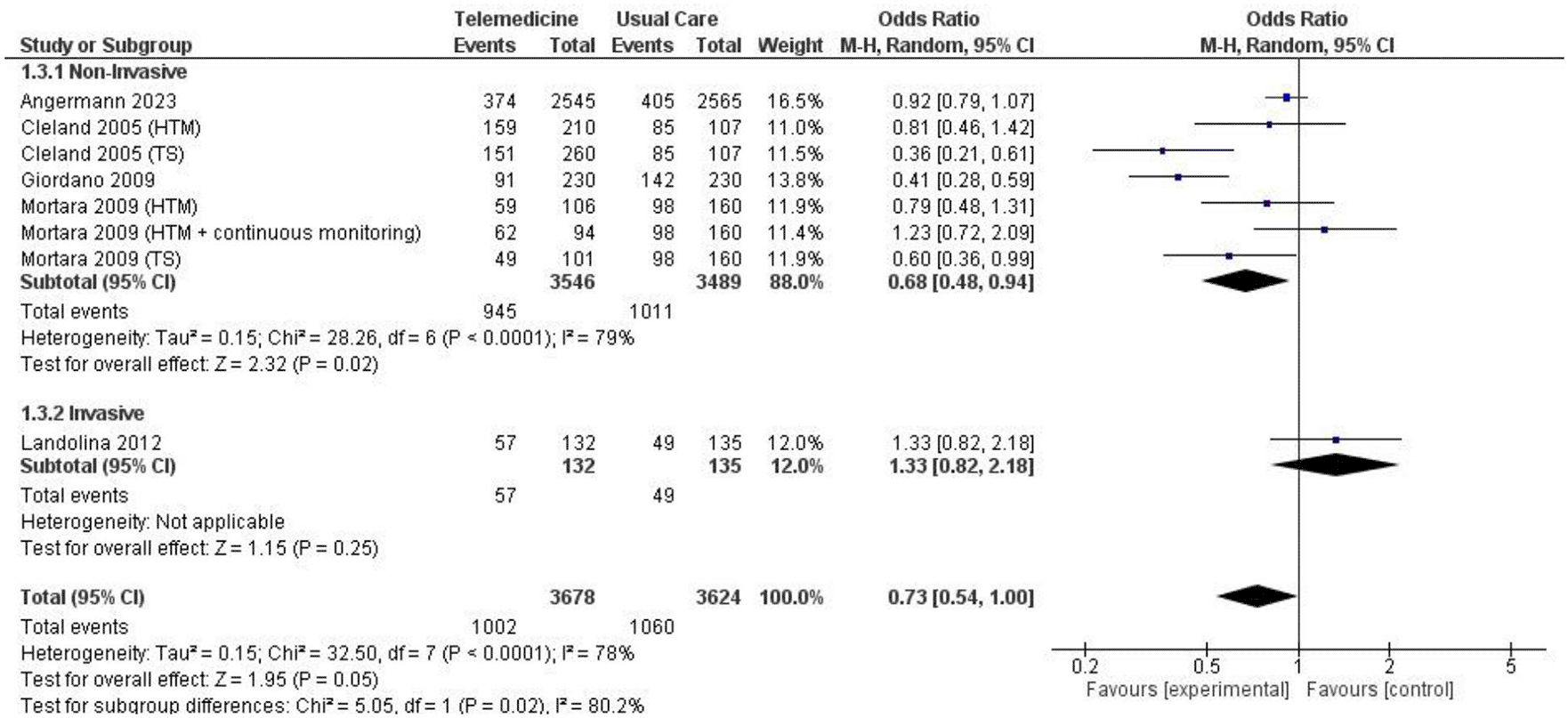
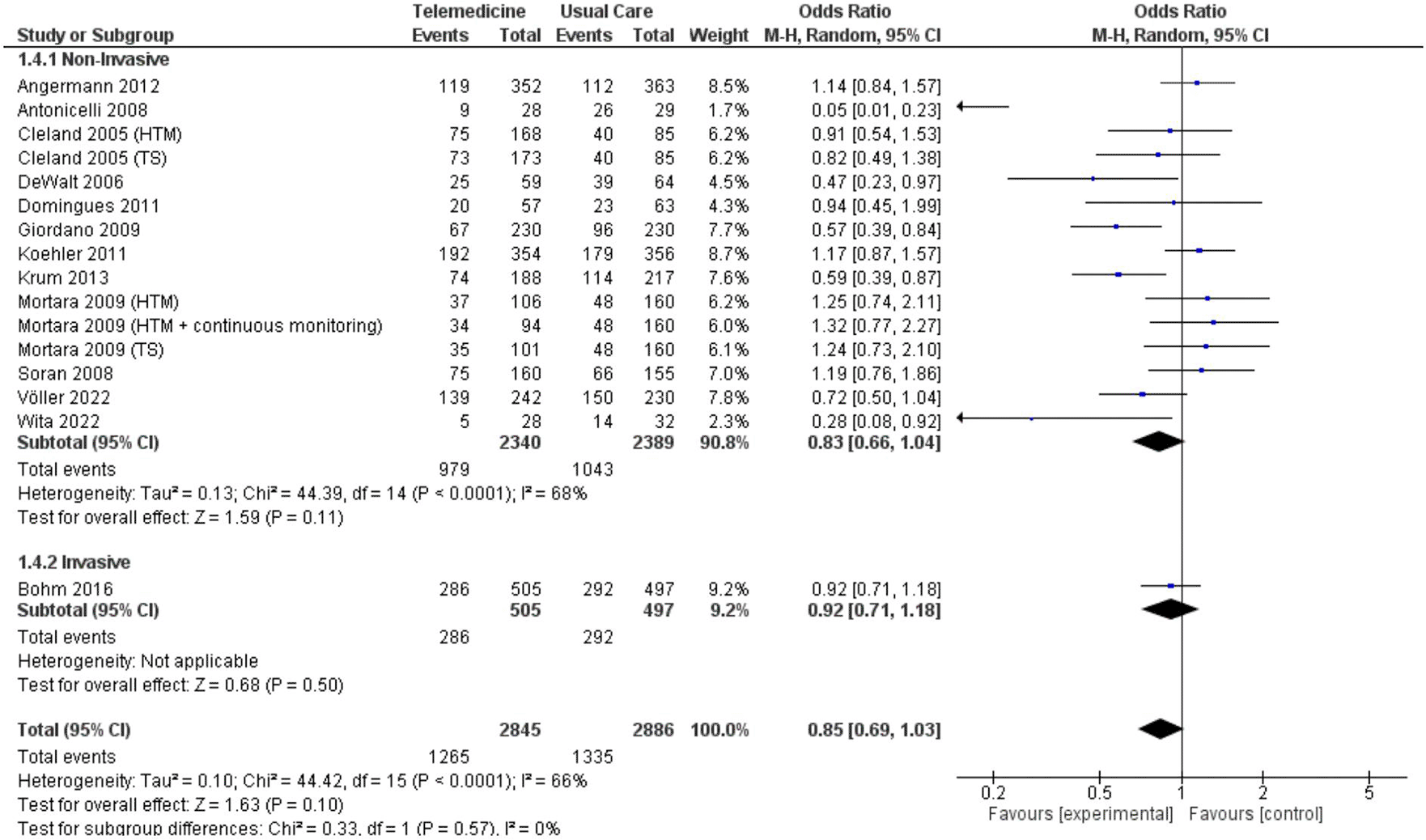
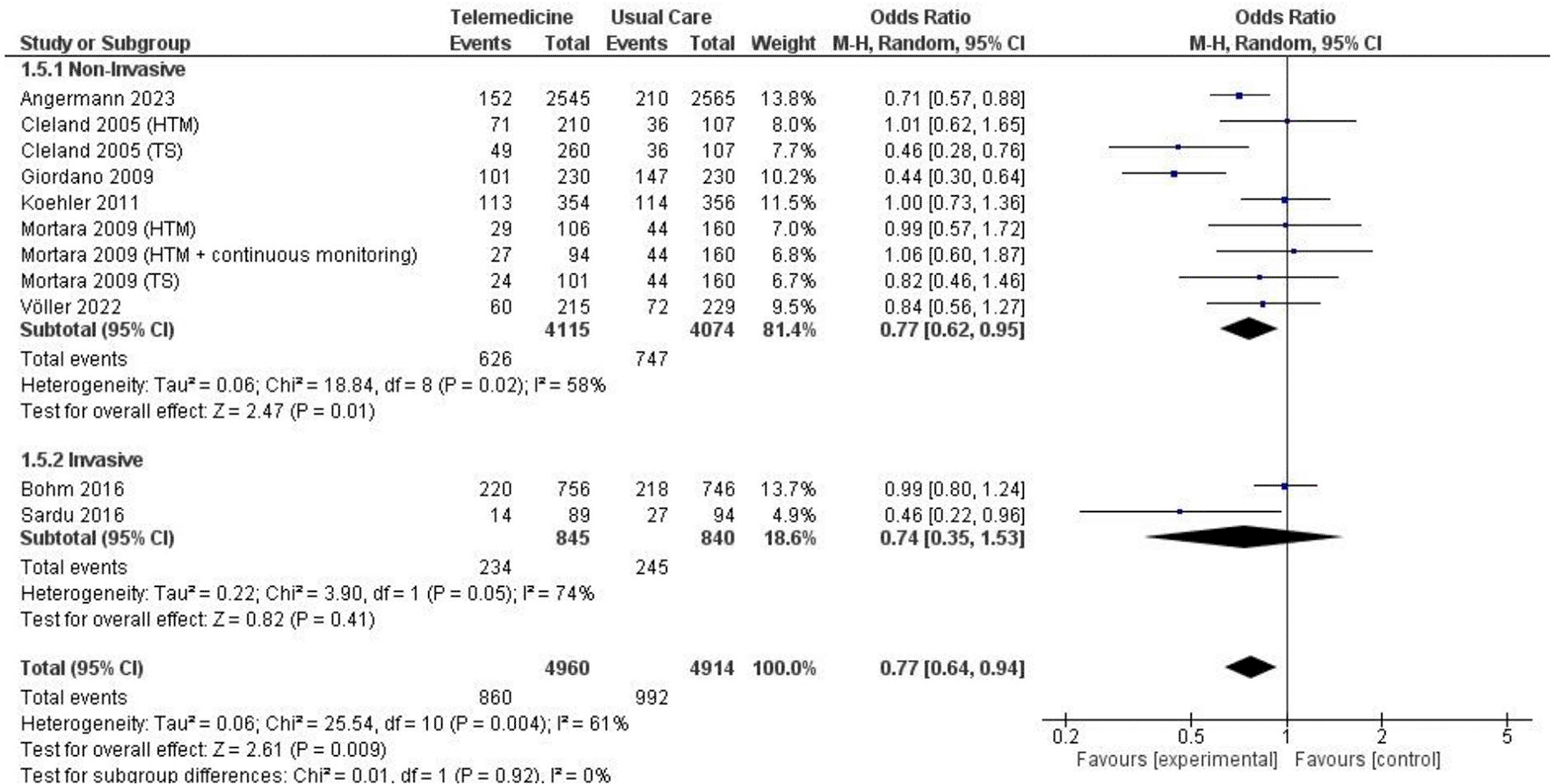
In two analyses focusing on all-cause hospitalization, one based on number of events and the other on the number of patients admitted. Findings from 5 studies totaling 7,302 patient-years reported incidents of hospitalization based on number of events ( Figure 7; Figure 8). However, these results did not show a significant reduction in the risk of all-cause hospitalization based on number of events (OR 0.73, 95% CI 0.54 – 1.00, p = 0.05, I2 = 78%) ( Figure 7). Thirteen studies with total participants of 5,731 mentioned studies of number of patients who went for admission for all-cause, also with insignificant reduction of the risk (OR 0.85, 95% CI 0.69 – 1.03, p = 0.10, I2 = 66%) ( Figure 8). Eight studies with total patient-year of 9874 reported HF-related hospitalization based on number of events with no significant reduction of the risk (OR 0.77, 95% CI 0.64 – 0.94, p = 0.009, I2 = 61%) ( Figure 9). Sixteen studies with total number of patients 7478, reported HF-related hospitalization based on total number of patients who went for admission, with significant reduction of the risk (OR 0.78, 95% CI 0.69 – 0.87, p < 0.0001, I2 = 0%) ( Figure 10).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
(HTM=home telemonitoring; TS=telephone support).
We performed a subgroup analysis comparing non-invasive and invasive approaches in terms of all-cause mortality. The non-invasive approach showed a significantly lower result (OR 0.69, 95% CI 0.59 – 0.80, p < 0.00001, I2 = 0%), whereas the invasive approach did not yield a significant result (OR 0.70, 95% CI 0.42 – 1.15, p = 0.16, I2 = 73%) ( Figure 11). In the non-invasive subgroup analysis of cardiovascular-related mortality, a lower significant outcome was observed (OR 0.69, 95% CI 0.58 – 0.82, p = 0.0001, I2 = 0%), while the invasive approach showed a similar outcome with no significant result (OR 0.63, 95% CI 0.36 – 1.08, p = 0.09, I2 = 51%) ( Figure 12). The sensitivity analyses of the all-cause mortality in non-invasive group achieved significant result, similar with the main analysis (see Extended data section for supplementary figures1-6). We were not able to conduct the same sensitivity analysis in the invasive group due to the lack of number of the studies.
We also conducted subgroup analyses for both non-invasive and invasive approaches, focusing on all-cause hospitalization events and the number of patients experiencing all-cause hospitalization and HF-related hospitalization. The non-invasive subgroup analysis of all-cause hospitalization based on the number of events yielded a significant result, albeit with high heterogeneity (OR 0.68, 95% CI 0.48 – 0.94, p = 0.02, I2 = 79%) ( Figure 13), while the non-invasive subgroup analysis of all-caused hospitalization based on the number of patients did not have any significant result (OR 0.83, 95% CI 0.66 – 1.04, p = 0.11, I2 = 68%) ( Figure 14). The non-invasive subgroup analysis of HF-related hospitalization based on the number of events showed a significant result (OR 0.77, 95% CI 0.62 – 0.95, p = 0.01, I2 = 58%) ( Figure 15). Unfortunately, the invasive subgroup analyses of all-cause hospitalization and HF-related hospitalization based on number of events could not be conducted due to a low number of studies. When considering the number of patients, the non-invasive subgroup analysis of HF-related hospitalization demonstrated a significant result (OR 0.77, 95% CI 0.65 – 0.90, p = 0.001, I2 = 15%), and a similar result was observed in the invasive subgroup analysis (OR 0.80, 95% CI 0.65 – 0.97, p = 0.03, I2 = 0%) ( Figure 16).
We attempted to conduct a sensitivity analysis for all-cause hospitalization by the number of patients and for HF-related hospitalization in the non-invasive group. The results indicated significance in studies with a follow-up period of ≥12 months in HF-related hospitalization, similar with the main analysis, but not in the all-cause hospitalization (see extended data: Supplementary figure 9; Supplementary figure 10). There were not enough studies with follow-up periods of < 6 and 6 – 12 months in non-invasive group in both all-cause and HF-related hospitalization, therefore the analyses were considered invalid. We also were not able to conduct the same sensitivity analysis in hospitalization based on number of events in non-invasive group, and in all of the outcomes in the invasive group, due to the same reasoning.
As the emphasis of contemporary healthcare is progressively transitioning from treating illnesses to proactively preventing them and supporting recovery, remote monitoring and telemedicine are poised to become pivotal areas of interest for both scientific research institutions and manufacturers in the coming years.40 The first definition was proposed by the World Health Organization (WHO) in 2007 as follows, “The delivery of health care services, where distance is a critical factor, by all health care professionals using information and communication technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and for the continuing education of health care professionals, all in the interests of advancing the health of individuals and their communities”.41
HF is a common, debilitating, unpredictable, gradually worsening condition with a poor outlook, which places a substantial strain on resources and finances. Objectives in managing HF encompass enhancing symptoms, functional capabilities, quality of life, and patient self-efficacy, while also aiming to lower hospital admissions, mortality rates, and the associated logistical and financial burdens.42 Despite recent pharmacological and therapy advancements, HF, particularly with reduced ejection fraction confers a high morbidity and mortality worldwide.43 The primary objective of remote monitoring for HF is to identify early signs of HF hemodynamic deterioration, enabling timely intervention and thereby preventing HF-related hospitalizations. Therefore, telemedicine might offer more frequent monitoring of patients’ status remotely and could be used as a tool to improve outcomes.41,44
Our meta-analysis, which comprises 27 studies focused on telemedicine in HFrEF suggests that telemedicine system significantly reduced all-cause mortality and cardiovascular-related mortality, in either main analysis, subgroup analyses, and sensitivity analyses. Our analysis also showed significant result in HF-related hospitalization in the main analysis. Previous meta-analysis of RCTs which included 29 RCTs showed that telemedicine significantly reduced all-cause hospital admission (OR 0.82, 95% CI 0.73-0.91, p <0.00001) and cardiac hospital admission (OR 0.83, 95% CI 0.72-0.95, p =0.003).44 Different from the meta-analysis of Zhu et al., we included both RCT and Non-RCT studies and specifically assessed HFrEF patients. Telemedicine via remote telemedicine (RTM) offers patients a structured approach to managing their health conditions and can empower them to take control of their well-being. The primary focus of telemedicine is the timely identification of deteriorating health conditions and the swift implementation of medical interventions.45
The management of HFrEF has advanced throughout the years.46 Despite well-established evidences and guidelines regarding GDMT, its implementation is still poor in clinical practice.47,48 The major challenges are concern of side effects and inadequate patients’ follow up. Close-monitoring of vital signs, weight and patients’ symptoms, are important to guide dose titration of GDMT medications, which may be difficult to obtain during daily out-patient visits, including for patients with HFrEF. Therefore, telemedicine strategy could become useful in tackling this obstacle.45 A pilot study of DAVID-HF trial by Wong et al (2022) showed that daily ambulatory remote monitoring system using arm-band monitor may be feasible for drug dose escalation in HFrEF.49 Other meta-analyses also have indicated that telemedical monitoring for chronic HF can lead to a decrease in overall mortality over a 6 to 12-month follow-up period.50,51 Findings from the Trans-European Network-Home-Care Management System (TEN-HMS) Study demonstrated that HF patients who were assigned to telemedicine immediately after being discharged from the hospital for HF experienced lower mortality compared to patients in the usual care group.26,52 These findings align with our sensitivity analysis, revealing significant results for all-cause and cardiovascular-related mortality across different follow-up durations (< 6 months, 6 – 12 months, and ≥ 12 months) (extended data: Supplementary figure 7; Supplementary figure 8). This suggests that telemedicine is beneficial for both short-term and long-term mortality outcomes. In the period preceding active promotion of GDMT, telemedicine emerged as a valuable tool, facilitating continuous assessment of treatment efficacy. This encompassed the monitoring of essential parameters such as body weight, urine output, blood pressure, electrocardiogram (ECG), heart rate, and the potential manifestation of side effects.25
The IN-TIME trial revealed three potential mechanisms that could enhance clinical outcomes and reduce overall mortality. These include the timely identification of life-threatening tachyarrhythmia, prompt recognition of suboptimal ICD/RCT-D function, and increased awareness among patients regarding the worsening of symptoms.21 A significant majority, around 80%, of patients experiencing HFrEF will exhibit frequent and intricate ventricular arrhythmias. Particularly in cases of HFrEF, these ventricular arrhythmias can adversely impact cardiac hemodynamics, worsening the HF syndrome. The timely identification of these arrhythmias is crucial as it can lead to more favorable clinical outcomes and mortality.53
In contrast, individual studies of the TIM-HF and the Tele-HF trial did not confer similar significant results and diverged from those of the TEN-HMS study and the pooled analyses.54,55 This variation could be explained by the diverse nature of studies on RTM that include HF patients with different risk profiles, varying durations of follow-up, and variations in RTM methodologies across trials. Furthermore, the meta-analyses and trials which showed significant results primarily encompassed a combination of telephone-based monitoring (with or without personal intervention) and technology-assisted monitoring reliant on information.25,26,52
In principle, the use of telemedicine is anticipated to reduce hospitalization rates among patients with HFrEF. Nevertheless, the outcomes from several trials have been varied. We uncovered a substantial overall decrease in HF-related hospitalization, but this reduction was not observed in all-cause hospitalization. To further scrutinize and challenge the analysis, we attempted a more detailed examination. Interestingly, the decrease in all-cause hospitalizations only exhibited statistical significance in the non-invasive group when considering the number of events. On the contrary, HF-related hospitalization consistently demonstrated significant results in the non-invasive group, particularly in sensitivity analyses for the ≥ 12 months group based on the number of patients. Unfortunately, the most of the analysis in the invasive group lacked a sufficient number of studies, but not in the HF-related hospitalization based on number of patients. The most reliably observed trend in terms of hospitalization is the decrease in all-cause hospitalizations based on number of events within the non-invasive group.
Giordano et al. reported a 36% decrease in total hospital readmissions and a 31% decrease in hemodynamic instability, with significantly lower HF hospitalization compared to the usual care group, similar to our main analyses of HF-related hospitalization.30 Conversely, Cleland et al. found no difference in the number of events between the telemedicine and usual care groups.26 The IN-CONTACT trial, which included telemetry, remote + phone, and usual care groups, reported no significant differences in hospitalization rates across all intervention arms.20 These findings align with earlier RCTs, such as the EVOLVO trial. In the EVOLVO trial, a combination of early issue detection through telemedicine and remote management proved more effective in reducing both the duration of hospitalization and mortality, rather than simply decreasing the number of hospitalizations.22 This discrepancy may be attributed to the occurrence of multiple hospitalization events in a single individual as well as low number of studies. Additionally, variations in baseline characteristics of HF patients across studies contribute to the differences, with Giordano et al. including NYHA class IV patients, while the IN-CONTACT trial and EVOLVO trial focused on NYHA class I-III patients, potentially explaining the higher hospitalization rates observed by Giordano et al.20,22,30 In terms of all-cause hospitalization, we could only pinpoint five studies with diverse methodologies and varying risk profiles of patients, all of which considered the number of events as an outcome. This diversity might explain the non-significant results observed in terms of all-cause hospitalization based on number of events ( Figure 7).
In both main analysis and non-invasive all-cause hospitalization based on number of patients, no significant results were attained ( Figure 8; extended data: Supplementary figure 9). However, we identified a significant favorable outcome in the sensitivity analysis of HF-related hospitalization based on number of patients, especially in the context of long-term outcomes in non-invasive group (≥ 12 months) (extended data: supplementary figure 10). Our research yielded results consistent with the findings of the Scholte et al. study, showing a significant decrease in hospitalization rates among patients, referred to as first hospitalization or in our study as hospitalization based on number of patients.10 Similar to their study, we observed that most studies demonstrating significant outcomes utilized either complex telemedicine or a combination of structured telephone and HTM. In the study conducted by Giordano et al, they employed a combined strategy, allowing patients to connect with predetermined nurses anytime and anywhere.30 Notably, they reported that the readmission curve did not diverge significantly until at least 100 days, possibly indicating a learning curve among staff in managing patients with telemonitoring. This observation may contribute to the varied prognosis seen in studies with longer follow-up periods. In contrast, we firmly believe, based on the study by Nunes-Ferreira et al., that the core of a successful telemonitoring program lies in the synergy of monitoring and regularly collecting biodata. Additionally, ensuring good patient adherence and compliance emerges as a fundamental key to the success of telemonitoring initiatives.17
Certain observational studies consistently demonstrate a reduction in hospitalization rates, specifically in arrhythmia patients. Piccini et al. conducted such a study and asserted that telemonitoring could alleviate the burden of hospitalization through the early detection of arrhythmia, lead malfunction, or device failure.56 They specifically highlighted the benefits for patients previously diagnosed with atrial fibrillation (AF), noting fewer hospitalizations for stroke, improved adherence to oral anticoagulation, and prompt detection of AF resulting in fewer transient ischemic attacks or strokes.56–58 These results might explain the significant result we had in hospitalizations based on number of events in the non-invasive group, which we further explain in the non-invasive approach section.
Non-invasive home telemonitoring includes HTM, TS, and complex telemonitoring using a combination of TS and/or 24-h call centre.
In our meta-analysis, we discovered notable benefits associated with a non-invasive approach in terms of all-cause mortality, cardiovascular-related mortality, all-cause and HF hospitalization based on the number of events, as well as HF hospitalization based on the number of patients. The sensitivity analyses also showed similar result with the main analysis (extended data: Supplementary figure 7 – 10). This study is, to our knowledge, the first comprehensive meta-analysis to showcase the advantages of a non-invasive approach for patients with HFrEF. Notably, Scholte et al. demonstrated significant results in reducing all-cause mortality, first hospitalization, and total hospitalizations in patients with HF overall.10 The E-INH trial, the latest study with the longest follow-up period of up to 120 months, yielded similar results with lower all-cause mortality and improved quality of life compared to the usual care group.9 The oldest study included in our meta-analysis, conducted by Gattis et al. in 1999, added that a straightforward evaluation by a clinical pharmacist significantly lowered all-cause mortality and HF events.29 Another study conducted in a rural area with 405 patients by Krum et al. demonstrated a reduction in the composite outcome and the number of patients hospitalized.35
Over the past two decades, even without a standardized protocol, the non-invasive approach has consistently demonstrated significant advantages through simple long-distance follow-up and evaluation. This might be due to, as previously mentioned, patients with HFrEF require improved compliance with GDMT to prevent further deterioration of the ejection fraction. Achieving this outcome necessitates continuous contact with specialists to maintain appropriate medication prescriptions, which might reduce the total number of hospitalizations required for the patients.9 We also believe that the heterogeneity within our non-invasive group is generally low, particularly regarding mortality outcomes, which aligns with findings from a previous meta-analysis by Scholte et al.10 They noted that the low heterogeneity observed in their study was attributed to the inclusion of stable HF patients (NYHA classes I-II). This observation supports the assertion that the non-invasive approach is genuinely advantageous in terms of reducing mortality across various patient profiles.
In addition to medical therapy, device utilization has become increasingly essential in HF management, particularly HFrEF. Device therapy includes cardiac resynchronization therapy (CRT) and implantation of intracardiac defibrillators, both for the primary and secondary prevention of sudden cardiac death (SCD).59 As HF patients are at increased risk for life-threatening arrhythmias and SCD, ICD should be considered in particular subset of HF patients.60 ICD has been shown to improve outcomes in eligible HF patients, particularly with reduced ejection fraction. It has been endorsed by current European guidelines.43,61 Contemporary ICDs have the capability to continuously assess the functionality of the implanted system, measure clinical parameters, and document the incidence of arrhythmias or other incidents. As a result, they have the potential to offer early alerts regarding alterations in cardiac conditions or safety concerns. All device manufacturers provide remote ICD monitoring technology, allowing physicians to access patient data from a distance and, consequently, minimizing the need for unnecessary routine and interim appointments.61
At present, studies on the invasive approach to telemonitoring are limited and exhibit diversity. Despite our efforts to perform subgroup and sensitivity analysis focusing on invasive devices, low number of studies, and the lack of standardized methodologies as well as substantial heterogeneity in baseline characteristics among the studies hindered the identification of any meaningful results. The only significant result in the invasive group was the HF-related hospitalizations based on number of patients, with only 4 studies included ( Figure 16). Additionally, multiple studies have confirmed the advantages of telemonitoring in invasive devices. The IN-TIME study has yielded promising randomized data that demonstrates the advantages of invasive remote monitoring for implanted devices in improving clinical outcomes. The combined clinical assessment, which considered factors such as overall mortality, HF hospitalizations, changes in the NYHA class, and shifts in patient self-assessment, showed better results in the group utilizing invasive remote monitoring.30 As previously mentioned, Hindricks et al. outlined a potential function of invasive telemonitoring, highlighting its ability to identify suboptimal ICD/RCT-D performance.21 One of the potential harm is that these devices may trigger unnecessary and inappropriate defibrillation shocks, leading to hemodynamic consequences, and associated with excess mortality.62 In this context, telemonitoring can promptly detect such issues and enable early intervention.30 These results align with the TELECART study, where telemonitoring was identified as a predictive factor for hospitalization in HFrEF patients undergoing treatment with invasive devices.23 The RESULTS study similarly supported this finding, revealing that the group with invasive devices exhibited a lower primary endpoint outcome (comprising all-cause mortality or cardiovascular-related hospitalization) and a 50% reduction in in-clinic visits.24 The utilization of invasive techniques is also able to detect imminent deterioration, such as an increase in pulmonary artery pressure.9 While not included in our study, findings from the PREFER study and CONNECT trial suggested that remote monitoring with automatic notifications significantly shortened the time to a clinical decision in response to clinical events, particularly atrial arrhythmias—a prevalent complication in patients with HFrEF.63,64 Scholte et al. reached a similar conclusion with our study, possibly influenced by the limited number of studies included. The analysis indicated that the subgroup analysis focusing on invasive approaches did not reveal significant benefits in terms of mortality and hospitalization.10 Nevertheless, their findings highlighted a specific positive outcome related to IHM, which was associated with a reduction in hospitalizations related to HF by enabling the modification of diuretic dosages to prevent decompensation. This is particularly important, as decompensation stands out as a primary factor contributing to recurrent hospitalizations in HF patients.10 The DOT-HF trial and EVOLVO study also validated this outcome, showing a substantial decrease in HF hospitalizations.22,65 Additional studies on the invasive approach to telemonitoring are necessarily required to validate and confirm the current theory.
The incorporation of telemonitoring into clinical practice offers considerable promise for the management of HFrEF. Telemonitoring enables the continuous observation of key health parameters, such as body weight, urine output, blood pressure, electrocardiogram (ECG), heart rate, and the identification of potential side effects, facilitating early detection of clinical deterioration and enabling timely and appropriate adjustments to treatment regimens. This proactive approach has the potential to mitigate hospitalization rates and reduce the need for emergency interventions, thereby improving patient outcomes and overall quality of care. In our study, we observed a statistically significant reduction in both all-cause mortality and HFrEF-related hospitalizations, emphasizing the potential clinical benefits of telemonitoring for this patient population. Moreover, the flexibility in follow-up durations, including < 6 months, 6–12 months, and ≥ 12 months, allows for the customization of care to meet the individual needs of patients. Non-invasive home telemonitoring systems—such as home telemonitoring (HTM), telemedicine services (TS), and more complex telemonitoring systems that combine TS with 24-hour call centers—empower patients to take an active role in managing their health while providing healthcare providers with real-time data to inform clinical decision-making. From a clinical perspective, the implementation of telemonitoring can improve patient adherence to prescribed therapies, reduce healthcare costs, and enhance access to care, particularly for patients in remote or underserved areas. Therefore, the integration of telemonitoring into standard clinical practice could serve as a valuable strategy for optimizing the management of HFrEF and advancing the delivery of efficient, patient-centered healthcare.
The primary factors contributing to the costs of telemedicine include the allocation of specialized human resources, the provision of appropriate technology, and the interaction between the medical team and the patient.1 Healthcare providers must carefully consider the potential limitations of these technologies, as they can incur significant costs, particularly for smaller healthcare facilities or hospitals. Additionally, telemedicine may not be suitable for patients who prefer face-to-face consultations with their physicians. Therefore, it is crucial for healthcare professionals to establish a strong rapport with patients to facilitate their comfort and acceptance of these emerging technologies. Moreover, it is important to recognize that no service is flawless, and telemedicine is no exception. Despite these challenges, numerous studies have consistently demonstrated that telemedicine can result in considerable savings in time and costs, as well as improvements in patient outcomes.
To the best of our knowledge, there are already multiple studies of HF patients with telemedicine/telemonitoring, but our study is one of the first telemedicine studies with HFrEF patients.
There are a couple of limitations which should be mentioned. Please consider that our meta-analysis identifies substantial concerns related to bias and heterogeneity. This may stem from the considerable diversity and lack of standardization in the interventions studied. Several of the included studies exhibited considerable bias risks due to inadequate descriptions of the randomization process and the absence of adjustments for confounding factors. Additionally, both subgroup and sensitivity analyses were conducted as part of our efforts to enhance the quality of our analysis. Furthermore, there was a discrepancy in the approaches used among the included studies, with some employing invasive strategies and others non-invasive ones. For instance, in 1999, Gattis et al. employed a clinical pharmacist to evaluate the overall health of patients, which included monitoring vital signs and making adjustments to medications.29 In contrast, the majority of other studies relied on a physician or a combination of a physician and a nurse for these assessments. The criteria for including patients with reduced EF also varied, with most invasive studies involving patients with EF less than 35%, often using ICD or RCT-D. There are also multiple studies which include or exclude chronic stable HF patients (NYHA I-II) or with clinically severe HF (NYHA IV). Additionally, several studies featured more than two intervention arms.20,26,33 The differences in follow-up times were also responsible for the high heterogeneity. We were not able to analyze the differences in subgroup analysis as most of the studies do not always have the preferable outcome of interest.
Subsequently, variations in the definition of “usual care” across these trials may contribute to the absence of a significant reduction in hospitalization. Another factor contributing to the challenge in our meta-analysis is the variation in rehospitalization definitions across multiple articles. Some studies report rehospitalizations based on the total number of events, while others use the total number of patients. The lack of standardized measurements makes it difficult for us to incorporate these diverse data into the meta-analysis.
In summary, we filtered the initial 4,947 articles down to 27 that were thoroughly reviewed. We found that telemedicine has demonstrated its effectiveness in patients with HFrEF. However, the high heterogeneity among the articles due to the variations in how telemedicine and usual care were conducted, the lack of invasive studies and standardized method and definition, as well as the insufficient assessment of confounding bias, limit our study from achieving its highest quality and potential. We expect that future researchers will explore invasive approaches further and more on standardizing effective telemedicine practices due to the existing variability in methods and clinical situation of the patients.
Reporting guidelines
Figshare: Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow-chart (PRISMA) flowchart for “Current real world health data of telemedicine for heart failure with reduced ejection fraction: A systematic review and meta-analysis”. https://doi.org/10.6084/m9.figshare.24995804.v366
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Figshare: Preferred Reporting Items for Systematic Reviews and Meta-Analyses Flow-chart (PRISMA) checklist for “Current real world health data of telemedicine for heart failure with reduced ejection fraction: A systematic review and meta-analysis”. https://doi.org/10.6084/m9.figshare.24995780.v367
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Figshare: Supplementary figures of “Current real world health data of telemedicine for heart failure with reduced ejection fraction: A systematic review and meta-analysis”. https://doi.org/10.6084/m9.figshare.25712379.v1.68
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors express their gratitude to Hariadi from the Department of Cardiology and Vascular Medicine, Faculty of Medicine, Public Health, and Nursing at Gadjah Mada University for providing updated insights into systematic review and meta-analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiovascular
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public health, Health science, Biotechnology, Biology, Plant propagation
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public health, Biotechnology, Biology, Plant propagation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 28 Apr 25 |
||
|
Version 2 (revision) 25 Oct 24 |
read | read |
|
Version 1 11 Jun 24 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)