Keywords
Viral Haemorrhagic Fever, Central Africa, SORT IT, Outbreak, Epidemic response, Decentralized care, Operational Research, Ebola
This article is included in the TDR gateway.
This article is included in the TDR: Ebola and Emerging Infections in West and Central Africa collection.
Traditionally in the Democratic Republic of the Congo (DRC), centralised Ebola treatment centres (ETCs) have been set exclusively for Ebola virus disease (EVD) case management during outbreaks. During the 2020 EVD outbreak in DRC’s Equateur Province, existing health centres were equipped as decentralised treatment centres (DTC) to improve access for patients with suspected EVD. Between ETCs and DTCs, we compared the time from symptom onset to admission and diagnosis among patients with suspected EVD.
This was a cohort study based on analysis of a line-list containing demographic and clinical information of patients with suspected EVD admitted to any EVD health facility during the outbreak.
Of 2359 patients with suspected EVD, 363 (15%) were first admitted to a DTC. Of 1996 EVD-suspected patients initially admitted to an ETC, 72 (4%) were confirmed as EVD-positive. Of 363 EVD-suspected patients initially admitted to a DTC, 6 (2%) were confirmed and managed as EVD-positive in the DTC. Among all EVD-suspected patients, the median (interquartile range) duration between symptom onset and admission was 2 (1-4) days in a DTC compared to 4 (2-7) days in an ETC (p<0.001). Similarly, time from symptom onset to admission was significantly shorter among EVD-suspected patients ultimately diagnosed as EVD-negative.
Since <5% of the EVD-suspected patients admitted were eventually diagnosed with EVD, there is a need for better screening to optimise resource utilization and outbreak control. Only one in seven EVD-suspected patients were admitted to a DTC first, as the DTCs were piloted in a limited and phased manner. However, there is a case to be made for considering decentralized care especially in remote and hard-to-reach areas in places like the DRC to facilitate early access to care, contain viral shedding by patients with EVD and ensure no disrupted provision of non-EVD services.
Viral Haemorrhagic Fever, Central Africa, SORT IT, Outbreak, Epidemic response, Decentralized care, Operational Research, Ebola
The following changes have been made in this version of the article
1. Methodology: More details on the designation of facilities as a centralised Ebola treatment centres (ETCs) or decentralised treatment centres (DTC), a figure showing the timeline of establishment of ETCs and DTCs and text on handling of missing data have been added.
2. Discussion: The conclusion has been expanded to explicitly mention the limitations of the study.
See the authors' detailed response to the review by Nlandu Roger Ngatu
See the authors' detailed response to the review by Francesco Branda
Ebola virus disease (EVD) is a rare but deadly viral haemorrhagic fever with an average case fatality rate of 50% (ranging between 25-90%) among those infected.1 Though vaccines and curative treatments are available, successful containment of an EVD outbreak is largely dependent on early detection, isolation, and treatment of cases.2
The Democratic Republic of the Congo (DRC) in Central Africa has experienced 15 EVD outbreaks since 1976.3 In the DRC, patients with EVD have been managed through an EVD-centric approach, at EVD treatment centres (ETCs) which are constructed in locations with large numbers of cases and which function parallel to the existing healthcare delivery system. However, recent outbreaks in the DRC and elsewhere revealed that local communities associated ETCs with remoteness and death and were reluctant to seek care from these facilities.4–7 These beliefs led to delays in admission which in turn adversely impacted survival.
To address these concerns, a strategy of decentralization was piloted during the 11th EVD outbreak, which occurred in 2020 in Equateur Province, DRC. Small teams, diagnostics, and supportive treatment services were deployed in existing local health centres in hard-to-reach areas to improve accessibility and allay communities’ apprehensions regarding ETCs. These were known as Decentralised Treatment Centres (DTCs). This approach aimed at reducing the risk of EVD transmission through early isolation of cases and improving patient outcomes through early access to diagnosis and supportive treatment while also ensuring that non-EVD health services continued to be provided to the communities.
The decentralized approach in the Equateur province has not yet been evaluated. The province merits attention in view of its large geographic expanse, tropical ecosystem conducive to re-emergence of EVD, presence of hard-to-reach pockets and a predominantly rural population which set it apart from most provinces of the DRC. Five of the fifteen outbreaks in the DRC have occurred in and around this province.3
This study therefore aimed to report on the utilization of decentralized facilities and whether these facilities helped promote early admissions and early diagnosis during the 2020 EVD outbreak in Equateur. This information can be used in preparation for future outbreaks, in terms of resource allocation, training of human resources and provision of EVD management infrastructure at existing health centres in the country.
This was a retrospective cohort study making secondary use of data collected primarily for clinical purposes.
General setting
The DRC is the largest country in central Africa with a population of 112 million as of 2023.8,9 It is one of the five poorest countries globally.8 Equateur is one of its 26 administrative provinces with a population of 1.6 million and is divided into 18 health zones (Figure 1). Each health zone provides services to a population of 100,000-200,000, and is further divided into health areas for every 10,000 population.10
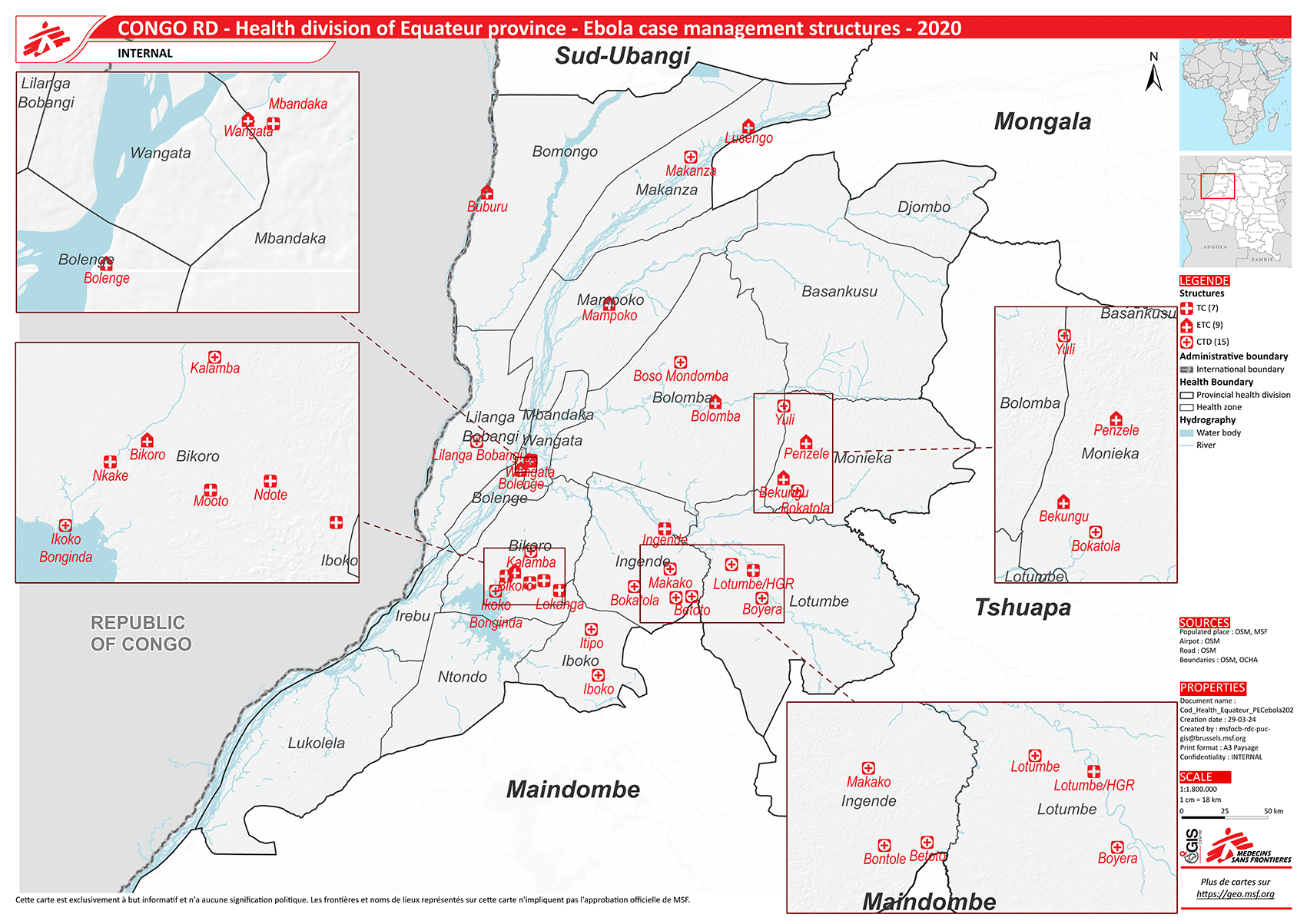
(Source: Geographic Information System Centre, Médecins Sans Frontières).
Abbreviations: ETC=Centralised Ebola treatment centre, CTD=Decentralised treatment centre, TC=Transit centre.
The DRC has a three-tier public health system: primary health centres in every health area, a secondary “General Reference Hospital” in each health zone and a tertiary “Provincial Hospital” in each provincial capital. Services in these facilities are provided on payment of user fees by the patients.10 Mbandaka, the provincial capital of Equateur, is around 1200 km by road from the national capital of Kinshasa. Within the province, the Congo river system is the major channel for transport and there is limited road connectivity.
Specific setting: EVD Outbreak of 2020
The outbreak occurred in Equateur between 1st June 2020 and 18th November 2020.11 It produced 130 cases (119 confirmed and 11 probable), of which 75 recovered and 55 died.3,12 Outbreak response was coordinated by the Ministry of Health (MOH) with technical support from multiple international aid organizations. Case definitions followed the World Health Organization recommendations.13 All EVD care services were provided free of cost during the outbreak.
In the initial period, suspected cases were admitted to ETCs which were set up exclusively for EVD care. These centres were equipped with isolation units, diagnostic facilities, and advanced treatment modalities including monoclonal antibodies (Table 1). A total of nine ETCs were newly constructed (mostly semi-temporary structures) during the outbreak (Figure 1).
| Centralized Ebola treatment centres | Decentralized treatment centres | |
|---|---|---|
| Main considerations for designation |
| |
| Location | Newly constructed during outbreak and located close to localities with large number of cases | Co-located with existing health centres in remote health areas |
| Mode of admission | ||
| Human resources |
| |
| Bed capacity | Variable (based on patient load, epidemiological trends, and the transmission chain); 10 – 43 beds | Limited; average = 6 beds |
| EVD Diagnostic facilities | Yes (GeneXpert PCR in all ETCs with repeat of GeneXpert PCR at ETC Wangata for confirmation) | Sample collection locally with transport to ETC Wangata for GeneXpert PCR |
| Treatment facilities | Yes (Investigational treatment like monoclonal antibody therapeuticsb) | Only supportive treatment available and referral to ETC for advanced therapeutics (including monoclonal antibody treatment) |
| Isolation facilities | Yes | Yes |
| Biomedical waste management | Yes | Yes |
| Safe and dignified burial of dead bodies | Supported by the team of the ETC | Supported by the team of the DTC |
| Management of non-EVD cases | For EVD-negative cases, samples are sent to Kinshasa to rule out other febrile illness including malaria and other viral haemorrhagic fevers. EVD-negative cases are transferred out to a different health care facility for treatment | For EVD-negative cases, samples are sent to Kinshasa to rule out other febrile illness including malaria and other viral haemorrhagic fevers, and treated within the same facility |
Seven transit centres (TCs) were also established in places where there was no testing capacity. Samples were taken from suspected cases and transported to ETCs. Patients were kept in isolation at the TCs while awaiting laboratory results. These TCs were also considered as centralized centres, functioning parallel to the existing medical system. Some of the TCs were converted into ETCs over time.
As the outbreak progressed, suspected cases were reported from remote health zones and a decentralized approach was piloted. This approach was developed by the MOH in consultation with Médecins Sans Frontières (MSF). Based on contact tracing and epidemiological investigations, health areas where cases would be expected were identified. In these areas, the existing health centres were equipped to be decentralized treatment centres (DTCs). The first DTC started functioning in July 2020; 15 DTCs were established during the outbreak. The decision on designation of a facility as an ETC or DTC was made by the outbreak response team led by the MoH. Once a facility was selected to function as a DTC, the outbreak response team demarcated a triage area and an isolation area, ensured supply of basic personal protective equipment and apparatus required for sample collection-transport and for providing supportive treatment for suspected cases were available and established a biomedical waste management system, including identification of safe burial spaces in consultation with the local community. Table 1 provides a description of the services provided at the ETCs and DTCs. Figure 2 shows the time trend of confirmed cases and the establishment of ETC and DTCs, based on available data.
All patients with suspected EVD admitted to any EVD health facility during the 2020 EVD outbreak in Equateur were included.
During the outbreak, the Médecins Sans Frontières (MSF) team under directions of the MOH developed a common form to collect the basic details of patients admitted as suspected cases to any EVD health facility. A healthcare worker was identified as a focal person in each facility and was responsible for data collection and updation of the form on a daily basis. A supervisor was identified within the MSF team who was responsible for collating the paper forms from a group of facilities and digitising them into a linelist. At the time of digitization, the supervisor would check for data completeness and whenever possible coordinate with the facility focal person to retrieve missing data from the facility treatment records and update the linelist. The compiled line-list of patients with suspected EVD admitted to any EVD health facility constituted the data source.
Data on patient demographic characteristics, clinical characteristics at the time of presentation, final diagnosis, and treatment outcomes were extracted from the line-list and analysed using STATA (version 16.0, StataCorpLLC, College Station, Texas, USA). R is an open-access software which can be used to conduct the same analysis.
The outcomes of interest were time to admission, time to diagnosis and final treatment outcomes. Time to admission was calculated as the duration between the date of symptom onset and date of first admission. Time to diagnosis was calculated as the duration between the date of symptom onset and date of the first positive PCR test (for confirmed cases) or date of the earliest negative PCR test (for non-EVD cases). Date of treatment initiation was not recorded in the line-list, and therefore time taken to initiate treatment could not be assessed. For patients transferred from one facility to another, the outcome reported at the final EVD health facility was considered as the final outcome.
Time to admission and diagnosis were summarized as medians with inter-quartile ranges and compared between the two types of facilities (i.e., ETC versus DTC) using Mann Whitney-U test. Final outcomes between the two were compared using the Chi-square test. A p-value < 0.05 was considered statistically significant.
Handling of missing data: The number of observations with missing data are reported for key variables. Since the outcome variables (time to diagnosis and time to admission) were non-parametric, imputation techniques and sensitivity analysis to assess impact of missingness in these variables were not performed. Statistical tests were performed after omitting the observations with missing data for the outcome variables pertaining to the test.
There were 2359 line-listed unique patients suspected of having EVD. Of these, the type of EVD health facility visited first was an ETC for 1996 (85%) patients and a DTC for 363 (15%) patients. Of the 1996 patients with suspected EVD who were initially admitted to an ETC, 72 (4%) were confirmed as EVD-positive. Of the 363 patients with suspected EVD who were initially admitted to a DTC, 6 (2%) were confirmed as EVD-positive in the same health facility and remained there for care (Figure 3).

*These two patients were diagnosed as EVD-negative in the first ETC but were eventually confirmed as EVD-positive in the next ETC that they consulted.
ETC=Centralised Ebola treatment centre, DTC=Decentralised treatment centre, EVD = Ebola Virus Disease, EVD+=EVD-positive; EVD-=EVD-negative.
Table 2 shows the demographic and clinical characteristics of patients with suspected EVD based on the type of EVD health facility first visited. The age and gender distribution were not significantly different between the two types of facilities. In total, 895 (45%) patients with suspected EVD who first visited an ETC and 137 (38%) who first visited a DTC were not vaccinated. At the time of admission, certain signs or symptoms were reported by a significantly higher proportion of patients with suspected EVD who visited an ETC first compared to a DTC, for example: fatigue (75% vs 54%), muscle pain (35% vs 20%), breathlessness (13% vs 4%), bleeding (7% vs 3%), and dysphagia (7% vs 3%). Apart from EVD, the most common final diagnosis included malaria in 954 (48%) patients first admitted to an ETC and 57 (16%) patients first admitted to a DTC.
| Characteristics | Type of facility visited first | P-value | |||
|---|---|---|---|---|---|
| Centralised Ebola treatment centre | Decentralised treatment centre | ||||
| n | (%) | n | (%) | ||
| Total | 1996 | (100) | 363 | (100) | |
| Age (in years) | 0.518 | ||||
| 0-4 | 348 | (17.4) | 69 | (19.0) | |
| 5-14 | 449 | (22.5) | 76 | (20.9) | |
| 15-29 | 418 | (20.9) | 78 | (21.5) | |
| 30-44 | 370 | (18.5) | 77 | (21.2) | |
| 45-59 | 242 | (12.1) | 35 | (9.6) | |
| 60 and above | 169 | (8.5) | ret | (7.2) | |
| Not recorded | 0 | (0.0) | 2 | (0.6) | |
| Gender | 0.152 | ||||
| Male | 1010 | (50.6) | 165 | (45.5) | |
| Female | 986 | (49.4) | 190 | (52.3) | |
| Not recorded | 0 | (0.0) | 8 | (2.2) | |
| Vaccination against EVD | <0.001 | ||||
| Vaccinated prior to admission | 71 | (3.6) | 4 | (1.1) | |
| Vaccinated after admission | 4 | (0.2) | 0 | (0.0) | |
| Unvaccinated | 895 | (44.8) | 137 | (37.7) | |
| Vaccinated but timing unknown | 515 | (25.8) | 24 | (6.6) | |
| Vaccination status unknown | 511 | (25.6) | 198 | (54.5) | |
| Signs and symptoms at admissiona | |||||
| Fever | 1639 | (84.3) | 290 | (80.1) | 0.050 |
| Fatigue | 1459 | (74.9) | 196 | (54.2) | <0.001 |
| Vomiting | 1006 | (51.9) | 176 | (48.6) | 0.250 |
| Diarrhoea | 713 | (36.7) | 131 | (36.2) | 0.849 |
| Muscle pain | 684 | (35.2) | 74 | (20.4) | <0.001 |
| Breathlessness | 252 | (12.9) | 16 | (4.4) | <0.001 |
| Bleeding | 143 | (7.4) | 10 | (2.8) | 0.001 |
| Dysphagia | 140 | (7.2) | 11 | (3.0) | 0.003 |
| Hiccups | 49 | (2.5) | 8 | (2.2) | 0.724 |
| Conjunctivitis | 27 | (1.4) | 4 | (1.1) | 0.665 |
| Final diagnosis | NAb | ||||
| EVD-positive | 70 | (3.5) | 6 | (1.7) | |
| EVD-positive and Malaria | 2 | (0.1) | 0 | (0.0) | |
| Malaria | 954 | (47.8) | 57 | (15.7) | |
| Typhoid fever | 5 | (0.3) | 1 | (0.3) | |
| Respiratory Tract Infection | 18 | (0.9) | 5 | (1.4) | |
| Intestinal parasitosis | 69 | (3.5) | 2 | (0.6) | |
| Othersc | 296 | (14.8) | 20 | (5.5) | |
| Not recorded | 582 | (29.2) | 272 | (74.9) | |
| Final EVD status | 0.056 | ||||
| Confirmed EVD | 72 | (3.6) | 6 | (1.6) | |
| Probable EVDd | 2 | (0.1) | 2 | (0.6) | |
| Suspected EVDd | 76 | (3.8) | 12 | (3.3) | |
| Non EVD | 1843 | (92.3) | 343 | (94.5) | |
| Not recorded | 3 | (0.2) | 0 | (0.0) | |
Table 3 shows a comparison of pre-diagnostic delays based on the type of facility visited first. When all patients with suspected EVD were considered, the duration between symptom onset and admission to an EVD health facility was significantly shorter among those first admitted in a DTC (Median: 2 days, Interquartile range [IQR]: 1-4 days) compared to an ETC (Median: 4 days, IQR: 2-7 days). Similarly, the duration between symptom onset and diagnosis was significantly shorter among those first admitted in a DTC (Median: 3 days, IQR: 2-6 days) compared to an ETC (Median: 4 days, IQR: 2-7 days).
| EVD patients and time periods | Type of facility visited first | P-value | |||||
|---|---|---|---|---|---|---|---|
| Centralised Ebola treatment centre | Decentralised treatment centre | ||||||
| Na | Duration (in days) | N | Duration (in days) | ||||
| Median | (IQR)b | Median | (IQR)b | ||||
| All suspected cases of EVD | |||||||
| Symptom onset to first admission | 1978 | 4 | (2-7) | 352 | 2 | (1-4) | <0.001 |
| Symptom onset to diagnosis | 1753 | 4 | (2-7) | 229 | 3 | (2-6) | <0.001 |
| Confirmed cases of EVD | |||||||
| Symptom onset to first admission | 72 | 5 | (2-8) | 6 | 3 | (2-14) | 0.727 |
| Symptom onset to diagnosis | 72 | 6 | (3.5-8) | 6 | 3 | (2-14) | 0.342 |
| Non-EVD cases | |||||||
| Symptom onset to first admission | 1903 | 4 | (2-7) | 346 | 2 | (1-4) | <0.001 |
| Symptom onset to diagnosis | 1681 | 4 | (2-7) | 223 | 3 | (2-6) | <0.001 |
Among patients with suspected EVD who were later confirmed to be EVD-positive, there was no significant difference in the time to admission and time to diagnosis based on the type of facility (Table 3).
Among the patients with suspected EVD for which the final status was EVD-negative, the duration between symptom onset and admission among those first admitted to a DTC (Median: 2 days, IQR: 1-4 days) was significantly shorter than among those who were first admitted to an ETC (Median: 4 days, IQR: 2-7 days). Also, the time to diagnosis was significantly shorter among EVD-negative patients first admitted to a DTC compared to an ETC (Table 3).
The final outcomes of the 78 EVD-positive patients are shown in Table 4. Among the 72 EVD-positive patients first admitted in an ETC, 60 (83%) were cured and 10 (14%) died. All six EVD-positive patients first admitted in a DTC were cured.
To our knowledge, this is the first study exploring the decentralised model of care piloted during the 2020 EVD outbreak in the Equateur province of DRC. The study has three key findings. First, one out of seven patients with suspected EVD was first admitted to a DTC. Second, DTCs managed to reduce the time to admission diagnosis in all patients with suspected EVD (including some diagnosed later as EVD-negative). Third, 3% of all patients with suspected EVD were confirmed to have EVD. There were 12 EVD deaths in ETCs and none in a DTC.
The piloting of the DTC model marks a paradigm shift in outbreak control in the region – from a mostly EVD-centric approach to a more community-centric approach.14–17 The DRC’s “Strategic response plan for the EVD outbreak: 2018” calls for strengthening existing heath facilities and empowering the existing health workforce to conduct efficient EVD triage, maintain continuity of EVD and non-EVD care, and take healthcare closer to communities so that individuals can seek care early.16
The study has certain limitations. Since the dates of treatment initiation were missing for the majority of the patients, we could not evaluate the time taken to initiate treatment at ETCs compared to DTCs. Confounders like severity of illness (cycle threshold values) and the geographic proximity of patients to an EVD health facility might have impacted the time to admission, but we were unable to adjust for these due to the non-availability of data. These parameters should be meticulously documented in future outbreaks to enable a comprehensive evaluation of the DTC model. Given the small number of cases in this outbreak, we are unable to comment on whether the reduction in time to admission led to a difference in outcomes among patients first admitted to a DTC compared to an ETC. We were also unable to conduct qualitative interviews among patients and caregivers in these facilities, which could have provided in-depth insights into patient and provider perspectives around care seeking and delivery during the outbreak.
Despite these limitations, this study has important implications, more so because this is the first study exploring a decentralised model in the Equateur Province. Only 15% of the patients with suspected EVD in the 2020 outbreak first sought care in a DTC. This could be due to the fact that the DTCs were piloted one month into the outbreak in a limited and phased manner. Therefore, the ETCs bore the brunt of cases during the outbreak.
The DTCs appear able to reduce the time taken to admit and diagnose (EVD or non-EVD) patients with suspected EVD. This has two implications. First, DTCs could provide diagnosis and care to patients with other conditions during the outbreak. In countries like the DRC, a wide spectrum of febrile illnesses like malaria and viral haemorrhagic fevers are prevalent.18–20 As care provision for these illnesses has been disrupted during previous EVD outbreaks in this region,21,22 the establishment of DTCs might help overcome this issue. Second, the median time to admission was three days in the DTCs which was slightly lower than that reported in previous outbreaks in the DRC.23,24 A reduction in time to admission and diagnosis among confirmed EVD patients could be crucial for initiating early EVD specific treatment and thus reducing mortality.1,25
All six of the patients with confirmed EVD who first visited a DTC were diagnosed and cured at the same facility (i.e., the one initially visited). These patients might have had milder forms of disease which did not require referral. While this represents too small a number from which to draw firm conclusions on the effect of decentralised care on patient outcomes among those EVD-positive and could also be influenced by the severity of illness in those presenting to the DTC, it is an encouraging finding.
There is need to look at this model critically. Only 3% of all patients with suspected EVD admitted to a treatment centre were eventually diagnosed as EVD-positive. The 2020 outbreak resulted in 130 cases, among which 55 died.3 However, only 78 patients (10 of whom died) were admitted to an EVD health facility. The rest of the patients were identified during contact tracing but could not be located or brought to a facility. The majority of deaths happened in the community, which indicates that severely ill patients who needed urgent care either did not seek care or could not be provided with care. More needs to be done to ensure that people have access to timely diagnostics and medical care. A qualitative exploration of the circumstances which led to these community deaths might be useful to understand why these individuals did not or could not access facility-based care. Also, since <5% of the patients admitted with suspected EVD were diagnosed as EVD, there is need for better screening to optimise resource utilization and infection control.
In conclusion, this assessment of the decentralised model of EVD care provision in Equateur was unable to draw inferences on the impact of the model on treatment outcomes due to the relatively small size of the 2020 outbreak and lack of data on potential confounding factors which could impact outcomes. Notwithstanding the limitations of this study, decentralized models of care offer an opportunity to potentially reduce community transmission of EVD and improve access to care for all diseases, especially in remote and hard-to-reach areas. At the same time, it is imperative to ensure availability of relevant, timely and quality assured data during any future outbreaks for monitoring the response and comprehensively assessing the utility of decentralized models in the context of the DRC.
Ethical approval with waiver of informed consent was obtained from (a) National Ethics Committee of the School of Public Health, University of Kinshasa, DRC (Approbation Number: ESP/CE/115/2023 dated 04 August 2023), (b) Médecins Sans Frontières Operational Center Brussels Ethics Review Board (24 July 2023) and (c) Union Ethics Advisory Group, International Union against Tuberculosis and Lung Disease, Paris, France (EAG Number: 17/2023 dated 08 September 2023). Permission to access the line-list data was obtained from the MOH of the DRC.
This reporting of this study followed the STROBE guidelines.
Repository: STROBE checklist for ‘Evaluation of centralised and decentralised models of care during the 2020 Ebola Virus Disease outbreak in Equateur Province, Democratic Republic of the Congo: A brief report’. 10.6084/m9.figshare.25983145.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
In accordance with WHO’s open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
There should be no suggestion that WHO endorses any specific organization, products or services. The views expressed in this article are those of the authors and do not necessarily reflect those of their affiliated institutions. The use of the WHO logo is not permitted.
Figshare: Evaluation of centralized and decentralized models of care during the 2020 Ebola Virus Disease outbreak in Equateur Province, Democratic Republic of the Congo: A brief report. https://doi.org/10.6084/m9.figshare.25634556. 26
The project contains the following underlying data:
“Finaldataset F1000.xlsx” (Anonymised line-list of patients with suspected Ebola Virus Disease during the 2020 2020 Ebola Virus Disease outbreak in Equateur Province, Democratic Republic of the Congo).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by TDR, the Special Programme for Research and Training in Tropical Diseases hosted at the World Health Organization. The specific SORT IT program that led to this publication is a SORT IT partnership with the WHO Emergency Medical Teams (Geneva), WHO-AFRO (Brazzaville), WHO Country Offices and Ministries of health of Guinea, Liberia, Sierra Leone, and the Democratic Republic of the Congo, the Infectious Diseases Data Repository (IDDO); The International Union Against Tuberculosis and Lung Diseases, Paris, France and South East Asia offices, Delhi, India; The Tuberculosis Research and Prevention Center Non-Governmental Organization, Yerevan, Armenia; I-Tech, Lilongwe, Malawi; Medwise solutions, Nairobi, Kenya; All India Institute of Medical Sciences, Hyderabad, India; and the National Training and Research Centre in Rural Health, Maferinyah, Guinea. We acknowledge the support of the Ministry of Health of the Democratic Republic of the Congo and appreciate the contribution of all the communities affected by EVD, the health workers and Aid partners. We are grateful to Bav Bavi Mayambula, GIS officer at Geographic Information System Centre, Médecins Sans Frontières, Belgium for developing the map of Democratic Republic of the Congo depicting the health zones and the EVD health facilities which has been used in this report.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My research interests are diverse, spanning various domains such as data analytics, epidemic intelligence systems, and public health risk studies. To address these questions, I developed novel methods that combine techniques from mathematical modelling, and statistical inference (including AI and Machine Learning). My work focuses to epidemiological and statistical consulting in hospital settings, applying statistical and molecular methods in clinical settings, and analyzing climate-sensitive diseases like Dengue and Chikungunya and outbreaks and pandemics such as SARS-CoV-2, Mpox, and Ebola.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental and occupational health; Global health; Infectious diseases.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental and occupational health; Global health; Infectious diseases.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My research interests are diverse, spanning various domains such as data analytics, epidemic intelligence systems, and public health risk studies. To address these questions, I developed novel methods that combine techniques from mathematical modelling, and statistical inference (including AI and Machine Learning). My work focuses to epidemiological and statistical consulting in hospital settings, applying statistical and molecular methods in clinical settings, and analyzing climate-sensitive diseases like Dengue and Chikungunya and outbreaks and pandemics such as SARS-CoV-2, Mpox, and Ebola.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 28 Aug 24 |
read | read |
|
Version 1 17 Jun 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)