Keywords
Prehabilitation, Postoperative complications, Surgery, Lifestyle
This study aimed to compare the effect on postoperative complications of combined prehabilitation targeting predefined co-existing risky SNAP factors with usual preoperative routines in surgical patients.
This systematic review followed the PRISMA 2020 guideline and the protocol (CRD42022282611). Five databases were searched from inception to November 7, 2022 for randomised controlled trials on prehabilitation targeting ≥2 predefined risky lifestyles compared with usual preoperative routines. Risky lifestyles included Smoking, Nutrition (malnutrition and/or BMI>25), risky Alcohol intake, and Physical inactivity (SNAP). Primary outcome was postoperative complications ≤30 days. Cochrane’s risk-of-bias tool 2 was used and meta-analyses were conducted. GRADE was used to assess certainty of evidence.
The search resulted in 20,862 records. At full-text screening, only two (120 participants) of 24 identified trials on combined SNAP intervention had ≥2 predefined risk factors and were included. One (n=110) on intensive physical and brief nutritional intervention to frail patients with colorectal cancer resection reported complication rates of 45% in both groups (relative risk (RR) 1.00, 95% CI 0.66 to 1.51). The other study (n=10, subgroup) on intensive alcohol and smoking intervention in patients with bladder cancer undergoing radical cystectomy, reported complications in 3/7 vs 3/3 participants. The meta-analysis estimated a RR of 0.79 (95% CI 0.41 to 1.51, I2 51%).
Two small of the 24 trials on prehabilitation targeted co-existing and predefined risky SNAP factors and the effect on postoperative complications is very uncertain. Future prehabilitation research involving patient needs is warranted.
Prehabilitation, Postoperative complications, Surgery, Lifestyle
Changes from the previous version:
This revised version addresses all peer-review comments from reviewer 1 and includes several important clarifications and improvements. The study aim has been harmonised across the abstract, introduction, and methods sections to consistently reflect the comparison of combined prehabilitation targeting predefined co-existing risky SNAP factors versus usual preoperative routines. Related terminology has also been standardized throughout the manuscript.
The Introduction has been expanded to explain the rationale more clearly for focusing on patients with two or more co-existing SNAP risk factors, supported by evidence that such combinations may potentiate postoperative risk. The eligibility criteria in the methods section have been revised for consistency and clarity, aligning with the stated aim.
The data synthesis and statistical analysis subsection now includes a more precise explanation of the rationale for using a random-effects model (modified Mantel-Haenszel) for the meta-analysis, due to expected heterogeneity in surgical procedures and interventions.
Figure and table legends have been reviewed and now include full definitions of all abbreviations. Minor textual issues, such as a missing word in Figure 1, have also been corrected.
The discussion has been strengthened with additional reflections on how the findings may inform future research design, including how treatment-as-usual (TAU) should be defined in the context of ERAS protocols, as well as considerations regarding resource prioritisation for high-risk patients.
The Conclusion has been revised to emphasise the surprising lack of studies evaluating tailored, combined prehabilitation interventions and the need for further high-quality research in this area.
See the authors' detailed response to the review by Tim Neumann
Surgery is a pivotal part of the treatment of numerous medical conditions. The estimated global volume of surgical procedures was 313 million procedures in 2012 and this has since increased.1 Development of complications is still a major challenge worldwide2,3 despite the improvements in perioperative care in the last decades including improved surgical and anaesthesiologic techniques, preoperative interventions targeting co-morbidities, and enhanced postoperative recovery programmes.4–6 The consequences of complications include prolonged recovery, reduced quality of life and life expectancy, as well as increased health care costs.7–10
Postoperative morbidity is in part related to preoperative modifiable risk factors such as smoking,11,12 nutrition (overweight/obesity, malnutrition),13–15 risky alcohol drinking,16 and physical inactivity17–19 (SNAP). The SNAP factors compromise several organ functions of importance for successful outcome after surgery.13,20–23 While daily smoking, risky alcohol intake, or malnutrition are followed by a general 50% increased complication rate across different types of surgery,11,13,16 overweight or obesity are associated with increased complications to a minor degree.24,25 Low physical activity level is associated with increased risk of complications, length of stay and postoperative mortality.18,26–29
The SNAP factors are modifiable by prehabilitation,20,22,30,31 but the impact on postoperative morbidity differs among the SNAP factors.32,33 Only the most intensive programmes targeting smoking, alcohol, and malnutrition reduce complication rates by half.13,32,33 Recent systematic reviews have shown an improvement after preoperative physical training programmes of functional capacity both pre- and postoperatively, however, no effect on postoperative complications has been shown.34,35 The effect of overweight or obesity interventions have only poorly been investigated outside bariatric surgery.36 Until now, the large majority of the evidence from SNAP interventions are based on investigating one risky SNAP factor intervention at a time.37,38 This is despite of up to half of hospital patients have co-existing SNAP factors,39–41 such as smoking and overweight or frailty involving both malnutrition and physical inactivity which significantly potentiate the risk at surgery.14,42 For example Park et al. found that the combination of being obese and smoking at the same time potentiated the risk of complications after surgery.14 As the risk of the two risk factors in combination seems to potentiate each other, it points even more towards the relevance of investigating the effect of combined prehabilitation.
This study aimed to compare the effect on postoperative complications of combined prehabilitation targeting predefined co-existing risky SNAP factors with usual preoperative routines in surgical patients. We hypothesised the combined prehabilitation targeting predefined co-existing risky SNAP factors would reduce the postoperative complications compared to usual preoperative routines.
This systematic review and meta-analysis was conducted and reported according to the Cochrane Handbook for Systematic Reviews of Interventions43 and in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)44 and AMSTAR (Assessing the methodological quality of systematic reviews)45 Guidelines. The protocol was registered on PROSPERO (CRD42022282611) before retrieval of data.
We included randomised controlled trials enrolling populations of adult patients (≥18 years) undergoing any surgical intervention and having predefined co-existing risky SNAP factors, defined as at least two of the five risky SNAP factors (daily smoking, alcohol intake > 2 drinks daily (= above 24 g ethanol) or 14 drinks weekly, malnutrition (defined as either weight loss of 10-15% within the last 6 months, BMI < 18.5, Subjective Global Assessment (SGA) Grade C, Nutritional Risk Screening (NRS) > 5, or preoperative serum albumin < 30 g/l), BMI > 25 and physical activity < 4 hours/week), or as described by the authors and receiving a combined prehabilitation initiated preoperatively and targeting those lifestyles. Populations in need for combined frailty intervention were accepted if the frailty screening tool involved at least two of the predefined risky lifestyles of interest in this review. Studies were excluded if their population did not have predefined risky lifestyles. Only face-to-face interventions (physical or online) were included. Intensive interventions were defined as having at least four sessions, each lasting longer than ten minutes and including education, motivational and (if relevant) pharmaceutical support.32,46 The intervention was compared with usual preoperative routines.
We searched Medline, Embase, Web of Science, Cochrane Central Register of Controlled Trials, and CINAHL on March 3, 2021 with an update on November 7, 2022. Our search strategy for MEDLINE was developed in consultation with an information specialist from the Cochrane Anaesthesia Group. It was combined with the Cochrane Highly Sensitive Search Strategy, with use of the sensitivity- and precision-maximising version, for identifying RCTs47 (see extended data file). Similar strategies were developed for the other databases. The search was performed without restrictions for language or publication year. In addition, we performed a simple keyword search (“multimodal prehabilitation surgery RCT”) in Google Scholar with a check-up of the first 200 references and a snowball search by manually scanning the reference lists of included trials and of topic relevant systematic reviews.
Screening of articles was conducted using Covidence® with an institutional subscription. Two reviewers screened all titles and abstracts, independently. Likewise, all full text articles were screened by two other reviewers. Any disagreements were discussed and resolved within the author group.
Data extraction was performed in collaboration of two reviewers with all discrepancies discussed and resolved within the author group. The following data were extracted from all studies into a pre-defined Excel-sheet: authors, year of publication, country, number of patients, patient characteristics (age, sex, ASA-score, SNAP factors), type of surgery, indication for surgery, intervention characteristics (targeted risky SNAP factors, intensity of intervention, preoperative duration, compliance measured as meeting adherence, additional interventions), definition of control groups, drop-outs, and length of follow-up.
The primary outcome was complications within 30 postoperative days, defined by requiring treatment and categorised according to a standardised methodology e.g., the Clavien-Dindo classification,48 comprehensive complication index,49 or as described by the authors.
We only included published randomized controlled trials (RCTs) including cluster and pilot RCTs. Secondary outcomes were extracted if present in the studies: length of stay (LoS), readmissions, adverse events of the lifestyle intervention, successful risk reduction perioperatively and long-term, and effect on patient reported long-term (one year) quality of life (QoL).
Successful risk reduction was defined as quit smoking, quit drinking, no overweight or obesity, no malnutrition, physical activity >4h/week as well as change of at least 1 step on the ASA-score50 regarding risky lifestyles or any change of at least 1 unhealthy lifestyle. For long-term successful risk reduction same definitions were applied except for alcohol which needed to be below risky limits instead of zero intake exclusively.
When doubts about extraction of data, trial authors were contacted by email for clarification. For studies that included a subset of eligible participants, we obtained data for only the subgroup of interest.
Risk of bias in the individual studies was assessed independently by two reviewers using the Cochrane Handbook’s risk-of-bias tool (RoB2) for RCTs.51 Any discrepancies or doubts were discussed and resolved together with a third reviewer. For each outcome in each study, all signaling questions were answered for the five bias domains: the randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. For each domain the responses were collated to a final judgement. In addition, the overall judgement of either low risk of bias, some concerns or high risk of bias was assigned based on the five bias domains.
To reduce the impact of bias due to missing data in the synthesis, we contacted the authors of the studies in case of missing estimates. In addition, in the data extraction we adhered to the best of our abilities to the intention-to-treat principle.52 Meta-analyses were performed using Review Manager 5.4® (RevMan [Computer program]. Version 5.4. The Cochrane Collaboration, 2020). The effect measure of choice for the binary outcomes (e.g., postoperative complications) was the relative risk (RR) with 95% confidence intervals (95% CI) in order to assess the likelihood of the event happening in the experimental intervention group relative to the control comparator.53 Secondary outcomes were expressed similarly except LoS and quality of life (continuous outcome measures), which were estimated based on the difference between means.54 If data were presented as median and IQR, the assumption was that the mean approximately corresponded to the median while SD was estimated as IQR/1.35.55
A meta-analysis for the relative risks of postoperative complications as well as for the secondary outcomes was conducted based on a random-effects model (modified Mantel-Haenszel)56 and the results presented in Forest plots. The random-effects model was chosen beforehand, as we planned to include different types of surgical procedures and different types of interventions targeting two or more predefined risky SNAP factors; thus, this systematic review would not fulfil the criteria for using the fixed model for the meta-analysis. Clinical heterogeneity was considered and evaluated qualitatively, and the statistical heterogeneity was assessed as I2-values as well as from the poor overlap in the visualized 95% CIs in the Forest plots.56 We considered two-sided P-values less than 0.05 and 95% CIs for the RR values for binary outcomes that did not include 1 to be statistically significant.
According to the new guidelines by The Institute for Quality and Efficiency in Health Care (IQWiG) it is recommended to use a fixed-effect model in the case of very few studies, so we also conducted these for the purpose of sensitivity analysis on the primary outcome.57
According to the original protocol, we also applied a network meta-analysis technique for postoperative complications in order to simultaneously compare three or more interventions in order to enable a comparison between multiple interventions since direct comparisons are limited. We estimated the odds ratios by default from the random effects network meta-analysis model with binomial likelihood and logit link.58 For these (arm based) network meta-analyses, we used generalised linear mixed models combining a series of 2×2 tables, with the odds ratio modelled as a linear combination of study level covariates and random effects, representing variation between studies.59
In addition, we planned subgroup analyses on different combinations of the five risky lifestyles, intensive interventions, elderly patient groups, and different types of surgery as well as sensitivity analyses.
Summary assessments of the quality of evidence for each important outcome were performed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach60,61 which includes four levels of quality of evidence (high, moderate, low, and very low) and presented in a summary of findings table.
As illustrated in the PRISMA flowchart, the literature search revealed a total of 20,863 different records identified from various sources; these were screened for eligibility based on title and abstract, and 188 studies were subsequently assessed in full text. A total of 24 studies apparently met the inclusion criteria except for the criterion of at least two predefined risky SNAP factors. Consequently, we were only able to include two RCTs fulfilling our stringent eligibility criteria62,63 ( Table 1).
| Author Year Country | Type of surgery (No randomized) | SNAP inclusion criteria | SNAP intervention (intensity) | Additional intervention | Type of control | Postoperative complication definition | Complications IG vs. CG NE/No included in analysis (%) |
|---|---|---|---|---|---|---|---|
| Included: Two preidentified risk-factors | |||||||
| Carli62 2020 Canada | Colorectal cancer resection (120) | N+P: Frailty (Fried) | P (I) N (B) S (B) A (B) | Psych. Int. | Rehab –programme identical to prehab. | Complications 30 days | See Figure 2 |
| Lauridsen63 2022 Denmark | Radical cystectomy for bladder cancer (13) | S (daily) + A (≥3u/day) | S (I) A (I) | - | Standard care | Complications 30 days | See Figure 2 |
| Excluded: One preidentified risk-factors | |||||||
| Barberan-Garcia 84 2018 Spain | Major abdominal surgery Benign or malignant (144) | P: Duke Activity Status Index | P (I) Nm (B) S (B) A (B) | - | P (B) Nm (B) S (B) A (B) | Complications 30 days | 19/62 (31) vs. 39/63 (62) |
| Goodman81 2007 UK | Coronary artery bypass surgery (188) | No: BMI>28 | P (I) N (I) | Medication optimization | Standard care | Postoperative complications | Data not shown |
| Kalarchian 201382,94 USA | Bariatric surgery (240) | No: BMI>40 or BMI 35-40 | P (I) No (I) | - | Standard care: P (B) N (B) | Adverse events 30 days | 1/71 vs. 0/72 |
| Liang83,95 2018 USA | Ventral hernia repair (118) | No: BMI 30-40 kg/m2 | P (I) No (I) | - | Standard counseling | 30 days surgical site occurrences | 3/44 (7) vs 6/34 (18) |
| McIsaac85 2022 Canada | Intra-abdominal or thoracic cancer resection (204) | Frailty (Clinical Frailty scale) | P (B) N (B) | - | Standard care | In-hospital complications | 42/94 (45) vs. 52/88 (59) |
| Sawatzky86 2014 Canada | Coronary artery bypass graft (17) | P: Sedentary | P (I) N (I) | Education on risk factor management | Standard care | Operative + postoperative complications 30 days | 4/8(50) vs. 4/7(57) |
| Excluded: No preidentified risk-factors | |||||||
| Allen65 2022 UK | Esophago-gastric cancer resection (54) | None | P (I) N (?) | Psych. and medical coaching | Standard care | Clavien–Dindo class ≥ IIIa | 7/22 (32) vs. 12/23 (52) |
| Ausania66 2019 Spain | Pancreatico-duodenectomy Malignant (48) | None | P (I) Nm (B) | - | Standard care | Complications 30 days | 6/18 (33) vs. 12/22 (54) |
| Bousquet-Dion 67 2018 Canada | Non-metastatic colon or rectal cancer resection (80) | None | P (I) Nm (B) | Anxiety-reduction | P (B) as rehab. Nm (B) | Complications 30 days | 14/37 (38) vs. 8/26 (31) |
| Ferreira68 2021 Canada | Lung cancer resection (124) | None | P (B) N (B) (prehab.+ rehab.) | Anxiety-reduction | P (B) N (B) (8 weeks rehab.) | Complications (Clavien-Dindo 1-5) | 27/52 (52) vs. 26/43 (61) |
| Fulop69 2021 Hungary | Colorectal resection Benign or Malignant (184) | None | P (I) N (B) A (B) S (B) | Anxiety-reduction | Standard care | Complications 30 days | 17/77 (22) vs. 16/72 (22) |
| Gillis70 2014 Canada | Colorectal cancer resection (89) | None | P (B) Nm+o (B) (prehab.+ rehab.) | Anxiety-reduction | P (B) N (B) (8 weeks rehab) | Complications 30 days | 12/38 (32) vs. 17/39 (44) |
| Kaibori71 2013 Japan | Liver resection Malignant (51) | None | P (?) N (B) | - | N (B) | Morbidity: Yes | 2/25 (8) vs. 3/26 (12) |
| Lawson72,96 2021 Canada | Thoracotomy for lung cancer (34) | None | P (I) N (B) S (B) | Anxiety-reduction | S (B) | Clavien–Dindo 1-3+ 30 days | 11/20(55) vs. 4/8 (50) |
| Liu73 2020 China | Thoracoscopic lobectomy Malignant (85) | None | P (B) N (B) | Mental relaxation skills | Standard care | Clavien–Dindo ≥ 2-3+ 30 days | 4/37 (11) vs. 5/36 (14) |
| López-Rodríguez-Arias 74 2021 Spain | Colorectal cancer resection (20) | None | P (B) N (B) | Anxiety-reduction | Standard care | Complications 30 days | 2/10 (20) vs. 5/10 (50) |
| Minnella75 2018 Canada | Esophago-gastric cancer resection (68) | None | P (B) N (B) | - | Standard care | Clavien-Dindo 1-5 complications 30 days | 14/24 (58) vs. 18/25 (72) |
| Minnella76 2021 Canada | Radical cystectomy Malignant (70) | None | P (B) N (B) | Anxiety-reduction | Standard care | Clavien-Dindo 1-5 complications 30 days | 16/30 (53) vs. 16/28 (57) |
| Molenaar77 2023 Netherlands | Colorectal cancer resection (269) | None | P (I) N (?) S(?) | Anxiety-reduction | Standard care | Complications 30 days | 39/123 (32) vs. 54/128 (42) |
| Nielsen78,97 2008 Denmark | Lumbar fusion (73) | None | P (B) S (I) A (I) | - | Standard care | Major complications 30 days | 8/28 (29) vs. 8/32 (25) |
| Nguyen79 2022 France | Total knee replacement (262) | None | P (I) N (B) | Anxiety-reduction | Standard care | Adverse events | ? |
| Stein80 1970 USA | Thorax, abdominal or other surgery (48) | None | P (I) - Chest physio. S (?) | Medication per indication | Standard care | Pulmonary complications grade 1-4 | 5/23 (44) vs. 15/25 (60) |
One of the two included studies was a multicentre RCT with stratification for smoking, alcohol and both had a small subgroup (n=10) investigating the effect of combined intensive smoking and alcohol cessation intervention for patients with these predefined risk factors undergoing radical cystectomy for bladder cancer.63 In total, the sub-group consisted of 13 (eight in intervention group + five in control group) patients, but three did not undergo surgery leaving ten patients (seven in intervention group + three in control group) ( Table 2). The other included study (n=110) investigated the effect of a brief nutrition and intensive physical activity intervention on patients undergoing colorectal cancer surgery.62 They included their patients based on being frail preoperatively according to the Fried Frailty Index,64 which includes both elements of physical capability and malnutrition. Patients who were current smokers or had a risky alcohol intake were counselled regarding cessation, but no information regarding counselling content or lifestyle improvement were described ( Table 2). None of the two included studies received funding from foundations involved in any part of the trials (designing of study, study conduction, dissemination of results etc) except the funding.
| Study | Carli 202062 | Lauridsen 202263 | ||
|---|---|---|---|---|
| Follow-up time | Before surgery + 4weeks after surgery | 6weeks + 3, 6, 12months after inclusion | ||
| Duration of intervention (protocol) | 4 weeks | 6 weeks | ||
| Group allocation | IG | CG | IG | CG |
| Baseline to surgery days, median (IQR) | 40 (28-50) | 35 (22-55) | 8 (7-22) | 13 (12.5-23) |
| Adherence to intervention mean (SD) | 80% (27%)a | - | 100%b | - |
| Number of patients undergoing surgery | 55 | 55 | 7 | 3 |
| Men, N (%) | 29 (52.7) | 23 (41.8) | 7 (100%) | 3 (100%) |
| Age, median (IQR) | 78 (72-82) | 82 (75-84) | 63 (58-69) | 71 (68-72) |
| ASA score, N (%) | ||||
| II | 19 (34.5) | 9 (16.4) | 2 (28.5) | 3 (100) |
| III | 33 (60.0) | 43 (78.2) | 3 (43) | 0 |
| IV | 3 (5.5) | 3 (5.5) | 0 | 0 |
| No information | 2 (28.5) | |||
| Current smokers, N (%) | 6 (10.9) | 5 (9.1) | 7 (100) | 3 (100) |
| Malnutrition, N (%) | B: 27 (50.0)c | B: 12 (26.7)c | 0d | 0d |
| C: 11 (20.4)c | C: 13 (28.9)c | |||
| BMI ≥ 30, N (%) | 14 (25.5) | 16 (29.6) | 1 (14.3) | 0 |
| Risky alcohol use, N (%) | 5 (9.1)e | 3 (5.5)e | 7 (100)f | 3 (100)f |
| Physical active ≤ 30 min/day, N (%) | - | - | 2 (29) | 1 (33) |
Risk of bias assessment for the two included studies are presented in Figure 1. Being behavioural intervention studies blinding of patients and study personnel was not possible. Otherwise, both studies were judged to have a low risk of bias in almost all domains for both primary and secondary outcomes. Exceptions were the secondary outcomes of successful risk reduction at the end of intervention in Carli et al.62 and at 12 months in Lauridsen et al.63 In Carli et al. less than 80% of the control group attended follow-ups. Furthermore, most of the non-attendances at follow-up were not justified. One could wonder whether the non-attendances at follow-up were the patients in the worst physical shape/with the least improvement, thereby inducing bias due to missing results. Therefore, the domain of missing outcome data was assessed as having a high risk of bias. The problems with follow-up data in Lauridsen et al. was explained by death of patients. One patient in each group died within 90 days due to complications, the rest due to recurrence of their bladder cancers, therefore the domain received some concerns instead of high risk of bias. To identify publication bias we planned to produce funnel plots but too few studies were identified for this.
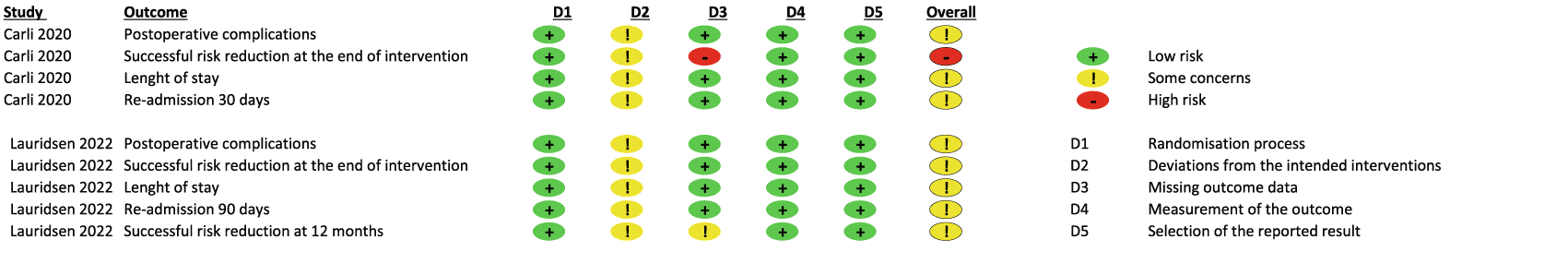
+: low risk of bias, !: some concerns, -: high risk of bias, D1: randomization process, D2: deviations from the intended interventions, D3: missing outcome data, D4: measurement of the outcome, D5: selection of the reported result.
None of the two included studies reported a statistically significant difference in postoperative complications within 30 days between intervention and control groups. Carli et al.62 reported complications in 25 of 55 (45.5%) participants in both intervention and control group. Lauridsen et al.63 reported complications in three of seven participants and three of three participants, respectively. The meta-analysis based on a random-effects model estimated a weighted relative risk of 0.79, 95% CI [0.41, 1.51] ( Figure 2). The meta-analysis based on a fixed-effect model estimated a weighed relative risk of 0.92, 95% CI [0.63, 1.34]; I2: 51%.
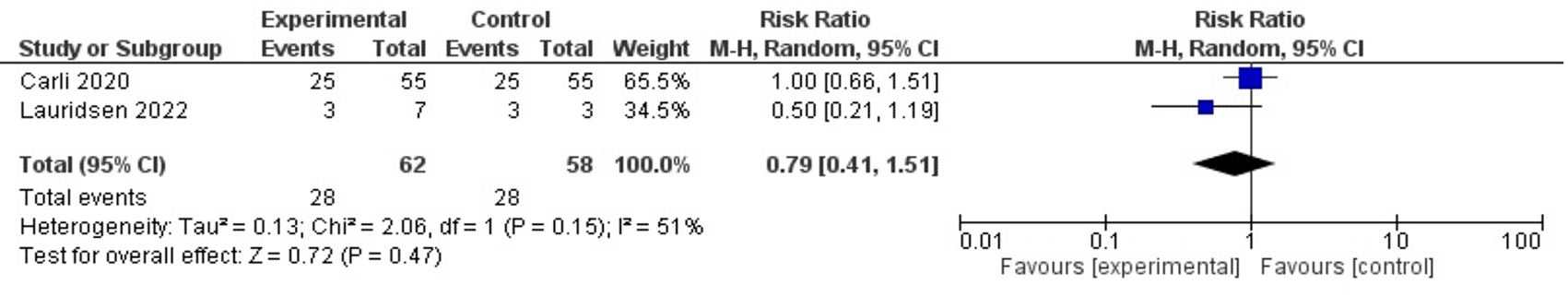
CI: confidence interval, df: degrees of freedom, P: p-value, Z: z-value.
According to the protocol, we performed a network meta-analysis comparing all intervention groups as if it had been compared in one large trial. When comparing an intensive (I) physical activity and brief (B) nutrition, smoking and alcohol cessation intervention (PINSAB) with an intensive smoking and alcohol cessation intervention (SAI) the risk of complications could potentially be unfavorable for PINSAB (OR = 1.19 [0.26 to 5.45]). SAI on the other hand might reduce the risk of complications when compared to treatment as usual (TAU) (OR = 0.79 [0.17 to 3.60]), but when TAU was compared to PINSAB there was no reason to suspect any difference in risk of complications (OR = 0.94 [0.46 to 1.92]).
Carli et al. reported an identical length of stay in both groups of 4 days (mean difference 0.00, CI 95% [-1.38, 1.38]) while Lauridsen et al. reported length of stay of 7.3 days in the intervention group versus 11.7 days in the control group. The mean difference was -4.40 with CI 95% [-9.10, 0.30] pointing towards a shorter length of stay in the intervention group but still not statistically significant. Meta-analysis estimated a weighted mean difference of -1.60 days ( Figure 3).
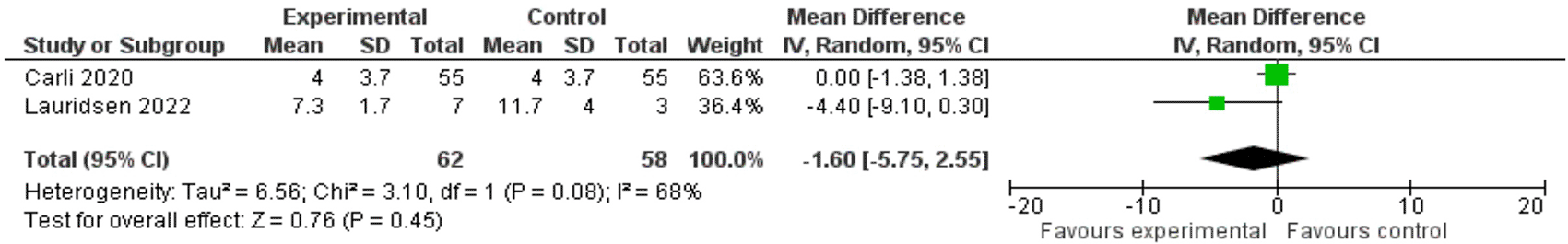
CI: confidence interval, df: degrees of freedom, IV = inverse variance, P: p-value, SD: standard deviation, Z: z-value.
It was not possible to conduct meta-analyses for the remaining secondary outcomes due to less than two studies reporting the outcomes. Carli et al. reported readmissions within 30 days in two of 55 (3.6%) participants versus five in 55 (9.1%) participants in the intervention and control groups, respectively (RR 0.40 [0.08, 1.97]). Lauridsen et al. reported readmissions within 90 days in five of seven participants versus two in two participants, respectively (RR 0.71 [0.45, 1.14]). Carli et al. did not report on long-term quality of life. Lauridsen et al reported one-year QoL and VAS QoL measured by the EQ-5D instrument. Mean difference for one-year QoL and VAS QoL was 0.06, 95% CI [-0.19, 0.32] and 2.4, 95% CI [-33.8, 38.6], respectively.
Regarding successful risk reduction, data were sparse and reported on few SNAP factors. All available results are reported as intention to treat in Table 3. Both studies reported no adverse events due to the interventions. The data available did not allow for any subgroup analyses to be made.
The GRADE assessments of the quality of evidence for the meta-analysis outcomes for postoperative complications and length of stay are summarized in Table 4, including descriptions for downgrading the quality of the evidence.
| SNAP prehabilitation compared to treatment as usual in adult patients undergoing surgery | ||||||
|---|---|---|---|---|---|---|
| Patient or population: Adult surgical patients Setting: Face-to-face interventions Intervention: SNAP prehabilitation Comparison: Treatment as usual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with treatment as usual | Risk with SNAP prehabilitation | |||||
| Postoperative complications | 483 per 1.000 | 381 per 1.000 (198 to 729) | RR 0.79 (0.41 to 1.51) | 120 (2 RCTs) | ⨁⨁◯◯ Lowa,b,c | |
| Length of Stay (LoS) follow-up: mean 30 days | MD 1.6 days lower (5.75 lower to 2.55 higher) | - | 120 (2 RCTs) | ⨁◯◯◯ Very lowc,d | ||
| Readmission 30 days | 91 per 1.000 | 36 per 1.000 (7 to 179) | RR 0.40 (0.08 to 1.97) | 110 (1 RCT) | - | |
| Readmissions 90 days | 1.000 per 1.000 | 710 per 1.000 (450 to 1.000) | RR 0.71 (0.45 to 1.14) | 9 (1 RCT) | - | |
Surprisingly, this systematic review identified only two randomized trials of prehabilitation before surgery targeting two or more co-existing and predefined risky SNAP factors (like physical activity below four hours per week and daily smoking) compared to treatment as usual or no treatment. More than 20 randomized trials on multimodal SNAP factor prehabilitation has been published and all but two had only one or no predefined risky SNAP factor as inclusion criteria. The lack of preidentified risk factors may result in populations with mixed needs for prehabilitation, thus, offering prehabilitation to both patients with and without risky SNAP factors thereby with or without a need for prehabilitation. We found no significant effect on postoperative complications in the two included studies.
The large majority of RCTs evaluating combined interventions mainly involved physical training and nutritional support, but they did not deliver the prehabilitation based on specified risky SNAP factors presented in the inclusion criteria ( Table 2). This contrasts with other prehabilitation programmes, e.g., to quit smoking and alcohol prior to surgery, which would not be offered to persons without risky alcohol or tobacco use.
Most of the excluded studies recruited participants on the basis of diagnosis or type of surgery rather than identified individual needs for prehabilitation.65–80 Another six studies on combined interventions included participants based on only one predefined risky SNAP factor; overweight81–83 or limited physical activity, function, or sedentary lifestyle.84–86 In the 22 excluded studies, there seems to be a discrepancy between the patients’ needs for prehabilitation and the programmes delivered. This may explain the inconsistency in impact on postoperative complications.
Frailty is a medical syndrome characterized by “diminished strength, endurance, and reduced physiologic function”,87 which leaves the individual with decreased tolerance of stressors and vulnerable to adverse outcomes.88 Numerous screening tools exist, however, we have only included randomized trials using tools that include two or more of the predefined risk factors of relevance for this systematic review e.g. the Fried Frailty Index,64 which involves weight loss and reduced physical activity in addition to muscular weakness, slow walking, and exhaustion.42 The Fried Frailty Index is used in the included study by Carli et al.62 and this frailty diagnosis is closely related to the development of postoperative complications.42 However, the evidence from RCTs of frailty intervention is sparse,89,90 but studies are ongoing.91,92
The included randomized trial on intervention for co-existing risky alcohol and tobacco use prior to major surgery is based on the major risk reducing effect on the separate preoperative intensive smoking or alcohol intervention.12,32,33 It is only a minor subgroup from a larger study, but we have not been able to identify other studies of combined intervention for these risk factors. However, studies on non-surgical patients have shown successful quitting with the combined intervention, thereby indicating that this could be successful in relation to surgery.93
A strength in this review is the substantial search of electronic databases using a highly sensitive search strategy developed in consultation with an information specialist. Another strength is that we only included studies where participants were selected and offered interventions based on five predefined risk factors associated with increased risk of postoperative complications, specifically according to the individual patient’s risk profile. One may consider the inclusion of any type of surgery as a weakness, however, several of the risky SNAP factors have been shown to increase complication rates with around 50% across different kinds of surgery.11,13,16
The limited number and the small size of the included studies provided a weak foundation for estimating the effect of combined prehabilitation. Especially, the combined alcohol and smoking cessation intervention constitutes a very small sub-group with unequal allocation to intervention- and control group despite of stratification, probably due to recruitment from five centres.63 The other included study evaluated frailty intervention based on Fried Frailty Index with five parameters in total. However, it is not clear to which degree the two SNAP factors weight loss and low physical activity were present in the included participants.62 None of the two included studies intervened or reported outcomes on all the risky SNAP factors identified in baseline data. The biases above further impact the limitation of this review. In addition, both studies were conducted on patient groups with specific cancers in Western high-income countries (Canada and Denmark). Therefore, the results must be interpreted with caution, and the generalisation should be carefully considered.
Prehabilitation aiming at combined risky SNAP factors seems to have potential to abate postoperative complications, and probably long-term health. This untapped potential has not been defined yet, which may have impact for the individual patients and their families as they will continue suffering from potentially avoidable complications after surgery.
Complications are also resource consuming,7 adding to the workload of the health professionals, the economic burden on the healthcare system and the society at large. Economic resources often are limited. As those with risky SNAP have an increased risk of complications compared to those without risky SNAP factors,11–19 it seems plausible that prioritizing SNAP prehabilitation to those with risky SNAP factors would likely derive the greatest benefit in terms of reduced postoperative complications. However, as this review shows, the effect of combined prehabilitation in patients with risky SNAP factors is still very uncertain. In the future, when more evidence is available, prioritizing those with the greatest benefit could potentially be relevant to minimize the socioeconomic costs of prehabilitation.
The findings of this review show a lack of studies providing combined prehabilitation based on patient needs present in the study population. Therefore, conduction of high-quality large-scale studies on combined prehabilitation targeting co-existing and predefined risky SNAP factors are strongly requested. The combined prehabilitation should build upon the individual patient’s needs from preoperatively identified SNAP factors to establish new evidence regarding impact on surgical outcome at short term and health on longer term. Future research should consider explicitly including a demand of predefined risk factors in the research question and in the inclusion criteria, ensuring that interventions are targeted to co-existing risky SNAP factors.
When designing the studies, researchers need to take into account the varying levels of im-plementation of ERAS that may add to narrowing the gap of the effect of the interventions. The standard care and ERAS components implemented vary across institutions, surgical specialties, and procedures.4 This makes it complex to estimate the actual intervention effect and compare effects properly across studies. TAU should align with the standard care pro-vided at the specific institution and for the relevant procedure to ensure that the TAU group does not receive inferior care compared to patients not participating in the studies. As a re-sult, the definitions of TAU groups will vary between studies, highlighting the need for a de-tailed description of TAU content including specifying adherence to ERAS guidelines and the intensity of potential SNAP interventions. Additionally, the content of the intervention group including the intensity of the interventions should be described with equal detail. The thorough description and transparency is essential for enabling meaningful future meta-analyses. Furthermore, due to the complexity alternative statistical methods such as Bayesian metrics could be relevant to consider as they can provide a more nuanced understanding of intervention effectiveness beyond traditional p-values.98
This review only identified two small trials that did not demonstrate statistically significant effects on postoperative complications after prehabilitation targeting co-existing and predefined risky SNAP factors. Although surprising, this important finding highlights the lack of randomised trials evaluating the effect of individualised, combined prehabilitation programmes delivered in accordance with individual patients’ preoperative risky SNAP factors. The meta-analysis did not demonstrate statistically significant effects on postoperative complications in this context. However, the study underscores the need for more prehabilitation studies with interventions targeting preidentified risky SNAP factors in order to assess the true effect on postoperative complications.
The corresponding author attests that all listed authors meet the ICMJE authorship criteria and that no others meeting the criteria have been omitted.
We have used the repository Open Science Framework for the PRISMA checklist and flowchart. The name of the project is ‘Impact on postoperative complications of combined prehabilitation targeting co-existing smoking, malnutrition, obesity, alcohol drinking, and physical activity: a systematic review and meta-analysis of randomised trials’. https://doi.org/10.17605/OSF.IO/RKAVF
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We used Review Manager 5.4® (RevMan [Computer program]. Version 5.4. The Cochrane Collaboration, 2020). for the meta-analysis. Review Manager 5.4® is no longer available for download, but a web version can be used with a paid licence. However, an open access alternative is OpenMeta[Analyst].
We used Covidence for the screening of references with a paid institutional license. However, a limited open access alternative is the web tool Rayyan.
We have used the repository Open Science Framework for extended data (complete search strings) and reporting guidelines (PRISMA checklist and PRISMA flowchart).99 The name of the project is: Impact on postoperative complications of combined prehabilitation targeting co-existing smoking, malnutrition, obesity, alcohol drinking, and physical inactivity: a systematic review and meta-analysis of randomised trials. https://doi.org/10.17605/OSF.IO/RKAVF.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Orthopedics, spine, rehabilitation
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: perioperative medicine, health promotion, substance use and addiction medicine
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: perioperative medicine, health promotion, substance use and addiction medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 08 Apr 25 |
read | read |
|
Version 1 26 Jun 24 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)