Keywords
Influenza, vaccination,immunisation,infants
The primary objective of this systematic review was to identify the optimal timing for immunizing pregnant women to confer the most protection by reducing the incidence of laboratory-confirmed influenza or influenza-like illness in infants less than 6 months of age. Currently, there are gaps in research regarding the timing of administration during the gestational period to provide maximum immunogenicity to the infant. The research question being addressed is: ‘When considering immunization of pregnant mothers with the influenza vaccine, implementing a vaccination program during which trimester in pregnancy would optimize benefits for infants less than 6 months of age in terms of the incidence of laboratory-confirmed influenza and influenza-like illness?’
Systematic review/Meta-analysis
Randomized controlled trials (RCT’s) and observational studies comparing health outcomes of infants and children up to 6 months of age born to women who received inactivated influenza vaccine during pregnancy with mothers who did not receive the vaccine or received a control vaccine. The primary outcome was laboratory-confirmed influenza infection in infants. Secondary outcome measures included influenza –like illness diagnosed by a clinician and acute respiratory illness.
7 studies were included: 2 primary RCT’s and 5 observational studies (prospective and retrospective cohort studies).5 of the 7 studies were suitable to be included in the quantitative synthesis part and and were compared. Forrest plot analysis revealed that vaccinating pregnant mothers in the second and third trimester when compared with any trimester (1,2 and 3) (OR 0.18 vs. 0.65) conferred less protection, promoting vaccination in the first trimester.
Vaccinating pregnant mothers in the first trimester conferred greater protection to infants than any other trimester.
Influenza, vaccination,immunisation,infants
- Up to date search of studies
- Changed our conclusions
- Added some more detail to our methods section
- Identified some more confounders and tried to control them
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Influenza viruses belong to the Orthomyxoviridae family and are classified into three types: A, B, and C. In humans, types A and B are the most prevalent and cause infection of the upper respiratory tract, leading to mild symptoms such as low-grade fever, sore throat, fatigue, and muscle aches.1 However, serious complications can occur in clinically high-risk groups, such as people with a weakened immune system, over 65-year-olds, pregnant women, and children under the age of 2. Complications include myocarditis, bacterial pneumonia, sepsis, and multi-organ failure, which can be fatal.2 During pregnancy, many natural changes in the body, such as a decrease in respiratory volumes and urinary stasis due to an enlarging fetus, can lead to a heightened risk of these complications, often leading to hospitalization, and can even cause death of the mother and fetus.3 In pregnant women, complications may include miscarriages and preterm deliveries. Newborns born when a woman is infected during pregnancy may have congenital anomalies such as leukemia, Parkinson’s disease, schizophrenia, congenital heart defects, and neural tube defects.4 This group of viruses are the most commonly known viruses causing respiratory infections that lead to high rates of mortality worldwide, affecting 11% of pregnant women worldwide.5
In 2012, the World Health Organization published a position paper stating that influenza vaccination during pregnancy provides protection to both mothers and infants. Evidence suggests that immunization at an earlier stage provides better protection to the woman, and during the later stages, it provides a longer duration of protection for the infant.6 Various studies have been conducted on maternal immunization for influenza virus, discussing the protection it provides for the mother and child. Infants under 6 months of age produce a weak antibody and T-cell response to viruses such as influenza due to an immature innate immune system. The adaptive immune system also produces an inadequate response to T cell-dependent antigens encountered upon exposure to the virus. The lack of a sufficient immune response as a result of the inability to produce antibodies on their owncan cause serious ramifications for an infant’s health. This shows that active immunity, and therefore vaccination, does not confer effective protection for infants.7 However, immunity against influenza is essential for infants to avert potential life-threatening consequences and to survive possible infections. Maternal vaccination is administered to achieve immunity without needing an immune response, that is, not via direct vaccination, and to reduce the rate of mortality due to influenza in the age group.8 Passive immunity is attained via transplacental transmission of antibodies and breastfeeding from the mother to the child without direct vaccination of the infant.9 It is the most effective method of protection as a child cannot be vaccinated directly since studies proving the safety of direct vaccination for influenza in under 6-month-old children have not yet been conducted.
However, misconceptions about vaccination leading to stillbirth have affected the number of people willing to take it.10 Safety concerns regarding possible teratogenic effects during organogenesis in the embryonic stage of development in the first trimester have led to low uptake within the subgroup.11 Despite scientific evidence suggesting that the vaccine is safe for pregnant women, only 45% received the vaccine during the 2018/2019 flu season in the UK.12 The low uptake results from a lack of awareness regarding the risks associated with influenza for the infant and mother, as well as the misconceptions previously mentioned. In addition, in the UK, guidelines suggest vaccination during any trimester, whereas Australian recommendations suggest vaccination during the second or third trimester.13 This shows that due to limited data availability, national policies differ greatly across the world. However, we must keep in mind that vaccinating pregnant women is often opportunistic, and the timing is related to the timing of the flu season and availability of vaccines.
The most recent systematic review and meta-analysis, conducted by Jarvis et al. in 2020, investigated the efficacy of maternal immunization for influenza on infant health. It was concluded that vaccination prevents lab-confirmed influenza in infants aged < 6 months.14 As well as that, the results showed that the immunity provided by the maternal vaccination decreased over time as the infant grew older. However, there are gaps in research regarding the timing of administration during the gestational period to provide maximum immunogenicity. Vaccination at the optimal time would offer the highest level of protection to mothers and infants. Our review aims to combine the evidence from previously conducted studies and use it to help determine the time for providing maternal influenza vaccination to confer optimal benefits to the infant until 6 months of age.
The primary objective of this systematic review was to identify the optimal timing for immunizing pregnant women to confer the most protection by reducing the incidence of laboratory-confirmed influenza or influenza-like illness in infants less than 6 months of age.
This meta-analysis was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist. The completed PRISMA checklist is shown below in Figure 1.0.31 The study protocol was not registered; however, registration of the study can be found at https://www.crd.york.ac.uk/prospero/display_record.php?IDCRD42021202624.
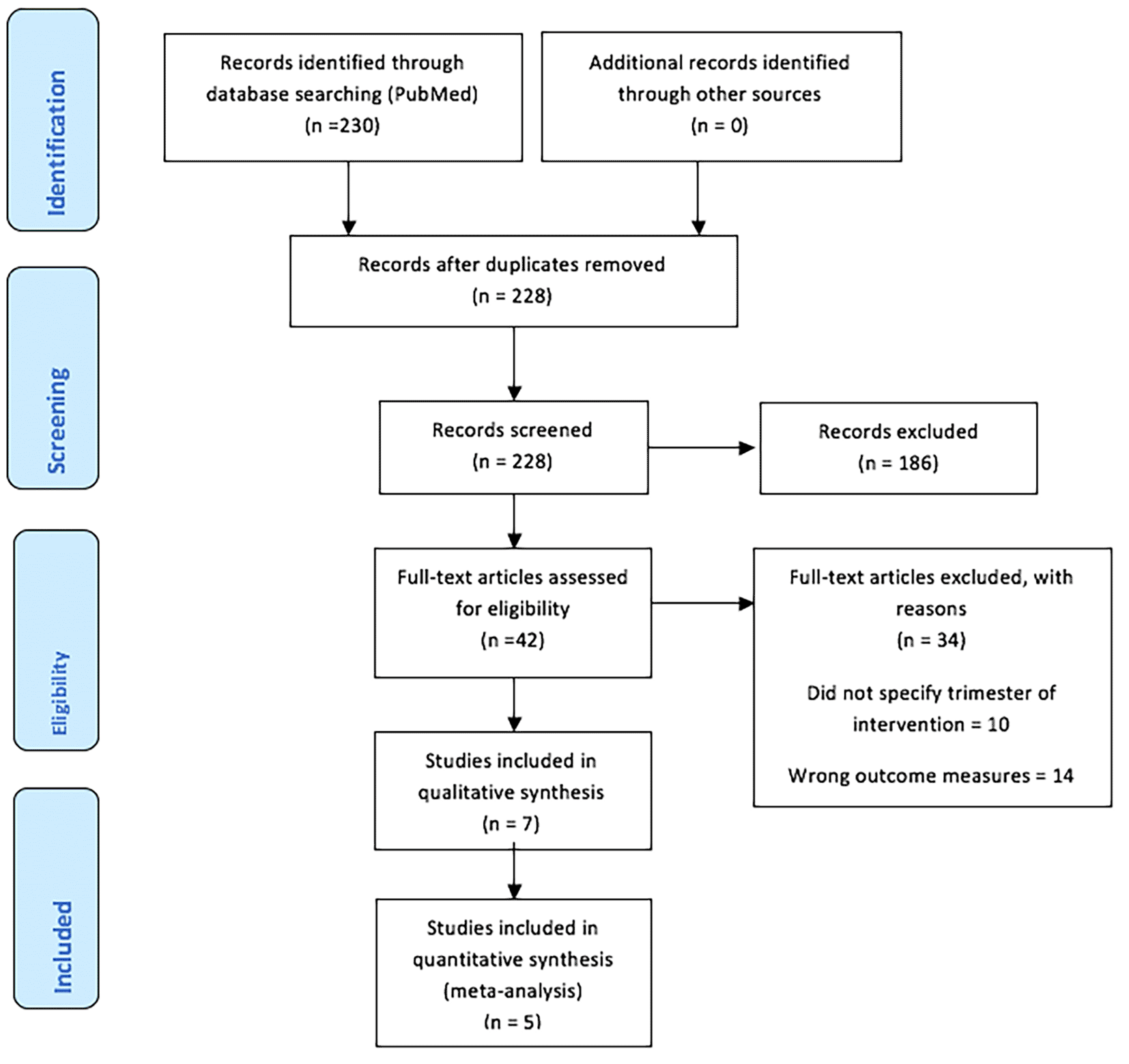
The search several electronic databases, however after applying similar strategies to other electronic databases such as Embase and Medline, no studies were found. The studies selected and screened by title and abstract only came from PubMed and not any of the other sources. Published studies ranged from the earliest known date of 17th of April 17, 1996, to the most recent date of January 21st 2021. The full search strategy is shown below. Manual searching of the reference lists was also undertaken by two peer reviewers on separate occasions.
(Pregnant women) AND (Influenza vaccination OR Influenza vaccine OR Influenza immunization) AND (Unvaccinated Pregnant women OR Control OR meningococcal vaccine OR pneumococcal vaccine) AND (laboratory confirmed influenza OR influenza-like illness OR Respiratory illness OR Respiratory infection OR influenza antibodies) AND (Newborns OR children less than six months OR Neonates OR Infants) AND (Randomized Controlled Trial OR Cohort Study OR Prospective Observational Study OR Case-Control study OR Cross-sectional study).
Full-text articles published as RCT’s or observational studies in the English language were included in our review. Keywords used to identify studies to be included in the study consisted of terms relating to the population of pregnant women and interventions such as influenza vaccination or immunization. Furthermore, studies that measured outcomes specifically related to laboratory-confirmed influenza or influenza-like illnesses in infants or newborns less than 6 months of age were included. Studies must also have a control group that includes either unvaccinated pregnant women, a control group, a meningococcal vaccine, or a pneumococcal vaccine. The length of follow-up of infants until an endpoint of 6 months of age was an important confounder to control; therefore, the duration of the study did not impact the outcome measures. Studies that did not have more specific outcome measures such as hospitalization rates, outpatient visits, or acute respiratory illness were excluded. Furthermore, studies that had outcome measures relating to higher severity such as hospitalization due to influenza or ICU admission-relating to influenza were also excluded. Studies that had the outcome measure of serology only or hemagglutinin inhibition titers were excluded. The study designs varied from randomized controlled trials to case-control studies. Studies in the form of case series, case reports, clinical practice guidelines, commentaries, conference abstracts, editorials, meta-analyses, narrative reviews, studies reporting estimated influenza rates based on ecological approaches, statistical modelling, or systematic reviews were all excluded. The following PICO keywords were used in the search:
Population: Pregnant Women
Intervention(s): Influenza vaccination OR Influenza immunization
Comparator (control): Unvaccinated Pregnant women OR Control group OR meningococcal vaccine OR pneumococcal vaccine
Outcome(s): laboratory-confirmed influenza OR influenza-like illness OR Respiratory illness OR Respiratory infection OR influenza antibody AND Newborns OR children younger than 6 months OR Neonates OR Infants
Study design: Randomized Controlled Trial OR Cohort Study OR Prospective Observational Study OR Case-Control study OR Cross-sectional study.
Data extraction was undertaken by one author, but then simulated again by another author to improve reliability using a Cochrane data extraction form for both RCT’s and observational studies. A meeting was then conducted with the project supervisor to improve inter-rater reliability. Key summary statistics included odds ratios, relative risk, risk ratios, and rate ratios.
The SIGN checklist by healthcare improvement Scotland was used to evaluate the risk of bias from the methodology of both Randomized controlled trials and observational studies.15 The overall quality rating of unacceptable, low quality, acceptable, and high quality was used to rate the studies for bias. Quality assessments for both RCT’s and observational studies were performed by two independent blinded reviewers. For unblinding, a decision was made on each quality assessment and overall ranking. If no agreement was reached, the third author decided the final rating.
We performed a preliminary analysis from initial searches using six studies that tested the efficacy of maternal immunization of infants with inactivated influenza vaccine on the incidence of laboratory-confirmed influenza episodes in infants in the first six months of life. The outcomes of the individual studies are presented in quantitative tables, and the studies had similar parameters. The meta-Analysis was performed using RevMan software (version 5.4 2020) requiring a minimum of two studies, with heterogeneity I2= 90%, which is greater than 55%; however, due to the sparse data sets, it was decided that a fixed-effects model was performed despite high heterogeneity. A plot was used to test the heterogeneity between studies. The analysis included only studies that reported outcomes using a polymerase chain reaction (PCR) test to confirm influenza infection; hence, studies that used a serology test to confirm influenza infection were excluded.
The studies included both prospective and retrospective randomized controlled trials and cohort studies. The studies assessed the efficacy of influenza vaccination in vaccinated groups to unvaccinated groups as a control. The total population size varied between studies; therefore, studies were weighted according to their population size to have a greater effect on outcomes. The odds ratio was calculated using the number of influenza events in the vaccinated group out of the total population of the vaccinated group compared to the number of influenza events in the unvaccinated group out of the total population of the unvaccinated group. Odds ratios was calculated for each of the six studies. The Odds ratio in our case is a statistical measure looking at the likelihood of influenza infection in vaccinated and unvaccinated groups. No additional analyses, such as subgroup or sensitivity analyses, were deemed necessary when discussed with the supervisor, as most studies were credible and similar in study characteristics.
A preliminary search using the search strategy on the 21stof May 2020 revealed 153 search results. However, an updated search was undertaken on the 8th of August 2024 which revealed that there were an additional 77 studies published, increasing the total to 230 search results from PubMed. After deduplication, 228 studies were finally included. After the first screening of titles and abstracts, 186 studies were excluded, and 42 full-text articles were assessed for eligibility. A total of 34 studies were excluded for three main reasons: not specifying the trimester of the intervention (10), wrong outcome measures (14), and wrong intervention (10). This resulted in eight studies being used for the qualitative synthesis of this review. However, only six of these studies were included in the quantitative analysis. The PRISMA flowchart provides a visual illustration of the following:32
The key study characteristics are shown in Table 133 in the supplementary material according to the quality assessment value and study design.
Two randomized controlled trials with a total of 4,533 participants were included in both the quantitative and qualitative analyses. Both studies had a similar proportion of vaccinated and unvaccinated participants, considerable randomization and blinding of participants, and similar exclusion criteria for participants, including characteristics such as previous complicated pregnancy or pre-term labor, recent administration of any study vaccines, and any systemic disease. Therefore, this allows for the control of any potential confounders that can affect the baseline characteristics of participants. One of the studies was conducted In Mali, where influenza occurs in a seasonal fashion, whereas the other was conducted in Bangladesh where Influenza occurs in a perennial fashion. All the studies carried out influenza testing in infants who presented with influenza-like illness or any illness. Zaman et al. included infants who had their first episode of laboratory confirmed influenza episode, the confirmation of influenza-like illness by a clinician and confirmation by a throat swab. In Tapia et al., they undertook weekly visits to detect influenza-like illness and severe acute respiratory infection. If the criteria of this was met, nasopharyngeal and oropharyngeal swabs were taken and tested.
Five observational studies with a total of 10,625 participants were included in the quantitative analysis, and two additional studies with a total of 1,474 participants were included in the qualitative analysis. Of the studies included in the quantitative analysis, two were prospective and one was a retrospective study. Of the two additional studies included in the qualitative analysis, one was a case-control study, and the other was a matched cohort study. All studies had a similar proportion of vaccinated and control participants, the desired outcome and intervention, and similar exclusion criteria, including seasonal variation in influenza infection rates and pre-term labor. Studies were conducted in many different countries and continents, and in all of them, influenza occurred seasonally rather than perennially. All the studies carried out influenza testing in infants who presented with influenza-like illness or any illness. Eick et al interestingly defines ‘influenza-like illness’ as a fever of 38.0°C or higher, diarrhea, or respiratory symptoms (including cough, runny nose, or difficulty breathing). In Singh et al, infants were categorized into groups A, B or C depending on the severity of symptoms. Group A included patients who presented with mild fever plus cough/sore throat with or without other coryzal symptoms (e.g body aches or diarrhea). Group B includes all the symptoms in A in a more severe from, such as high grade fever and severe sore throat. Group C included patients who had the above symptoms with the most severe symptoms such as breathlessness, chest pain drowsiness etc. In Maltezou et al.’s study, women and their infants were telephoned weekly by health professionals asking about any onset of fever or new symptoms such as respiratory symptoms, myalgias or headache. Data was also collected on healthcare seeking, hospitalization or admission to ICU. In Omer et al. infants were assessed weekly for illness through active surveillance and samples were then tested by PCR.
After assessing the quality of the studies for their respective methodologies, six of the seven studies were rated as high-quality, suggesting that most of the studies were rated as highly internally valid and had a relatively low risk of bias. Only one study (Singh et al.) was rated as acceptable quality and was one of the studies included in the quantitative synthesis as well despite this. Only studies rated as acceptable or of high quality were included in the final quantitative synthesis.
All studies reported a lower incidence of influenza in infants born to vaccinated mothers than in those born to unvaccinated mothers. A study by Zaman et al.,16 Benowitz et al.,17 and Tapia et al.18 reported vaccine efficacies of 63%, 91.5%, and 31%, respectively, in a study by Eick et al.19 reported a risk reduction of 41% and Singh et al.,20 Maltezou et al.21 and Omer et al.22 compared the prevalence of influenza in infants born to vaccinated and unvaccinated mothers, and found a lower prevalence in those who were vaccinated. A general trend was observed where studies that vaccinated mothers in the first two trimesters reported a higher vaccine efficacy than those that either vaccinated mothers in any of the trimesters or in the second and third trimesters, possibly indicating that vaccination in the first trimester is responsible for the higher reported vaccine efficacy.
The RevMan software, which was used for Quantitative Analysis, could not compute the results of case-control studies, and so studies by Eick et al.19 and Benowitz et al.,17 were excluded. The studies included in the quantitative analysis were those conducted by Zaman et al.,16 Tapia et al.,18 Maltezou et al.,21 Singh et al.,20 and Omer et al.22 Omer et al.22 was then further divided as it included trials in three different countries: Mali, where pregnant women were vaccinated in the third trimester; Nepal; and South Africa, where they were vaccinated in the second or third trimester. This was to allow for a comparison of the incidence of influenza when vaccinating in different trimesters in pregnancy.
The five studies consisted of 15,046 participants, including a range of trimesters during which the influenza vaccine was administered. In 2 studies,16,18 the vaccine was administered in the third trimester only, in two studies20,21 the vaccine was administered in one of all trimesters, and in one study,22 the vaccine was administered in either the second or the third trimester. The combined forest plots for each study group are shown in Figure 1.1, 1.2, and 1.3.
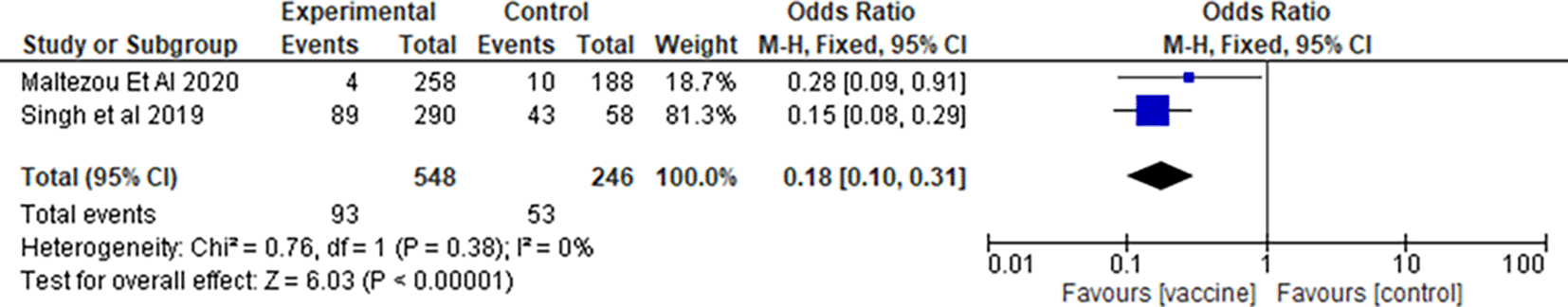


To determine the vaccination in which the trimesters provided the greatest protection against influenza infection, we used an elimination-based approach by grouping studies according to trimester and created forest plots comparing the two groups of studies at a time. The forest plot results were used to determine whether a difference in the trimesters correlated with a lower occurrence of influenza infection.
4.6.1 Influenza vaccination in any trimester of pregnancy
The forest plot shown in Figure 1.1 shows the studies who vaccinated in any trimester in pregnancy. The Forrest plot showed that maternal influenza vaccination was effective and consistently showed a lower occurrence of influenza infection in children less than 6 months of age, where an Odds Ratio<1 correlated with a lower rate of influenza infection, and all the studies showed a negative effect size in relation to the line (Pooled OR 0.21, 95%CI (0.15, 0.31)). There was no significant heterogeneity between the studies, as found in the forest plot (χ2=1.52, df=1(P=0.22); I2=34%, Z=8.25(P<0.00001)), allowing us to disregard the null hypothesis.
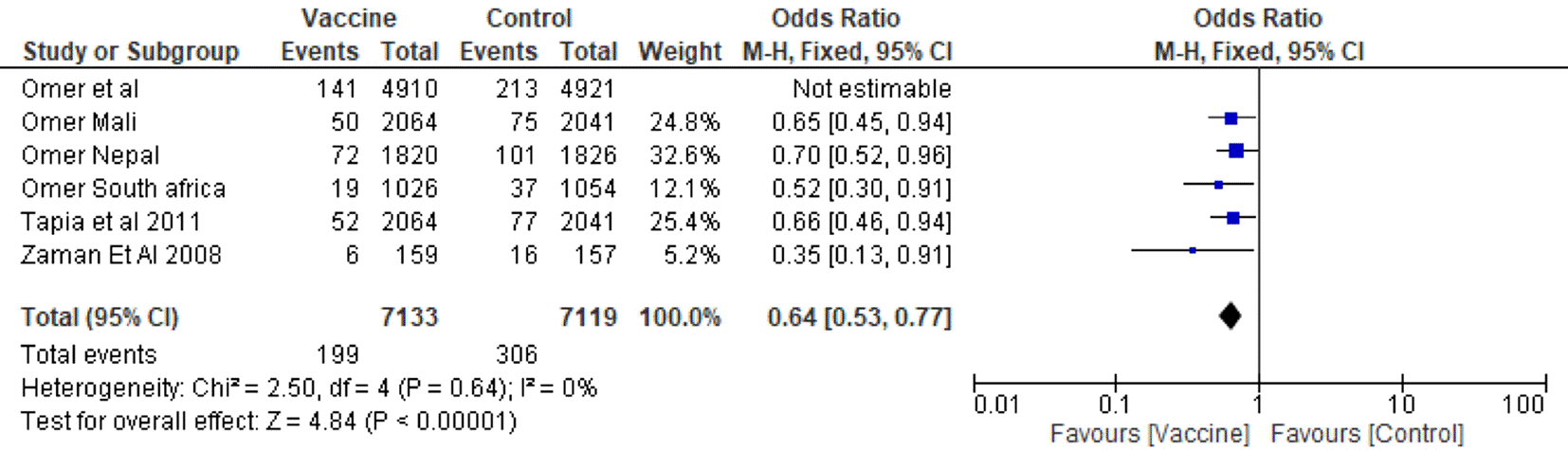
4.6.2 Influenza vaccination in Third trimester compared to Second or Third trimester of pregnancy
The forest plot shown in Figure 1.2 compares studies in which the vaccine was administered in the second and third trimesters and Figure 1.3 shows those in which it was administered in the third trimester only. The Pooled Odds Ratio was 0.64, and the test for heterogeneity found χ2=1.63 df=2 (P=0.44) I2=0%) and test for overall effect Z=4.83 (P<0.00001). The comparison shows that the pooled Odds Ratio is less than that reported by the second- and third-trimester studies depicted in Figure. 1.2. This allows us to conclude that there is no difference in efficacy between vaccinations in the second or third trimester.
4.6.3 Influenza vaccination in Third trimester compared to Any Trimester of pregnancy
A forest plot in Figure 1.3 was created using studies in which the vaccine was delivered in the third trimester and those in which it was delivered in any of the trimesters had a pooled Odds Ratio of 0.47[0.33,0.58], which is greater than the pooled Odds Ratio for only the latter group of studies shown in Figure 1.1 which was 0.21 [0.15,0.31], and also depicted a more negative effect size (95%CI below the negative line) when compared to the pooled OR for the former set of studies. Assessment of the heterogeneity between the studies showed p value<0.05, which highlights statistical significance (χ2=16.06 (p=0.001), I2=81%, Z=5.80 (P<0.00001)). A comparison of the vaccine efficacies of the two groups showed a higher vaccine efficacy with the inclusion of the first and second trimesters, suggesting a correlation between the inclusion of these trimesters and a higher vaccine efficacy.
4.6.4 Influenza vaccination in Second or Third trimester compared to Any Trimester of pregnancy
The test for heterogeneity showed the following results: χ2=18.91, df=2 (P<0.0001), I2=89%, Test for Overall Effect=5.96 (P<00001), allowing us to rule out the null hypothesis in this scenario as well. The pooled Odds Ratio for the comparison (0.55[0.45,0.67]) was greater than that for those that were not selective with the trimester shown in Figure. 1.1 (0.18[0.10,0.31]), indicating greater vaccine efficacy in the latter group. As both studies consisted of subjects administered the vaccine in the second and third trimesters, it can be concluded that the administration of the vaccine in the first trimester could be responsible for the increase in efficacy. These three comparisons allowed us to interpret that vaccinating pregnant mothers in the first trimester provides the greatest vaccine efficacy for protection against influenza infection in infants. However, more data are required to draw robust conclusions.
The results from the quantitative studies, five total (Zaman, Tapia, Singh, Maltezou, Omer) which were included in our final results, had a total of 15,133 pregnant women who showed maternal influenza vaccination in trimesters one, two, or three; this was protective against influenza in infants < 6 months of age. We compared the odds ratio of the studies reflecting the trimesters in which the mother received the vaccination such that we could isolate the impact of each trimester on the efficacy of influenza vaccination. Grouping the specific trimesters during which the vaccine was administered enabled a comparison through forest plots for each study and thereafter a comparison of the odds ratio. The results showed with statistical significance that maternal influenza vaccination in the first trimester conveyed the most significant efficacy in the reduction of infants aged under 6 months and laboratory-confirmed influenza. The clinical importance of our systematic review is that it allows for better protection of infants who are at greater risk of complications due to their susceptibility to infections as a result of their immature immune system. Coupled with influenza vaccinations licensed only for 6-month-old infants and above reinforces the significance of protecting young infants from complications related to influenza.
The strengths of the included studies include the collection of data on multiple outcomes. Most of the studies used laboratory-confirmed influenza as an outcome measure, which is known to be a highly sensitive and specific diagnostic test compared to other outcome measures such as ILI or serological testing.23 Tapia and Singh et al. reported the occurrence of influenza-like illnesses (ILI).18,20 Singh et al., in a retrospective study, utilized ILI cases to prevent an underestimation of the influenza burden.20 However, despite laboratory-confirmed influenza as a primary outcome measure, it would have allowed for improved internal validity.
Moreover, Eick et al. observed laboratory-confirmed influenza (LCI) as well as 4 fold or greater increase in serum antibodies collected at intervals and compared with previous specimen samples, indicating an influenza infection in that interval period.24 This robust methodology improved the accuracy of the results.
All studies included in the review were considered to be of high quality according to the SIGN tool. The studies controlled for confounders using similar exclusion criteria, that is, maternal breastfeeding as a confounder, as well as the exclusion of pre-term babies from the studies (as pre-term babies lose exposure to antibodies, reducing the number of antibodies. These criteria enhance the strength of the individual studies, allowing them to be more comparable. All studies that were of low quality were excluded. The advantage of utilizing high-quality studies is that it avoids carrying out a sensitivity analysis to exclude low credible results; therefore, it allows for better comparison between studies. Study designs such as case reports, systematic reviews, and case series were also excluded in the selection process to allow for better comparison between studies.
A final advantage of the selected studies was the inclusion of studies measuring a wide range of outcomes in different settings, from outpatient visits to hospitalizations, to provide a clearer understanding of the influenza burden. This would allow for greater coverage of cases, as most influenza cases are present in the outpatient department, and not all influenza-like illness cases would require hospitalization.
The limitations include the heterogeneity of the study designs, as observational studies and RCTs were used, making it difficult to compare them.25 Different tests have been used to confirm the presence of influenza, although PCR is the gold standard. Benowitz used a direct fluorescent antibody (DFA) test (the sensitivities of DFA and PCR were 82% and 95%, respectively), whereas Zaman used influenza antigen tests.26 The differences in sensitivity and specificity can cause detection bias, which reduces the accuracy of the results of the included studies. However, most studies used PCR to confirm LCI.
Moreover, the outcomes measured in the studies varied such that they did not mention the definition of ILI.18 This increases the methodological heterogeneity between the studies and reduces the accuracy of the results from specific studies. Few studies, such as Eick et al., have mentioned the inclusion criteria for defining ILI. Future studies should be more specific in defining influenza-like illnesses to allow for standardization when reviewing the outcome measures for individual studies.
The strengths of this review include a range of high-quality studies ranging (from RCT and observational studies) with apparent exclusion (i.e., laboratory-confirmed influenza or influenza-like illness) and inclusion criteria. A range of health databases were used, such as PubMed, Embase, and Cochrane, for the search strategy, which yielded a large number of studies, implying that the search was comprehensive to encompass all relevant articles.
Although the synthesis of RCTs is considered the highest level of clinical evidence, given the nature of our systematic review, we cannot control all variables due to the prospective nature of the study; hence, observational studies were the best fit. Observational studies are conducted in the natural environment; therefore, they are more reliable than RCTs owing to their higher external validity. This implies that the data can be extrapolated and applied to other similar contexts. Despite different study designs, strict inclusion criteria were implemented through the inclusion of studies that only measured laboratory-confirmed influenza or influenza-like illnesses as outcome measures. Therefore, making the studies even more comparable and reducing reporting bias.
The weighting for the meta-analysis was based on population size rather than study design.25 Since all studies were acceptable and of high quality when assessed for the risk of bias, a sensitivity analysis was not needed. The studies used in this systematic review all had a similar methodology in terms of outcomes measured, confounders, and measurements. An example of this is illustrated by the majority of studies using PCR to measure influenza cases and implementing regression models to control for variables such as breastfeeding.
Implementing internationally renowned quality assessment tools such as the SIGN tool allows for rating of the quality of evidence. However, in the future, it would be worth implementing a supplementary tool known as the grading of recommendations. The significance of this is to further rate the credibility of the findings from the perspective of a guideline author, who will extrapolate these recommendations and implement them in a clinical setting.
One Limitation of this review was the use of a combination of RCTs and Observational studies. Observational studies used for meta-analyses are generally considered suboptimal compared to those using only randomized control trials. This is a result of methodological heterogeneity, which could be present, as well as an increased risk of bias in observational studies.25 This may pose challenges in interpreting the results and exaggerating the effects of the study. The primary endpoint of all the studies was 6 months of follow-up with infants, which controlled for differences in the study periods.
Furthermore, observational studies have confounders that cannot be completely addressed, which increases the difficulty of comparing them with RCTs. A confounder between the studies was the variable timing of influenza seasons, that is, in Bangladesh, influenza is perennial, while in the USA, it is seasonal.16 This could have been controlled by carrying out a subgroup analysis and comparing the perennial and seasonal study outcomes separately. However, the limited number of perennial studies and the difficulty in classifying studies as either perennial or seasonal makes it difficult to carry out such an analysis.
It was difficult to completely isolate the variable of severity of influenza infection. This was due to the limited number of studies which isolated the confounder of severity of Influenza. Helpfully studies such as Maltezou and Singh et al had categorized patients into severity groups or mentioning the number of infants which were hospitalized for influenza. This had allowed for the exclusion of such patients from the studies. In Maltezou et al, patients diagnosed with influenza, had acute respiratory infection or had influenza-like illness were included in the study. In Singh et al, infants who fell under categories A or B were included but C were excluded due to the severity of infection. The rest of the studies included had clearly specified that participants were visited weekly and if were symptomatic were test for influenza antigens and included. There was no specific mention of infants with a high severity outcome such as hospitalized infants or ICU admitted patients and were therefore assumed to have mild symptoms.
After conducting extensive searches, it was difficult to find studies that compared the timing of maternal immunization for influenza among individual studies in the form of a meta-analysis, nor did any study aim to carry this out. A study carried out by Jarvis et al. in 2019 aimed to determine the effectiveness of influenza vaccination during pregnancy on child health outcomes.14 The systematic review concluded that maternal influenza vaccination is inversely correlated with infant hospitalization due to LCI. The meta-analysis, which included 19 studies, also reported that vaccine efficacy did not vary when administered in the third or second trimester. This supports the findings of our meta-analysis, as well as studies that investigated vaccine efficacy at different trimesters by measuring transplacental antibody transfer, suggesting that vaccine efficacy did not vary by gestational age at vaccination.27 This suggests that implementing maternal immunization programs is easier when women present for care later in pregnancy. Only one randomized controlled trial investigated the trimester of maternal vaccination with respect to infant outcomes.27 However, vaccination during the first trimester was not included in this study. They concluded that the results were too narrow to detect any differences in vaccination timing. Current research supports the idea that antibody transfer occurs during the second trimester of pregnancy. However, Katz et al. (2018) found higher antibody levels in women vaccinated later in pregnancy, but this was not considered statistically significant.28 Despite these studies’ findings, there is some indication that vaccinating earlier in pregnancy would elicit a more mature antibody response, which is consistent with the results of this analysis.14 Further to that, vaccinating earlier would also protect the mother and ensure protection throughout gestation.
An updated WHO position paper published in May 2022 on vaccines against influenza suggests that pregnant women are a high-risk group and should be vaccinated in any trimester of pregnancy.29 This recommendation is based on the evidence of a significant risk of severe disease in this group. Some studies have expanded on this point by emphasizing that mothers are at the greatest risk of severe infection in the third trimester, so vaccinating earlier yields greater benefit to the mother.29 Despite theoretical evidence that vaccination in later trimesters would increase antibody titers and provide longer-lasting immunity to infants, this is not currently recommended. Our review focused on outcomes for infants and suggested that studies that included infants born to mothers vaccinated in the first trimester had a reduced incidence of influenza infection until 6 months of age. This would be useful in recommending a future policy whereby earlier vaccination of pregnant mothers would benefit not only the infant but also the mother by preventing severe disease.
In our study, we attempted to answer a question regarding the gestational timing of maternal vaccination, which optimizes infant outcomes. We aimed to carry out a multicenter prospective cohort study in which pregnant mothers were vaccinated when they were confirmed to be pregnant. Infants were followed up for outpatient visits and hospitalizations, and influenza infection was confirmed using polymerase chain reaction (PCR). This would allow for a study that aims to compare the outcomes of infants born to mothers vaccinated in different trimesters of gestation to assess the reliability of our current findings. The current meta-analysis combines the outcomes of five studies that were comparable in terms of methodology and outcome measures, and a future meta-analysis that utilized the findings of more studies would also be useful, as meta-analyses are at the highest rank in the hierarchy of evidence. It is difficult to establish a cause-effect relationship as a very low number of studies actually isolated the variable of trimester vaccination, thereby exemplifying the need for more studies investigating optimization of the timing of influenza vaccination. Another unanswered question would be to look into the differences in studies conducted in studies where there is influenza is seasonal and where it is perineal. The studies included had a wide spectrum with regards to this with some countries having it seasonal (USA), others with 2-3 peaks such as Nepal and Mali and one study which has influenza all year around (Bangladesh). Comparing countries which only had influenza seasonally with those having influenza all year-round would help control this important confounder which impacts the number of cases. Due to the limited number of studies which have a single season and all year round fashion, it was difficult to compare the impact this had on the data set.
Our meta-analysis showed that studies that included mothers vaccinated in any trimester had beneficial effects in conferring protection to newborn infants from influenza infection until 6 months of age. However, the results also suggest that vaccinating in earlier trimesters has shown to confer greater protection to infants and requires further investigation into this. It was difficult to find studies which isolated the variable of trimester timing, but had a variety of trimesters where they were given the vaccine. This makes it very difficult to answer the research question of which trimester confers the most benefits in terms of influenza infection in infants. However, the data may suggest an inference that vaccinating in earlier trimester such as the first or second may confer greater benefits with regards to infant outcomes.
Data was made publically available on Fig share and licensed. Respective citations have been included in the bibliography and with the data set.
1. Figshare, PRISMA Checklist. https://doi.org/10.6084/m9.figshare.25907518.v1 31
2. Figshare PRSIMA Flow Diagram. figshare. https://doi.org/10.6084/m9.figshare.25907527.v1 32
3. Figshare Table 1 F1000.docx. https://doi.org/10.6084/m9.figshare.25907551.v1 33
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
No
Are the conclusions drawn adequately supported by the results presented in the review?
No
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioinformatics and Biostatistics
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pathogen Biological research on influenza virus, hepatitis E virus and evaluation of vaccine products
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
No
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Influenza virus infection, host immune response to virus infection, vaccine against virus infection
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
No
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public health, infectious disease epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 07 Oct 24 |
read | |||
|
Version 1 13 Jun 24 |
read | read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)