Keywords
CDK4/6 inhibitors, Working-age women, Metastatic Breast Cancer, Quality of Life, Work Activity
This article is included in the Oncology gateway.
Breast cancer frequently leads to reduced work capacity and increased absenteeism among working-age women diagnosed with this condition. In this study, we aimed to assess the effect of CDK4/6i combined with aromatase inhibitors (AIs) or fulvestrant on quality of life (QoL) and work activity in a cohort of Portuguese patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) metastatic breast cancer who started this treatment regimen.
This unicentric prospective observational cohort study was conducted in 35 Portuguese women with stage IV HR+/HER2- breast cancer receiving CDK4/6i combined therapy. The objectives of the study were evaluated using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 version 3 (QLQ-C30) and the breast cancer-specific Questionnaire (EORTC QLQ-BR23), and the Work Productivity and Activity Impairment questionnaire - General Health (WPAI:GH) questionnaires at four different time points throughout treatment.
The results obtained revealed a significant improvement in EORTC QLQ-C30 score from baseline in emotional functioning, social functioning, pain, dyspnea, insomnia, and financial difficulties, as well as in future perspective and breast symptoms, as assessed by EORTC QLQ-BR23 score. However, a significant deterioration from baseline in systemic therapy side effects was also observed. Despite the limitations regarding the cohort size, an increase in work absenteeism was also observed, as determined by WPAI:GH scores. This can possibly be attributed to pain-related bone metastases observed in those patients.
To the best of our knowledge, this is the first study to assess the impact of combined CDK4/6i therapy on the QoL and work activity of Portuguese patients with metastatic breast cancer. Our results indicate that although patients’ QoL did not appear to be affected, there was a significant decrease in work activity, as evidenced by preliminary results of the increased rate on work absenteeism.
CDK4/6 inhibitors, Working-age women, Metastatic Breast Cancer, Quality of Life, Work Activity
Globally, breast cancer is the most common form of invasive cancer and the leading cause of cancer-related death in women.1,2 It is also the most diagnosed cancer in the world, with 2.26 million new cases and around 684,000 deaths in 2020.3 Advanced breast cancer, encompassing both locally advanced (stage IIIB/C) and metastatic (stage IV) stages, presents significant clinical challenges due to its aggressive nature and limited treatment options.2,4 Particularly, metastatic breast cancer with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) is the most common subset of breast cancer, accounting for at least 60% to 75% of all breast cancer cases.2,4 Therapeutic strategies for metastatic HR+/HER2- breast cancer often involve endocrine therapy, such as selective estrogen receptor modulators (SERMs) like tamoxifen, aromatase inhibitors (AIs) such as letrozole and anastrozole, and fulvestrant, an estrogen receptor antagonist, as hormone receptors play a pivotal role in tumor growth and progression.5 While these may lead to initial benefits, resistance can develop over time, leading, ultimately, to disease progression and posing the need for alternative treatment approaches.2,5 One of the groundbreaking advancements in the management of HR+/HER2- metastatic breast cancer has been the introduction of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i).5,6 CDK4/6is, including palbociclib, ribociclib, and abemaciclib, have shown remarkable efficacy by disrupting the cell cycle (suppressing the activity of CDK4 and CDK6) and inhibiting tumor growth, and were approved as first line (in combination with AI) and second line (in combination with fulvestrant) treatment in patients with HR+/HER2- metastatic breast cancer regardless of menopausal status, age, endocrine sensitivity, and type of metastasis.2,6 For instance, the PALOMA-2 trial7 and MONALEESA-2 trial8 demonstrated improved progression-free survival and overall response rates with palbociclib and ribociclib, respectively, in combination with letrozole compared to letrozole alone. Similarly, the MONARCH 2 trial9 revealed the efficacy of abemaciclib plus fulvestrant compared to fulvestrant alone.
However, evaluating new treatments, particularly combination regimens, extends beyond the mere efficacy reported by randomized controlled trials (RCTs). It is paramount to measure the quality of life (QoL) gained by delaying disease progression through patient-reported outcomes (PROs) for comprehensive benefit-risk assessments. Notably, several studies, including PALOMA-2,7,10 MONALEESA-2,8 MONALEESA-3,11–14 MONALEESA-7,15 MONARCH-2,9 MONARCH-3,16 and two systematic reviews have evaluated the QoL in HR+/HER2- patients treated with CDK4/6 inhibitors. The results showed that HR-QoL was generally maintained or improved with CDK4/6i.2,17 Despite these insights, there is still a lack of real-world evidence regarding the effectiveness of this treatment specifically for HR+/HER2- subpopulations, including working-age women, and the associated indirect costs.
In light of the above, this study aims to generate pivotal data on PROs, assessing the quality of life of this subpopulation, thereby filling the existing knowledge void and guiding future therapeutic decisions.
The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was submitted to the ethics committee of Portuguese Institute of Oncology (IPO) of Coimbra and approved on January 24, 2022 (TI 33/2021). Participants written and signed Informed Consent Form was obtained prior to any study-related activity.
This is a unicentric prospective observational cohort study assessing the PROs on QoL and work activity of working-age women with HR+/HER2- metastatic breast cancer who started treatment with a CDK4/6i combined with AI or fulvestrant. We also intended to evaluate the patient-reported adverse events.
The recruitment period of the study occurred from April 2022 to March 2023, and patients were enrolled from the IPO of Coimbra, in Portugal. All patients that were about to start treatment with a CDK4/6i combined with AI or fulvestrant, irrespective of the line of treatment, were identified by the clinical research team as potential eligible participants.
To be enrolled in the study, participants must meet all the following inclusion criteria and none of the exclusion criteria. Inclusion criteria: i) Women aged 18 years old or older; ii) any menopausal status; iii) working-age women, including homemakers, unemployed, students or women on sick leave; and iv) histologically confirmed diagnosis of HR+/HER2- metastatic breast cancer and be followed in Medical Oncology consultation of the IPO of Coimbra were eligible to enroll the study. Exclusion criteria included: i) Male patients; ii) retired women; and iii) patients already undergoing treatment with CDK4/6is.
The study cohort included 35 women with ages ranging between 26 and 67 years old.
After signing the informed consent form, participants were asked to complete three questionnaires concerning their health-related quality of life (HRQOL) at four different time points: at baseline (C0), on day 1 of cycles 2 (C2), 3 (C3), and 7 (C7). In addition, questionnaires were filled out whenever there was disease progression, or the treatment was discontinued. PROs were analyzed using the validated European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 version 3 (QLQ-C30)18,19 and the breast cancer-specific Questionnaire (EORTC QLQ - BR23),20,21 and the translated version of the Work Productivity and Activity Impairment questionnaire - General Health (WPAI:GH).22
The EORTC QLQ-C30 version 3 is a 30-item questionnaire composed of a global QoL subscale, five multi-item functional subscales (physical, role, emotional, cognitive, and social functioning), three multi-item symptom scales (fatigue, nausea/vomiting, and pain), and five single-item symptom scales assessing other cancer-related symptoms (dyspnea, sleep disturbance, appetite loss, constipation, and diarrhea).23 The questionnaire consists of 28 4-point Likert scale items, with options from “not at all” to “very much”, to assess functioning and symptoms, and two 7-point Likert scale items for global health and overall QoL.
The EORTC QLQ-BR23 is a 23-item breast cancer-specific companion module to the EORTC QLQ-C30. It consists of four functional scales (body image, sexual functioning, sexual enjoyment, future perspective) and four symptom scales (systemic side-effects, breast symptoms, arm symptoms, upset by hair loss).
Responses to all items of EORTC QLQ-C30 and EORTC QLQ-BR23 were converted into a 0–100 scale, using a standard scoring algorithm. For functional and global QoL scales, higher scores represent a better level of functioning/QoL than lower scores. On the other hand, for symptom-oriented scales, higher scores represent greater symptom severity.
The WPAI:GH is a 6-item questionnaire composed of the following questions: Q1 = currently employed; Q2 = working hours missed due to health problems; Q3 = working hours missed due to other reasons; Q4 = hours worked; Q5 = health condition affected productivity during work and Q6 = health condition affected daily activities. WPAI outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity, reflecting worse outcomes. Absenteeism is defined as the percentage of time absent from work, in the last week, due to health conditions. It is calculated as Q2/(Q2 + Q4) × 100%. Presenteeism is measured by the how health problems affected work productivity, over the last 7 days, on a rating scale ranging from 0 to 10, with 0 indicating that health problems had “no effect on my work” and 10 indicating that “health problems completely forbid me from working”. The outcome is expressed as a percentage of impairment due to health issues, during work. Higher scores indicate greater impairment and, consequently, less productivity. This is calculated as (Q5/10) × 100%. Overall percentage of work impairment due to health conditions is calculated as follows Q2/(Q2 + Q4) + [(1-(Q2/(Q2 + Q4))) × (Q5/10)]. The percentage of activity impairment due to health problems is calculated as Q6/10.
Adverse Events were also evaluated based on data collected from medical records, according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5. This evaluation was carried out on baseline, day 1 of cycles 2, 3, and 7, and whenever disease progression was observed, or when the treatment was discontinued.
Additionally, demographic, and clinical data were collected from medical records from Department of Medical Oncology. Those including age, employment status, comorbidities, ECOG performance status, menopausal status, stage at initial diagnosis, hormone receptor status, prior endocrine therapy and chemotherapy, as well as metastatic sites.
The participants were informed that no personal, health, or genetic information would be provided to third parties unless required by law, health authorities, or ethics committees in order to verify the information obtained in this study. As predicted by Portuguese Law 58/2019, implementing the General Data Protection Regulation (GDPR), all personal data will be considered confidential. To ensure the anonymization of participants personal data and identity, questionnaires were de-identified with a code. The association between the code and the identity was protected by an authorized person of the research team; strict measures were taken to make such information available only to authorized personnel, who will not be able to disclose the participant identity to third parties.
Sample size was calculated using the t-test for paired samples (two-sided), assuming a moderate effect with a Cohen’s d of 0.5, a 0.05 significance level and a power of 80%. From this, sample size was calculated as n = 34.
Data analysis was performed using RStudio (RRID:SCR_000432) with R Project for Statistical Computing (RRID:SCR_001905), version 4.2.2. Descriptive analysis of the variables was performed using mean (standard deviation) and median (interquartile range, minimum, and maximum) for quantitative variables and counts (percentages) for qualitative variables. For all analyses, a confidence interval (CI) of 95% was used. Longitudinal analysis was performed using linear mixed models using the glmer function from the R package: lme4 (RRID:SCR_015654).
Between April 2022 and March 2023, 35 patients were enrolled in the study, with ages ranging from 26 to 67 years old and a median age of 47 years. All participants presented with advanced metastatic disease at baseline. Of these, a total of 13 individuals (37%) presented with initial stage IV disease.
Regarding employment status, only 10 patients were actively working (either full-time or part-time employed). The remaining participants were homemakers, unemployed, students or on sick leave.
Most patients were previously submitted to endocrine therapy (74.3%) and/or chemotherapy (57.1%). Patient demographic, clinical and pathological characteristics at baseline, are summarized in Table 1.43
ECOG, Eastern Cooperative Oncology Group (ECOG); ER, estrogen receptor; PR, progesterone receptor.
In total, 32 patients (91.4%) received ribociclib, two (5.7%) received palbociclib and one patient (2.9%) received abemaciclib. CDK4/6i therapy was combined with fulvestrant in 12 patients (34%) and with letrozole in 23 patients (66%). Most patients received a CDK4/6i combined therapy as first-line treatment (29 patients, 82.9%), whereas six (17.1%) received CDK4/6i combined therapy as second-line treatment.
The questionnaire completion rates for the three questionnaires during the treatment period were 100% at baseline (C0), about 83% at cycle 2 (C2), 74% at cycle 3 (C3), and approximately 49% at cycle 7 (C7) of treatment with the CDK4/6i combined therapy (Table 2).
EORTC, European Organization for Research and Treatment of Cancer; QLQ-C30, Quality of Life Questionnaire C30 version 3; QLQ-BR23, Quality of Life breast cancer-specific Questionnaire; WPAI:GH, Work Productivity and Activity Impairment questionnaire - General Health; QoL, quality of life; C0, Cycle 0 (baseline); C2, Cycle 2 of CDK4/6i treatment; C3, Cycle 3 of CDK4/6i treatment; C7, Cycle 7 of CDK4/6i treatment; N, Total of results.
At the data cut off, 04/30/2023, stable disease (SD) was presented in 24 (69%) patients, seven (20%) had a partial response (PR) and four (11%) patients had disease progression (DP). From the four patients who presented DP, only two have responded to the questionnaires at the time of DP. Therefore, data from those participants were not included in the analysis.
Throughout the duration of the study, mean global QoL scores among participants receiving CDK4/6i combined therapy were maintained when compared to the baseline assessment.
Regarding the five QLQ-C30 functional scales (physical, role, cognitive, emotional, and social functioning) assessed at baseline, significant differences were identified. A significant enhancement from baseline to C2 and C7, and only to C7 in emotional functioning [10 (95% CI 3.3 to 17); P = 0.004] and social functioning [11 (95% CI 3.4 to 19); P = 0.005], respectively, were evident for participants receiving CDK4/6i combined therapy (Table 3; Figure 1). Contrarily, overall changes from baseline scores for physical, role, and cognitive functions did not attain statistical significance.
EORTC, European Organization for Research and Treatment of Cancer; QLQ-C30, Quality of Life Questionnaire C30 version 3; QLQ-BR23, Quality of Life breast cancer-specific Questionnaire; WPAI:GH, Work Productivity and Activity Impairment questionnaire - General Health.

Relative to the QLQ-C30 symptom scale, a significant decrease from baseline in pain and in dyspnea related to pulmonary metastasis were observed at C3 [−8.6 (95% CI −16 to −1.3); P = 0.021 and -5.3 (95% CI -9.7 to -0.83; P = 0.020, respectively] (Table 3; Figure 1). Furthermore, a significant decrease in both insomnia [-11 (95% CI -21 to -0.54); P = 0.039] and financial difficulties [-19 (95% CI -30 to -9.2); P < 0.001] were also observed at C7 when compared to baseline values (Table 3; Figure 1). However, there was a significant worsening in diarrhea at C3 [7.6 (95% CI 1.8 to 13); P = 0.010] (Table 3; Figure 1). No significant differences were observed for the other EORTC QLQ-C30 symptoms.
Regarding QLQ-BR23 functional scales, the sample size for sexual enjoyment parameter was smaller when compared to other scales (Table 2), as only sexually active participants respond to this parameter (48 vs. 107 answers, in total of the scale). A statistically significant improvement in future perspective was observed at C3 of CDK4/6i combined therapy [12 (95% CI 3.7 to 21), P = 0.005] (Table 3; Figure 2). Comparative evaluations of other breast cancer-specific functional scales to the baseline values did not yield statistically significant results.
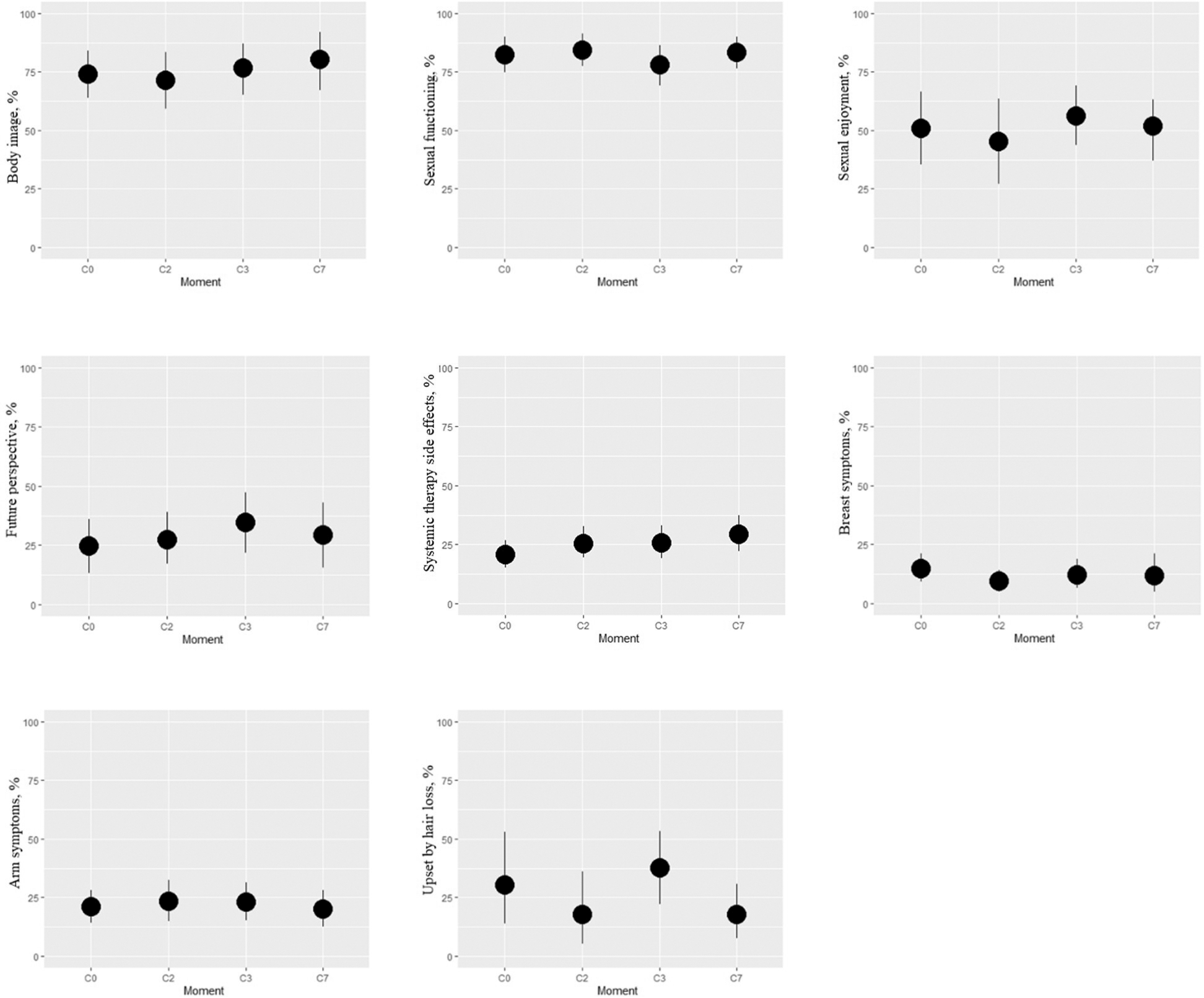
With respect to the QLQ-BR23 symptom scale, it is important to clarify that the item hair loss was exclusively addressed to patients who effectively experienced hair loss, resulting in a considerably diminished cohort size (53) for the symptom scale “upset by hair loss” relative to other scales (Table 2). A statistically significant decrease from baseline to C2 and C7 in this parameter was observed [-17 (95% CI 33 to -1.9); P = 0.028] (Table 3; Figure 2). Conversely, a statistically significant deterioration from baseline to the same time points was observed for systemic therapy side effects [6.7 (95% CI 2.1 to 11); P = 0.005] (Table 3; Figure 2). Furthermore, on C2 of CDK4/6i combined therapy heralded a statistically significant improvement in breast symptoms [-6.6 (95% CI -11 to -1.9); P = 0.006] (Table 3; Figure 2).
Within the study cohort, it was observed that two participants, initially classified as “not currently employed” prior to the initiation of CDK4/6i combined therapy, resumed occupational activities during the course of treatment. Conversely, two participants, categorized as “currently employed” at baseline, ceased their occupational activities at C3 assessments. Analysis of the WPAI-GH metrics revealed a statistically significant increase in the number of working hours lost by C3 [17 (95% CI 0.54 to 33); P 0.043] (Table 3; Figure 3). Comparative evaluations of the other WPAI:GH parameters from baseline values did not yield any statistically significant variations.
Adverse events, as classified per CTCAE version 5, were documented in 34 participants (representing 97% of the cohort) for the duration of this study. The most common adverse events encompassed hematological disorders, namely neutropenia (82%) and anemia (19%), accompanied by gastrointestinal disturbances, such as nausea and vomiting (13%). From the 29 patients presenting with neutropenia of any grade, 14 participants (comprising 40% of the cohort) experienced grade 3 neutropenia, while 15 experienced grade 1-2 neutropenia. No other adverse events reaching grade 3 severity were recorded. Importantly, it is not clear whether the underlying cause of the AEs observed can be attributed to CDK4/6i combined treatment or not. A detailed list of adverse events is shown in Table 4.
The main goal of advanced breast cancer treatment is to delay disease progression and chemotherapy initiation, while maintaining QoL for as long as possible, thus minimizing treatment-related adverse events.
The association between QoL and PFS in oncology clinical trials has not been well established yet.24,25 The clinical benefit may be defined as improvement in efficacy, safety, and absence of a decline in QoL.26 Patient QoL is known to be impacted by both the efficacy and tolerability profile of a therapeutic agent. Moreover, it is well-known that treatment-related toxicities can negatively affect the QoL of patients with advanced breast cancer.27,28 Indeed, the impact of a treatment on patient’s QoL also affects professional ability and work activity. Conventional therapies, like chemotherapy, can cause several serious adverse events and have been shown to have a significant negative impact on QoL.29 However, with recent advances in the available therapies for metastatic/recurrent breast cancer, preserving patient QoL has become more feasible due to the development of more tolerable agents, including hormone therapy and CDK4/6i.30,31 As there are many working active age women undergoing these treatments, it is crucial to determine if these treatments positively affect the work activity of those patients. PFS benefits have been observed with ribociclib combinations in all three MONALEESA trials,11–14 with palbociclib combinations in PALOMA-27,10 and PALOMA-332 and with abemaciclib combinations in MONARCH-29 and MONARCH-3.16 The recently published European Society for Medical Oncology Magnitude of Clinical Benefit Scale guidance emphasized the importance of a holistic assessment of the value of a medicine that includes Patient Reported Outcomes (PROs), in addition to efficacy and safety.33 Moreover, in this article, the author emphasized the importance of QoL for their Clinical Benefit Scale. They state that there is an upgrade of the ESMO-MCBS score if QoL is improved compared to the control arm, while a downgrade in the score is observed if an improvement in progression-free survival is not paralleled by an improvement in QoL.33
Moreover, in a cross-sectional study by Hollen et al., patients with breast cancer rated maintaining QoL, independence, being able to perform normal activities, and controlling pain as more important than controlling breast cancer symptoms.34
To the best of our knowledge, this is the first study evaluating the impact of CDK4/6i combined therapy on the QoL and work activity of working-age women with metastatic breast cancer in a Portuguese cohort.
The overall results supported the positive risk-benefit profile of CDK4/6i combined with fulvestrant or AI. These treatments seem to maintain patient’s QoL. Considering the results from our 35-patient cohort, we observed a statistically significant improvement in the EORTC QLQ-C30 score from baseline in several parameters, such as emotional functioning (baseline to C2 and C7), social functioning (baseline to C7), pain (baseline to C3), dyspnea (baseline to C3), insomnia (baseline to C7), and financial difficulties (baseline to C7). The analysis of the EORTC QLQ-BR23 score showed an improvement from baseline in future perspective (baseline to C3) and in breast symptoms (baseline to C2), namely in the swollen and sensitivity, and skin complaints in or around the affected breast area. Furthermore, a significant decrease from baseline in “upset by hair loss” parameter was perceived by C2 and C7 of CDK4/6i combined therapy. On the other hand, we noticed a significant deterioration from baseline in systemic therapy side effects at C2 and C7, with respect to parameters such as dry mouth, hair loss, sickness, hot flashes, and headache. Although some of these symptoms have been described as characteristic of CDK4/6i combined therapy, we do not have sufficient data to attribute them to the treatment regimen in our cohort.
Regarding the impact of treatment on work activity, we verified a significant increase from baseline in absenteeism (working hours lost due to health complications) by C3. However, it is important to highlight that only 10 patients were actively working (employed full-time or part-time) at baseline, and 9 out of 10 had bone metastasis. It is well described that bone metastases are one of the main causes of pain in advanced breast cancer patients, and it is responsible for a decrease in QoL, negatively impacting patient’s mood, work capacity, relationships, the ability to walk, and sleep.35,36 Thus, one can speculate that bone pain can contribute to absenteeism in these patients. In addition, among the group of 10 employed patients, seven of them reduced their workload while the remaining three maintained their working hours (Table 5). It was noted that the seven patients who decreased their workload experienced a decline in their functional scale, which encompasses both physical and emotional functioning, as well as future prospects. However, there was no discernible change in their symptom scale, indicating that their symptoms continued to be effectively managed despite the decrease in their functional capabilities. One of the possible explanations might be the interconnected dynamics of three factors—metastatic breast cancer, mental health status, and work absenteeism, which are underpinned by their collective impact on a patient’s QoL. While the physical burden of metastatic breast cancer and its treatment can directly reduce the QoL, the onset or exacerbation of depression in response to the disease further compounds this reduction. This intertwined relationship, where the decline in QoL leads to increased absenteeism from work, and vice versa, creates a feedback loop that can be challenging to break with poor outcomes. In this regard, a recent cross-sectional, observational preliminary study including 61 patients with metastatic breast cancer reported absenteeism in 23% of cases, impairment at work in 31%, overall work productivity loss in 45%, and 40% of patients reported daily activity impairment. Moreover, the authors reported depression among the significant risk factors correlated with absenteeism (p = 0.008).37 According to another study, severe anxiety and moderate to severe depression were reported in 44.2 and 36.9% of the patients, respectively.38
| Patients who reduced working hours, N (%) | Patients who maintained working hours, N (%) | Patients who increased working hours, N (%) | Total N (%) | |
|---|---|---|---|---|
| Employed patients | 7 (20) | 3 (8.5) | - | 10 (28.5) |
As expected, treatment was well tolerated. The most common adverse events were neutropenia (any grade), as well as anemia, nausea/vomiting, and fatigue (grade 1-2). Although we could not establish a causal relation between the AEs and the treatments, the AE were not surprising as it were already described in other breast cancer studies using CDK4/6i39 and combination therapies. Our study did not observe grade 3/4 adverse events such as leukopenia, diarrhea, and fatigue, which were reported in the three MONARCH trials.40 However, MONARCH studies evaluated patients taking abemaciclib, whereas only one patient of our cohort was receiving abemaciclib. Moreover, there were no treatment-related grade 4 adverse events or deaths.
In the real-world scenario, the data available about the QoL among patients treated with CDK4/6i is scarce and most of the evidence comes from participants treated with palbociclib.17 In general, none of the studies showed a decline in the QoL of patients receiving CDK4/6i.17 In line with our results, the prospective, observational multicenter POLARIS study showed an improvement in QoL, specially a trend toward pain improvement.41,42 On the other hand, it was not observed a significant change from baseline concerning QoL and symptom scores in the black, indigenous and people of color (BIPOC) study subpopulation.41
Although these results are encouraging, some limitations should be pointed out, such as the size of the cohort and the number of participants actively working at baseline. Additionally, the distribution of patients across the different CDK4/6i was not balanced, as we only had one patient treated with abemaciclib and two patients treated with palbociclib. Considering that the side-effects induced by the different CDK4/6i can be different among patients, it is difficult to establish the impact on patients’ QoL. Finally, it was not possible to evaluate how the progression of disease impacts the QoL and work capacity, since only two patients presented with disease progression.
The landscape of metastatic breast cancer treatment has evolved significantly, with a focused objective to not only delay disease progression but to uphold and possibly enhance the QoL of the patients. The findings from this study emphasize the therapeutic advantages of CDK4/6i combined therapy, both in terms of clinical outcomes and QoL metrics, for women diagnosed with HR+/HER2- metastatic breast cancer. Moreover, they point towards the tangible link between treatment modalities, QoL, and work activity. The increase in absenteeism noted in our cohort, although preliminary, offers a window into the multifaceted challenges faced by working-age women with metastatic breast cancer. While the physical symptoms and associated treatment effects certainly play a role, it is crucial to also recognize the intertwined relationship of their mental health status and its impact on work absenteeism, all culminating in the broader narrative of QoL. However, to ascertain the true magnitude and implications of this observed trend on work activity, further comprehensive studies, ideally with larger cohorts and longer follow-up durations, are imperative.
Figshare: Underlying data for ‘the impact of CDK4/6 inhibitors on the quality of life of a Portuguese cohort of working-age women with metastatic breast cancer’, https://doi.org/10.6084/m9.figshare.24602550.v2. 43
This project contains the following underlying data:
- quality of life of working-age women with metastatic breast cancer.xlsx (results of the questionnaire)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank the patients who participated in the study. We also thank the study personnel for valuable comments and suggestions on the study design and manuscript. Statistical analysis assistance was provided by Vera Afreixo, PhD, of Mathematics Department of University of Aveiro, funded by Pfizer Pharmaceutical Company. Medical writing support was provided by Fernando Cristo, PhD, funded by Pfizer Pharmaceutical Company. All individuals named in this section have granted their permission for their names and affiliations to be included in this publication.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)