Keywords
COVID-19, cancer, comorbidities, mortality, hazard ratio, risk factor, ISARIC, SORT IT
This article is included in the TDR gateway.
This article is included in the TDR: Ebola and Emerging Infections in West and Central Africa collection.
This article is included in the Coronavirus (COVID-19) collection.
The coronavirus disease 2019 (COVID-19) has caused substantial morbidity and mortality on a global scale. A strong correlation has been found between COVID-19 treatment outcomes and noncommunicable diseases such as cancers. However, there is limited information on the outcomes of cancer patients who were hospitalised for COVID-19.
We conducted an analysis on data collected in a large prospective cohort study set-up by the World Health Organisation (WHO) International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). All patients with laboratory-confirmed or clinically-diagnosed SARS-CoV-2 infection were included. Cancer was defined as having a current solid organ or haematological malignancy. The following outcomes were assessed; The hazard ratio of 30-day in-hospital mortality, intensive care unit (ICU) admission, length of hospitalization and receipt of higher-level care.
Of the 560,547 hospitalised individuals who were analysed, 27,243 (4.9%) had cancer. Overall, cancer patients were older and had more comorbidities than non-cancer patients. Patients with cancer had a higher hazard ratio of 30-day in-hospital mortality than non-cancer patients (29.1.3% vs 18.0%) and longer hospital stays (median of 12 days vs 8 days). However, patients with cancer were admitted less often to intensive care units than non-cancer patients (12.6% vs 17.1%) and received less invasive mechanical ventilation than non-cancer patients (4.5% vs 7.6%). The hazard ratio of dying from cancer, adjusted for age, sex and country income level was 1.18 (95%CI: 1.15-1.2).
This study’s findings underscore the heightened vulnerability of hospitalized COVID-19 patients with cancer, revealing a higher mortality rate, longer hospital stays, and an unstructured pattern of care that reflects the complexity of managing severely ill patients during a public health crisis like the COVID-19 pandemic.
COVID-19, cancer, comorbidities, mortality, hazard ratio, risk factor, ISARIC, SORT IT
We incorporated a Cox proportional hazard model in the analysis and statistical methods, and patients with missing covariates were excluded. Patients with complete variables were analysed, and the results were presented in the tables.
Furthermore, we have re-run the analysis in a further sensitivity analysis by restricting the data to covariates that have <10% missingness. Reassuringly, the obtained hazard ratio from the new multivariable model remains relatively robust.
See the authors' detailed response to the review by Tom Fowler
See the authors' detailed response to the review by Chih-Yuan Hsu
Early in the COVID-19 pandemic, data were collected to identify risk factors for poor outcomes that could inform a risk-based approach to health policy and patient management. Risk factors including age, sex, and several comorbidities were reported to be associated with an increased risk of death.1,2 The most common comorbidities identified in hospitalised patients during the first wave of the COVID-19 pandemic were chronic cardiac or cardiovascular diseases, diabetes mellitus, hypertension, non-asthmatic chronic pulmonary disease, obesity, and chronic kidney disease.1,3–6 Understanding which individuals are likely to have a poor prognosis could help inform vaccine prioritisation, shielding policies, or allocation of healthcare resources and patient management in future infectious disease outbreaks and pandemics.
Several studies have reported COVID-19 patients with cancer to be at higher risk of adverse outcomes compared with COVID-19 patients without cancer.7,8 In a study from China, COVID-19 patients with cancer had higher observed increased rates of death, intensive care unit (ICU) admission, and need for invasive mechanical ventilation.9 A study of COVID-19 patients in the United States of America reported that cancer patients were at higher risk of death and hospitalisation but were not found to have significantly different rates of ICU admission or ventilator use compared to non-cancer patients.10 Data from the United States Centre for Disease Control showed that in 2020 and 2021 respectively, 2.0% and 2.4% of people who died of cancer had COVID-19 listed as the underlying cause of death.11 There is a dearth of evidence on the outcomes of patients with cancer in middle- and low-income countries.
The studies referenced above and other national studies have shown that patients with cancer have worse outcomes than those without cancer when hospitalised due to COVID-19.12,13 However, to our knowledge, no study has been conducted to evaluate the association between cancer and hospital outcomes among hospitalised COVID-19 patients using an international data set. This study, seeking to build on the collection of existing evidence, uses secondary COVID-19 patient data, collected in 54 countries via the Clinical Characterisation Protocol designed by the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) and the World Health Organisation (WHO).14,15 We investigated the association of cancer as a comorbidity with 30-day in-hospital mortality, ICU admission, length of hospitalization and receipt of higher-level care in COVID-19 patients with and without cancer.
This was a prospective cohort study that utilised secondary data from the COVID-19 clinical database hosted by the Infectious Diseases Data Observatory (IDDO). The database contains individual patient data from more than 800,000 hospitalised patients in more than 1,200 institutions from 54 countries across 6 continents. The data were collected using the ISARIC-WHO case report form as a part of the ISARIC-WHO Clinical Characterisation Protocol.15,16
We included hospitalised patients of any age with clinically or laboratory-diagnosed SARS-CoV-2 infection. Patients were enrolled between 30th January 2020 and 10th January 2023. Patients with unknown cancer status were excluded. Patients admitted for complications due to COVID-19 were followed from the time of hospital admission to discharge or death.
We compared the differences in demographic characteristics, comorbidities, treatment with intensive interventions, length of hospitalisation, death (defined as the hazard ratio of 30-day in-hospital mortality), and hospital outcomes to characterise hospitalised COVID-19 patients with and without cancer.
Severe disease was defined as treatment with higher-level care, including one or more of the following events: admission to an ICU, treatment with invasive mechanical ventilation (IMV), non-invasive ventilation (NIV), high-flow nasal cannula (HFNC), inotropes and/or vasopressors. Length of hospital stay was censored at 100 days.
The presence of cancer was self-reported by patients or relatives and recorded as a binary variable classified as malignant neoplasm in the ISARIC-WHO case report form. Cancer was defined as having a current solid organ or haematological malignancy. Malignancies that had been declared ‘cured’ ≥5 years with no evidence of ongoing disease, non-melanoma skin cancer and benign growths or dysplasia were not included in this definition. Those with unknown cancer status were excluded.
We used prospectively collected, international observational data on demographics, clinical features and outcomes of patients hospitalized with COVID-19 with or without cancer (coded as ‘malignant neoplasm’). Data were collected using the ISARIC-WHO Clinical Characterisation Protocol and contributed to a central repository at the University of Oxford, England. Participating sites used the ISARIC-WHO case report form to enter data onto a Research Electronic Data Capture (REDCap, https://www.project-redcap.org/ version 8.11.11, Vanderbilt University, Nashville, TN) database or used local databases before uploading to the central data repository.17 Open Data Kit is a suitable open access alternative (https://getodk.org). Centrally collated data were wrangled and mapped to the structure and controlled terminologies of the Study Data Tabulation Model (https://www.cdisc.org/standards/foundational/sdtm, version 1.7, Clinical Data Interchange Standards Consortium, Austin, TX) using Trifacta® software version 9.7.1 (http://trifacta.com). OpenRefine is a suitable open access alternative (https://openrefine.org/) to using Trifacta®. The data collection, aggregation, curation, and harmonisation process has been previously described.16 Though more than 50% of the data were collected from low- and middle-income countries, most data on patients with cancer were collected from patients in higher income countries, per World Bank classification. Our statistical analysis plan was designed to explore differences between patient outcomes between these two economic regions as a proxy for the quality of the healthcare setting in a country.
Continuous variables such as age and length of hospital stay were summarised as means with standard deviations or medians with interquartile ranges depending upon the distribution of data. Categorical variables (sex, presence of cancer, hospital exit outcomes, etc) were summarised as frequencies and percentages.
Categorical variables such as death and treatment with intensive interventions between patients with cancer and those without cancer were compared using the chi-square test. Continuous variables such as length of hospital stay were compared between the two groups using the unpaired t-test or Mann Whitney U test depending on the distribution of data. A Kaplan-Meier curve was plotted to show the cumulative incidence of mortality during hospitalization. To assess the independent effect of cancer on mortality in hospitalized COVID-19 patients, a Cox proportional hazard model was fitted to the data. Patients with missing covariates were excluded from the analysis and results of completely analysed data were presented in the tables. The model was adjusted for the following confounders: age, sex, and country income-level with no explicit adjustment made for further co-morbidities. Unadjusted and adjusted hazard ratios with 95% confidence intervals were reported as measures of association. In addition, we undertook two further sensitivity analyses using different adjustment sets. Denominators on individual analyses differ due to availability of data on different variables across the dataset. A P-value of <0.05 was considered statistically significant.
Information on country income level was obtained from the World Bank (https://datacatalog.worldbank.org/search/dataset/0038543).
All analyses were performed using R version 4.2.2 (IDE PBC, Boston, MA, USA), an open access software. (R: The R Project for Statistical Computing (https://www.r-project.org/).
Execution of the ISARIC-WHO Clinical Characterisation Protocol was approved by the WHO Ethics Review Committee (RPC571 and RPC572, 25 April 2013) and by local or national ethics committees for participating sites. Approvals (dates unknown) include the South Central—Oxford C Research Ethics Committee for England (Ref. 13/SC/0149), the Scotland A Research Ethics Committee (Ref. 20/SS/0028) for Scotland, and the Human Research Ethics Committee (Medical) at the University of the Witwatersrand in South Africa as part of a national surveillance programme (M160667), which collectively represent most of the data. Written patient consent for data to be collected and used in research was obtained or waived according to local norms determined by the responsible Ethics Committee. The data were collected using the ISARIC-WHO case COVID-19 report form, locally-tailored versions of the form, or independently designed forms. Arrangements surrounding the pooling, storage, curation and sharing of these data are covered by the IDDO Governance processes.18
All data were deidentified and ensured of low risk for identification of individuals by a statistical disclosure process prior to sharing. Data were shared under a Data Access Agreement following approval from the IDDO Data Access Committee.19 Execution of this secondary analysis was approved by the Union Ethics Advisory Group of the International Union against Tuberculosis and Lung Disease, Paris, France (EAG number 18/23, dated 8th September 2023).
Among 841,640 individual records in the dataset, 560,547 (66.6%) met the criteria for analysis. Of those that did not, 73,327 (8.7%) did not have clinical or laboratory confirmation of SARS-CoV-2 infection; a further 3,879 (0.5%) were not admitted to hospitals between January 30th 2020 and January 10th 2023; and 203,887 (24.2%) did not have information on cancer status available.
Of the 560,547 individuals analysed, 27,243 (4.9%) had cancer. Furthermore, 219,922 (39.2%) individuals that met the criteria for analysis were hospitalised in high-income countries. There were differences in age, sex, country income level, and other comorbidities in the group of patients with cancer versus those without cancer. Those with cancer were older (84.4% versus 46.3% aged ≥60 years), were more likely to be male (58.1% versus 49.1%) and were more likely to come from a high-income country (90.6% versus 36.6%). Of the 10 comorbidities most common in the whole population, all except obesity were more prevalent in the group of patients with cancer ( Table 1).
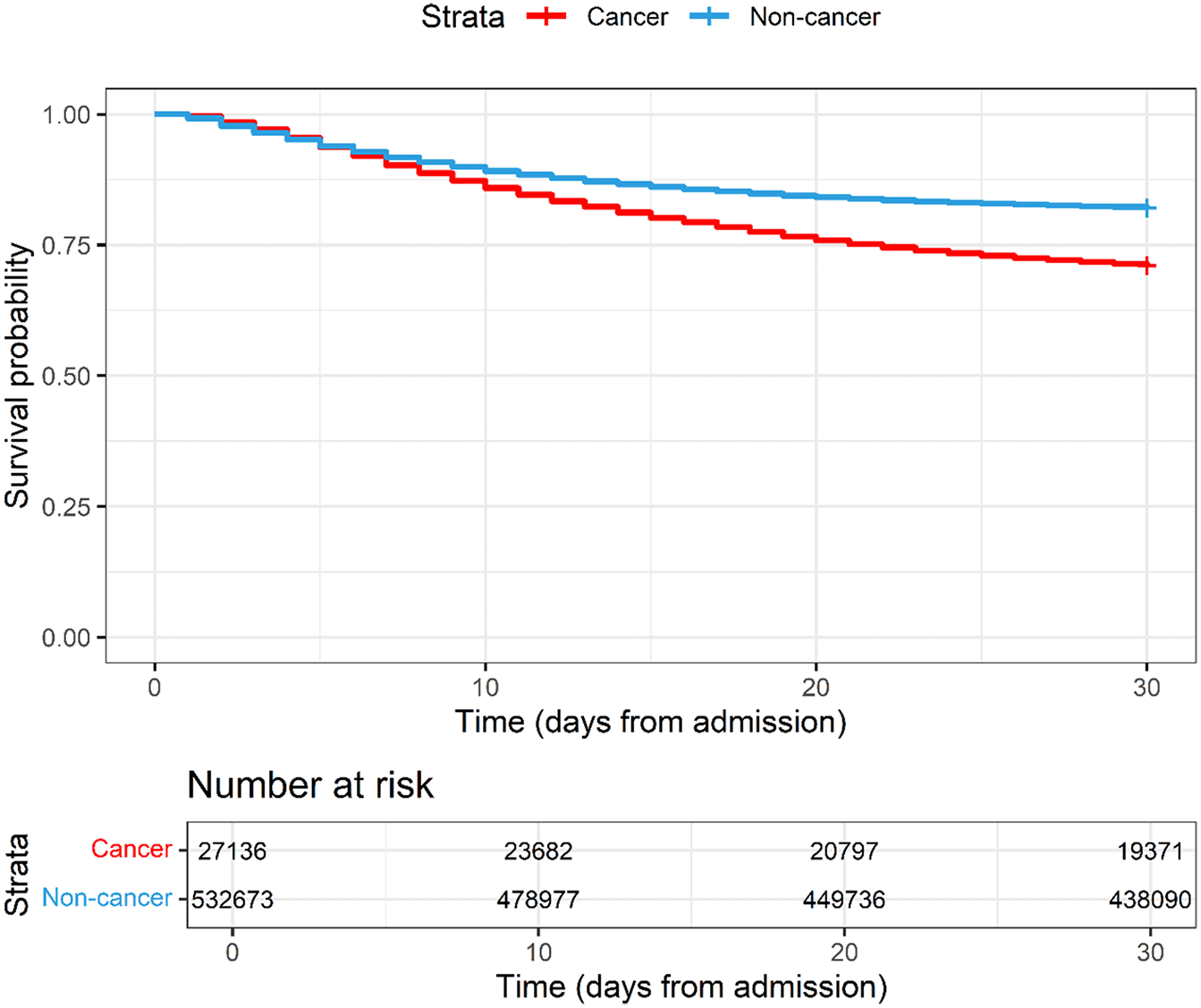
Patients with cancer had a higher hazard ratio of 30-day in-hospital mortality (29.1% vs 18.0%) and longer duration of hospitalization (median of 12 days (IQR 6.0-22.0) vs 8 days (IQR 4.0-14.0)) compared with those without cancer ( Table 2 and Figures 1 and 2).
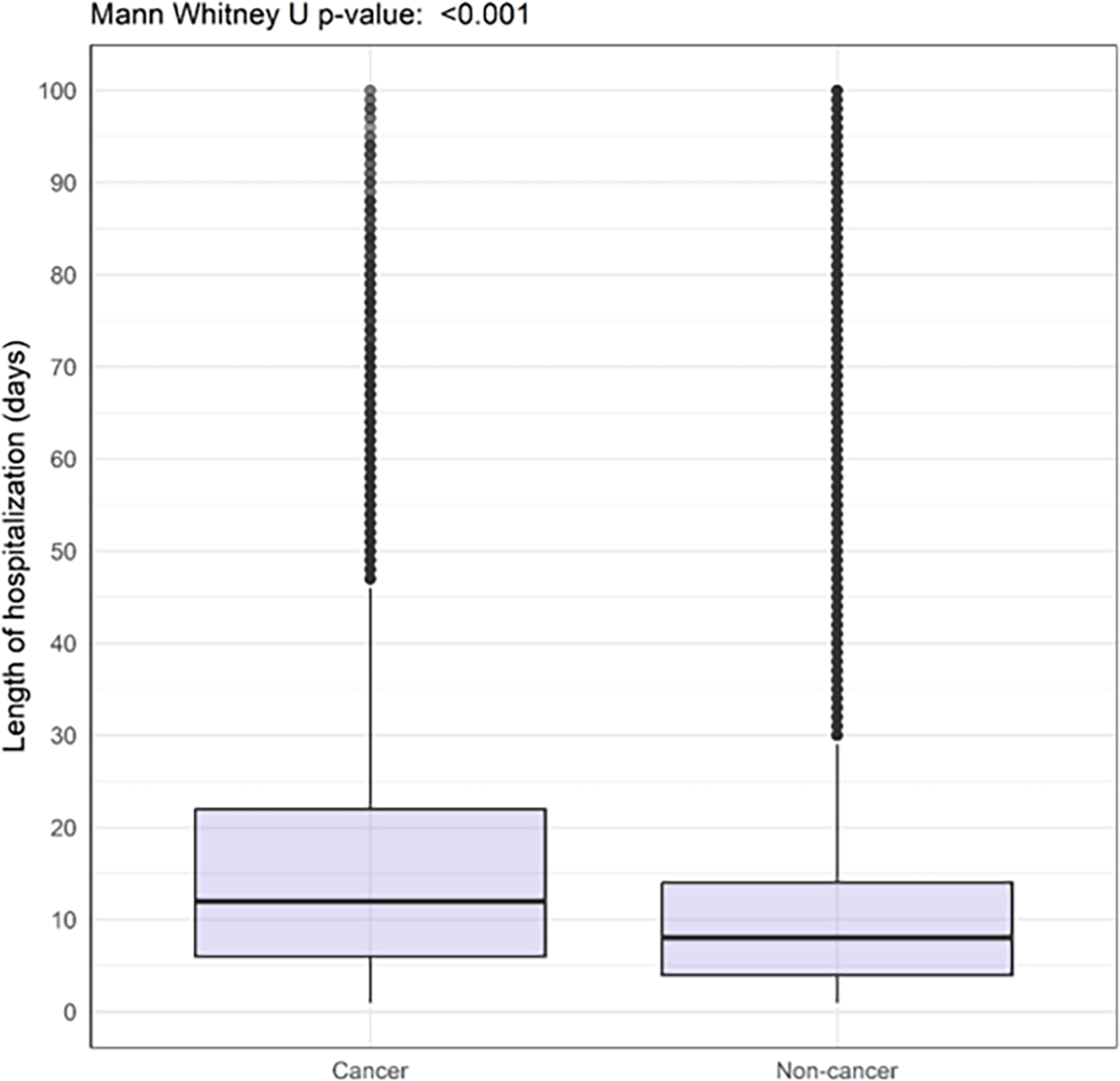
However, patients with cancer were reported to have received higher-level care slightly less often than those without cancer (28.9% vs 29.8%) including lower rates of ICU admission (12.6% vs 17.1%) and invasive mechanical ventilation (4.5% vs 7.6%). There were similar levels of treatment with high-flow nasal cannulas (17.5% vs 16.1%), extracorporeal membrane oxygenation (0.1% and 0.5%), non-invasive ventilation (11.6% vs 11.7%), and treatment with inotropes or vasopressors (3.5% vs 4.5%) across both groups ( Table 2).
The effect of cancer and other comorbidities on 30-day in-hospital mortality among COVID-19 patients is reported in Table 3. Hospitalised COVID-19 patients with cancer had a higher hazard ratio risk of 30-day in-hospital mortality compared to those without cancer. The hazard ratio of dying from cancer, adjusted for age, sex and country income level was 1.18 (1.15-1.2).
| Total (N=560547) | Deaths (N=103836) | Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio* (95% CI) | |
|---|---|---|---|---|
| Age | ||||
| 60 years and above | 270128 | 76514 | 2.01 (1.98-2.04) | 2.43 (2.39-2.46) |
| 0-59 years | 290247 | 27309 | ref | ref |
| Diabetes mellitus | ||||
| Yes | 132205 | 34293 | 1.4 (1.38-1.42) | 1.32 (1.31-1.34) |
| No | 416451 | 67133 | ref | ref |
| Chronic pulmonary disease | ||||
| Yes | 47480 | 13571 | 1.31 (1.28-1.33) | 1.30 (1.28-1.33) |
| No | 509044 | 89157 | ref | ref |
| Gender | ||||
| Male | 277291 | 56727 | 1.11 (1.1-1.12) | 1.19 (1.18-1.21) |
| Female | 282830 | 47020 | ref | ref |
| Cancer | ||||
| Yes | 27243 | 7940 | 1.16 (1.13-1.18) | 1.18 (1.15-1.2) |
| No | 533304 | 95896 | ref | ref |
| Chronic cardiac disease | ||||
| Yes | 70242 | 20692 | 1.2 (1.19-1.22) | 1.15 (1.13-1.17) |
| No | 485975 | 81965 | ref | ref |
| Obesity | ||||
| Yes | 42745 | 8327 | 0.97 (0.95-0.99) | 1.15 (1.13-1.18) |
| No | 253547 | 48963 | ref | ref |
| Hypertension | ||||
| Yes | 197592 | 48449 | 1.37 (1.35-1.38) | 1.13 (1.12-1.15) |
| No | 332886 | 47568 | ref | ref |
| Dementia | ||||
| Yes | 24610 | 8212 | 1.51 (1.48-1.55) | 1.08 (1.05-1.1) |
| No | 208109 | 35207 | ref | ref |
| Smoking | ||||
| Yes | 66094 | 14328 | 1.04 (1.02-1.06) | 1.06 (1.04-1.08) |
| No | 134883 | 22727 | ref | ref |
| Asthma | ||||
| Yes | 48683 | 8790 | 0.93 (0.91-0.95) | 1.04 (1.02-1.07) |
| No | 507639 | 93839 | ref | ref |
| Chronic neurological disorder | ||||
| Yes | 25611 | 6384 | 1.13 (1.1-1.16) | 1.02 (0.99-1.04) |
| No | 214546 | 38379 | ref | ref |
| Chronic rheumatological disorder | ||||
| Yes | 27055 | 6398 | 1.13 (1.1-1.16) | 0.96 (0.94-0.99) |
| No | 206354 | 37244 | ref | ref |
Adjusted hazard ratios were higher for age and gender compared with those for cancer. Adjusted for sex and country income level, individuals aged ≥ 60 years had the highest hazard ratio 2.43 (2.39-2.46). Adjusted for age and country income level, male sex had a hazard ratio of 1.19 (1.18-1.21).
Among all comorbidities, only diabetes mellitus (HR: 1.32, 95%CI: 1.31-1.34) and chronic pulmonary disease (HR: 1.30, 95%CI: 1.28-1.33) were more strongly associated with an increased risk of death compared with cancer, after adjusting for age, sex and country income level. Two sensitivity analyses were conducted and the results are presented in Table 4 and Table 5. The findings of the sensitivity analyses indicate that the quantified hazards ratio for cancer remained unchanged when adjusted for different comorbidities ( Table 4). In addition, the quantified association between any of the predictors and outcome remained relatively stable with some/minor differences in the estimated hazards ratio, apart from chronic neurological disorder ( Table 5). However, it has to be cautioned that such a multivariable model with all the predictors included is subject to large missingness.
| Hazards ratio [95% confidence interval] | |
|---|---|
| Results presented in Table 3 | |
| Not adjusted for any variables (from Table 3) | 1.16 (1.13-1.18) |
| Adjusted for age, sex, and income levels (from Table 3) | 1.18 (1.15-1.20) |
| Sensitivity analysis by adjusted for following comorbidities in addition to age, sex, and income levels: hypertension, diabetes, COPD, obesity, chronic cardiac diseases, dementia, asthma, neurological disorder, rheumatological disorder | 1.18 (1.14-1.21) |
| Unadjusted hazards ratio (95% confidence interval) (from Table 3) | Adjusted hazards ratio (95% confidence interval) | Adjusted hazards ratio (95% confidence interval) [Excluding predictors >10% missing] | |
|---|---|---|---|
| Cancer (reference: no) | 1.16 (1.13-1.18) | 1.20 (1.16-1.26) | 1.17 (1.14-1.21) |
| 60 years and above (reference: 0-59y) | 2.01 (1.98-2.04) | 2.63 (2.50-2.77) | 2.26 (2.22-2.29) |
| Diabetes mellitus (reference: no) | 1.4 (1.38-1.42) | 1.20 (1.17-1.24) | 1.29 (1.27-1.32) |
| Chronic pulmonary disease (reference: no) | 1.31 (1.28-1.33) | 1.33 (1.28-1.38) | 1.30 (1.27-1.33) |
| Male (reference: female) | 1.11 (1.1-1.12) | 1.24 (1.20-1.28) | 1.13 (1.11-1.15) |
| Chronic cardiac disease (reference: no) | 1.2 (1.19-1.22) | 1.26 (1.21-1.30) | 1.18 (1.15-1.20) |
| Obesity (reference: no) | 0.97 (0.95-0.99) | 1.10 (1.06-1.15) | Excluded |
| Hypertension (reference: no) | 1.37 (1.35-1.38) | 1.10 (1.01-1.14) | 1.12 (1.11-1.14) |
| Dementia (reference: no) | 1.51 (1.48-1.55) | 1.16 (1.10-1.22) | Excluded |
| Smoking (reference: no) | 1.04 (1.02-1.06) | 1.04 (1.00-1.08) | Excluded |
| Asthma (reference: no) | 0.93 (0.91-0.95) | 1.03 (0.98-1.08) | 0.93 (0.92-0.96) |
| Chronic neurological disorder (reference: no) | 1.13 (1.1-1.16) | 0.95 (0.91-0.99) | Excluded |
| Chronic rheumatological disorder (reference: no) | 1.13 (1.1-1.16) | 0.96 (0.93-1.00) | Excluded |
We note more than >50% of patients had missingness for smoking, dementia, Chronic neurological disorder, Chronic rheumatological disorder, and obesity. Understanding the underlying mechanism of missingness for some of the variables can be challenging. The standard implementation of multiple imputation assumes that missingness arises through a missing at random (MAR) mechanism. However, such tacit assumption can be questionable for some of the covariates. For example, it can be argued that missingness for dementia can be thought of arising from a not at random (MNAR) process – where the standard implementation of multiple imputation can be questioned. A rigorous investigation of the missingness mechanism and considerations for using principled statistical approaches is considered beyond the current scope of work. However, we re-ran the analysis in a further sensitivity analysis by restricting the data to covariates that have <10% missingness. Reassuringly, the obtained hazards ratio from the new multivariable model remains relatively robust.
Our study findings underscore the heightened vulnerability of cancer patients hospitalized with COVID-19, revealing a higher 30 days in hospital mortality rate, longer hospital stays, and a nuanced pattern of care that reflects the complexity of managing severely ill patients during a public health crisis. These outcomes align with the existing literature on the association of cancer with COVID-19 prognosis and treatment approaches during the pandemic.13,20 In keeping with our findings, other studies conducted in high-income countries have also documented that the proportion of COVID-19 patients with cancer and other comorbidities is higher in the elderly (>60 years) as compared to the general population.13,21,22
A meta-analysis of 4 studies (4691 non-cancer patients, 154 cancer patients) that looked at mortality in cancer patients versus non-cancer patients reported a pooled odds ratio of death of 3.91 (95%CI: 2.70-5.67).12 This is higher than reported in our study. This could be explained by the lack of adjustment for potential confounders in the meta-analysis. It is also unclear whether or not the patients in these studies were primarily admitted for COVID-19, for cancer, or for other reasons. When considering other significant risk factors for mortality, we observed that cancer ranked prominently. Cancer demonstrated a stronger association with mortality compared to all other comorbidities, except for diabetes mellitus and chronic pulmonary disease.
Despite the higher mortality risk, cancer patients in our study were slightly less likely to receive higher-level care compared to patients without cancer (28.9% vs 29.8%). Specifically, cancer patients were less frequently admitted to the ICU (12.6% vs. 17.1%) and had invasive mechanical ventilation less often (4.5% vs. 7.6%). These findings diverge from the expectation that higher-risk patients would necessitate more aggressive treatment. Though these event rates align with other studies of cancer patients, few comparators with non-cancer patients hospitalised for COVID-19 are in the literature. Marta et al. (2020) reported ICU admission rates of 39.1% in cancer patients with COVID-19 and use of invasive mechanical ventilation in 84.4%.23 Elgohary et al.’s (2021) systematic review and meta-analysis of cancer patients with COVID -19 reported an ICU admission rate of 14.5% (95% CI: 8.5-20.4) and a mechanical ventilation rate of 11.7% (95% CI: 5.5-18).12 When comparing cancer patients with non-cancer patients, Abuhelwa et al. (2022) found cancer patients hospitalized for COVID-19 had similar rates of invasive mechanical ventilation compared to those without cancer (10.14% vs 9.36.%).13
We found differences in mean hospital stay between patients with cancer and those without cancer. The longer hospital stay might be related to cancer patients having several other comorbidities and/or the cancer-related management. However, we cannot explain why they stayed longer in hospital but received less high-level care compared to COVID-19 patients without cancer. Abuhelwa’s 2022 nation-wide study reported no difference in hospital stays between these patient groups (8.07 vs 7.46 days). The difference between these findings and ours may reflect differences in admission policy or availability of hospital beds. The lower mortality rates in Abuhelwa’s study as compared to our findings may indicate less severe disease, and therefore a population requiring less in-hospital care.
One key strength of our study was the use of a large sample size, orders of magnitude larger than most previous studies. Therefore, our estimates should be more generalisable and should have a higher power to demonstrate significant associations than previously published studies. We adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting study findings.
This analysis has several limitations. Though recruitment of patients included in the database used for this analysis targeted those admitted for complications due to COVID-19, the reason for other admissions is not recorded in the database and therefore we cannot verify other reasons for admission but analysed the hospital outcomes of patient with cancer and COVID -19. Any inclusion of patients admitted for other reasons may have had an impact on the subsequent treatment pathway. This study encompassed all patients with current cancer, but we couldn’t distinguish between various cancer types as detailed information on each patients’ cancer diagnosis, staging and treatment modalities were not available. However, other studies have highlighted lung cancer and haematological cancers as being those most closely linked to mortality in COVID-19 patients.13,24,25
Differences in reporting of the type and details of cancer diagnosis across the available literature make it challenging to make comparisons. Studies that analysed data through the use of electronic health records may have included patients in remission.
Our study includes patients from January 30, 2020, to January 10, 2023. During this period, COVID-19 underwent significant changes in genomics, treatment, and epidemiology, with vaccines introduced at varying times across countries. However, our dataset lacks genotyping and reliable vaccination information, which are crucial for analysing temporal changes accurately. Without data on these key factors, especially vaccination status, we cannot provide a robust analysis of changes over time. The impact of evolving vaccination rates on outcomes is likely substantial but impossible to calculate with our current data.
We acknowledge this limitation, and it has informed changes to ISARIC’s case report forms for future outbreaks to address these data gaps. The majority of data on patients with cancer (90.6%) were collected from patients in high income countries. So, no inferences could be drawn from patient outcomes linked with World Bank income classifications.
Furthermore, we acknowledged the result presented in Table 5 is an extreme sensitivity analysis for the primary analysis presented in Table 3, imputation approaches were not considered. Understanding the mechanism of missingness requires nuanced exploration of the data and mapping the data generating mechanism, which we consider beyond the scope of the current work.
Our study found that patients with cancer were older with more comorbidities. They had an increased risk of mortality with longer duration of hospital stay as compared to non-cancer patients but received less high-level care including ICU admission and invasive mechanical ventilation. This highlights the importance of collecting accurate data in emerging infections to identify at-risk groups, facilitating appropriate resource allocation and patient management and informing policy decisions aimed at resource allocation during health emergencies. The availability and collection of data on our platforms were predominantly from high-income countries. To prepare for a future pandemic, data availability and coverage must be more universal. More must also be done to support data collection and the capacity to analyse those data within low- and middle-income countries for appropriate evidence generation and proper patient care.
Conceptualization and methodology, ATJ, LM, DN, SH, YST, RFT; formal analysis and visualisation, DN, SH; supervision, project administration and funding acquisition, RFT, LM; writing—original draft preparation, ATJ, LM, SH, RFT; writing—review and editing, ATJ, LM, DN, SH, IFK, IN, SMT, YST, MK, SL, DSG, RJS, RK, RFT. All authors have read and agreed to the last version of the manuscript.
In accordance with WHO’s open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
The data that underpin this analysis are available via a governed data access mechanism following review of a data access committee. Data can be requested via the IDDO COVID-19 Data Sharing Platform (http://www.iddo.org/covid-19 ). The Data Access Application, Terms of Access and details of the Data Access Committee are available on the website. Briefly, the requirements for access are a request from a qualified researcher working with a legal entity who have a health and/or research remit; a scientifically valid reason for data access which adheres to appropriate ethical principles. The full terms are at: https://www.iddo.org/document/covid-19-data-access-guidelines . These data are a part of https://doi.org/10.48688/cpwp-ft84.
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by TDR, the Special Programme for Research and Training in Tropical Diseases hosted at the World Health Organization. The specific SORT IT program that led to this publication is a SORT IT partnership with the WHO Emergency Medical Teams (Geneva), WHO-AFRO (Brazzaville), WHO Country Offices and Ministries of health of Guinea, Liberia, Sierra Leone, and the Democratic Republic of the Congo, the Infectious Diseases Data Repository (IDDO); The International Union Against Tuberculosis and Lung Diseases, Paris, France and South East Asia offices, Delhi, India; The Tuberculosis Research and Prevention Center Non-Governmental Organization, Yerevan, Armenia; I-Tech, Lilongwe, Malawi; Medwise solutions, Nairobi, Kenya; All India Institute of Medical Sciences, Hyderabad, India; and the National Training and Research Centre in Rural Health, Maferinyah, Guinea.
The views expressed in this article are those of the authors and do not necessarily reflect those of their affiliated institutions.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Single-cell RNA seq analysis, Survival analysis, Clinical trial
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: NCD, Operational research, Implementational research, Preventive oncology
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: NCD, Operational research, Implementational research, Preventive oncology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: global public health, cancer, palliative care, rare disease caregivers, qualitative and mixed-methods research
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Single-cell RNA seq analysis, Survival analysis, Clinical trial
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Starkey T, Ionescu MC, Tilby M, Little M, et al.: A population-scale temporal case-control evaluation of COVID-19 disease phenotype and related outcome rates in patients with cancer in England (UKCCP).Sci Rep. 2023; 13 (1): 11327 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Public Heath, Genomics, Pandemics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 21 Nov 25 |
read | read | read | |
|
Version 2 (revision) 07 Apr 25 |
read | read | read | |
|
Version 1 21 Jun 24 |
read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)