Keywords
Hepatocellular carcinoma (HCC), conventional transarterial chemoembolization (cTACE), lipiodol staining, Hounsfield unit (HU), tumor recurrence
The degree of intratumoral lipiodol staining after conventional transarterial chemoembolization (cTACE) has the potential to predict tumor responses and disease prognosis. This study is aimed at evaluating the correlation between the lipiodol retention of the tumor with a complete response after cTACE and tumor recurrence by quantitative assessment.
From January 2013 to September 2023, every patient receiving cTACE for HCC was recognized. Inclusion criteria were patients with ≤6 HCCs and sizes 1-6 cm, with at least one tumor obtaining a complete response after cTACE, and available baseline and follow-up CT studies. Tumoral, cTACE procedural, and lipiodol staining parameters were analyzed. Using univariate and multivariate analysis, significant factors associated with tumor recurrence were identified. ROC curve analysis was used to identify the optimal cutoff point for the statistically significant factors, predicting tumor recurrence.
The final population included 39 patients with 63 HCCs. Tumor recurrence was detected in 18/63 (28.6%) at a mean of 27.8 months. On univariate analysis, the incidence of tumor recurrence significantly increased with increased tumor size (p = 0.007), an inhomogeneous lipiodol staining pattern (p<0.001), a low minimum lipiodol radiodensity (p = 0.012), and a high lipiodol washout rate (p = 0.046). On multivariate analysis, an inhomogeneous lipiodol staining pattern (p<0.001) and a high lipiodol washout rate (p = 0.012) were significant predictors for tumor recurrence. On ROC analysis, a lipiodol washout rate of greater than 6.44 HU/month was related to tumor recurrence (sensitivity 83%, specificity 51%).
Inhomogeneous lipiodol staining pattern and lipiodol washout rate of >6.44 HU/month were predictors for recurrence of HCC after a complete response after cTACE. These correlations may provide useful guidance for subsequent imaging surveillance and treatment approaches.
Hepatocellular carcinoma (HCC), conventional transarterial chemoembolization (cTACE), lipiodol staining, Hounsfield unit (HU), tumor recurrence
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related deaths globally.1 Surgical, locoregional, and systemic therapies are treatment options for patients with HCC, contingent on the liver function, the tumor burden, and the patient's performance level. Given that a small percentage of patients receive an early diagnosis, palliative image-guided intraarterial therapies are important forms of treatment.
Transarterial chemoembolization (TACE) is the standard treatment for intermediate-stage HCC. Conventional TACE (cTACE), a treatment method involving chemotherapeutic agents emulsified with ethiodized oil (lipiodol), followed by embolic agents delivered via tumor-supplying arteries, is widely used and has shown significant patient survival benefits.2
Lipiodol used in cTACE has three specific functions: tumor-seeking drug carrier, transient micro-embolic agent, and radiopacity property. It serves as an agent for imaging in intraprocedural fluoroscopy and cone-beam computed tomography (CBCT), as well as postprocedural multidetector CT.3 Lipiodol staining also has the potential to predict tumor responses and disease prognosis, as many previous studies showed that the degree of lipiodol deposition within the tumor has been correlated with pathological tumor necrosis, tumor recurrence, and survival rate.4–13 However, there are few studies quantifying the assessment of lipiodol deposition.4,5
The main purpose of this study is to analyze quantitatively the correlation between the treated tumor's lipiodol retention and tumor recurrence. The secondary goal is to determine the cutoff value of lipiodol retention for predicting tumor recurrence.
The human research ethics committee of Thammasat university (Medicine) approved this study on August 30, 2022. The number of certificate of analysis (COA) was 180/2022 and the project number was MTU-EC-RA-0-162/65. The informed consent was waived because the research involved no more than minimal risk to subjects.
We retrospectively reviewed CT images and analysed the Hounsfield Unit of Lipiodol staining.
All cTACE procedures for patients with HCCs were retrospectively reviewed on the Radiology Information System. Only patients who had ≤ 6 tumors with a size of 1-6 cm at preprocedural imaging were included. The selection criteria for the treated target tumor included: 1.) the tumor that obtained a complete response according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) on triphasic computed tomography (CT) or magnetic resonance imaging (MRI) with additional noncontrast CT within 8 weeks after cTACE. This imaging study was referred to baseline CT. 2.) the tumor that had at least one follow-up imaging with triphasic CT after baseline CT. This imaging study was referred to follow-up CT. 3.) the tumor that had follow-up imaging with triphasic CT or MR for at least 6 months after cTACE. The imaging study that first detected tumor recurrence or the most recent imaging study in case of no tumor recurrence would be selected and referred to the final imaging. The exclusion criteria included: 1.) the tumor that received a particulate embolic agent such as PVA or microsphere during cTACE. 2.) the tumor that received any other combined or additional treatment such as ablation, radiotherapy, or systemic therapy; and 3.) the tumor with unmeasurable lipiodol density.
A tumor board consisting of hepatic surgeons, radiologists, interventional radiologists, hepatologists, and oncologists discussed whether or not to do cTACE for each patient. Patients received information regarding the intent and method of the cTACE procedure, as well as the likelihood of post-procedural complications. cTACE procedures were performed in the Angio suite using a Philips Allura Xper FD20/20 biplane X-ray system and were performed by one of three interventional radiologists (6 years’, 11 years’, and 13 years’ experience in cTACE). Local anesthesia was used for all of the cTACE procedures. A 5F introducer sheath was installed in the right common femoral artery. The celiac trunk and/or SMA were catheterized using a 5F Yashiro or Simmon angiographic catheter. Digital subtraction angiography and/or 3-D CBCT angiography were used to identify each tumor and arterial feeders. Selective catheterization of tumoral feeders was carried out under fluoroscopic guidance using 1.7-2.1F microcatheters. Chemoembolization was performed by delivering the lipiodol emulsion, which was made by mixing Mitomycin-C (Atlanta Medicare, 10 mg/vial, up to 20 mg) with lipiodol (Guerbet Australia Pty Ltd, 10 mL/vial, up to 10-15 mL) via the feeding arteries as selectively as possible. Some patients received additional 5-FU (Boryung Pharmaceutical, 500 mg/10 mL, up to 10 mL) following lipiodol emulsion. The amounts of chemotherapeutic drugs delivered were modified based on liver and renal function, tumor size and number. After that, embolization was carried out using a gelatin sponge slurry until flow stasis was accomplished. The second cTACE procedure was planned for the next 4-8 weeks in case where there were supposed to be residual tumors. Triphasic CT or MR with additional noncontrast CT would be scheduled at 4–8 weeks in cases where complete treatment is obtained after cTACE.
CT protocol
CT examinations were performed with a multidetector CT (128-slice model; Siemens or 256-slice model; Philips). A multiphase acquisition was obtained as follows: after an unenhanced abdominal scan, a non-ionic iodinated contrast agent containing 300 mgI/ml was administered intravenously through an 18-20-gauge cannula with a volume of 2 ml/kg and a rate of 2.5 ml/sec. Late arterial, portovenous, and delayed phase acquisition were obtained at 10 seconds after bolus tracking, 60 seconds after bolus tracking, and 3 minutes after contrast injection, respectively. A slice thickness of 5 mm was obtained for each acquisition. Image reconstruction in the axial, coronal, and sagittal planes was done.
MRI protocol
MRI examinations were performed with a 1.5T scanner (Magnetom Aera; Siemens) or a 3T scanner (Magnetom Skyra; Siemens). Precontrast images including coronal T2-ssTSE, axial T1 in phase and opposed phase, axial T2W and T2W FS, axial T2-long TE, axial T2-Trufi, and DWI/ADC (b value: 0/50/500/1000) were performed. Post-contrast dynamic T1W/FS on arterial, portovenous, interstitial, and hepatobiliary phases (20 minutes after gadoxetate injection or 60 minutes after gadobenate injection) was done. Subtracted images were used as needed.
Patient demographic data and laboratory results were collected from the electronic medical record. The liver function test and coagulogram at 1-2 days before the cTACE procedure and the serum alpha-fetoprotein (AFP) at the time of HCC diagnosis were used.
One board-certified interventional radiologist and one senior abdominal radiologist retrospectively reviewed the images in consensus on the Picture Archiving and Communication System (PACS) of the hospital. The latest preprocedural abdominal CT or MR, the angiographic imaging of the cTACE procedure, the baseline CT or MR with additional plain CT, the follow-up CT, and the final imaging were all analyzed.
The data collected from the latest preprocedural abdominal CT or MR were tumor morphology (uninodular or multifocal), number of tumors, tumor distribution (unilobar or bilobar), tumor thrombus in the portal vein or hepatic vein, and presence of ascites and varices. The data of the selected target tumor were also documented as follows: tumor size, tumor location (right lobe, left lobe, caudate lobe), tumor region (subcapsular or non-subcapsular), and the presence of a tumor capsule. The maximum tumor diameter measured in the axial plane was used to define the tumor size. The tumor capsule was defined as the presence of the thin enhancing rim in the portovenous or delayed phase.
The angiographic imaging of the cTACE procedures for the selected target tumor was analyzed. The collected information included selectivity of the microcatheter (segmental, subsegmental, subsubsegmental),14 degree of portal vein reflux (grade 0, grade 1, grade 2),14 number of cTACE procedures for the selected target tumor, and the usage of CBCT guidance.
The baseline CT or MR with additional plain CT was used for confirmation of the complete response of the target tumor. The lipiodol staining pattern (homogeneous, inhomogeneous) was assessed on non-contrast baseline CT images in the axial plane under a window level of 50 and a window width of 350 (WL/WW = 50/350). Homogeneous staining was defined as a completely dense lipiodol accumulation. If there was any area of lipiodol defect, it would be considered inhomogeneous staining.
At both baseline CT and follow-up CT, the maximum, minimum, and mean radiodensities of the lipiodol staining on the target tumor were measured using the Hounsfield units (HUs) on the non-contrast CT images. At the axial slice, where the tumor had a maximal diameter, they were measured by manually drawing a region of interest (ROI) along the edge of the tumor. The size and area of the tumor were also documented. If there were more than one follow-up CT, the latest CT before the final imaging would be chosen for the analysis. The lipiodol washout rate was calculated by dividing the mean HU difference between the baseline CT and the follow-up CT by the time interval between the baseline CT and the follow-up CT (HU/month). If, as was occasionally seen with the contracting tumor after treatment, the mean HU of the follow-up CT was higher than that of the baseline CT, the washout was recorded as 1.0 HU. The rates of decreased tumor diameter (cm/month) and area (mm2/month) were also calculated in the same way as calculating the lipiodol washout rate.
The final imaging was reviewed for evaluation of local tumor recurrence, which was defined as a new arterial lesion with portovenous and/or delayed washout occurring in contact with or inside the treated tumor. The interval between the cTACE and the final imaging was designated as the follow-up time period.
All statistical analyses were performed with the R program (version 4.2.1, R Foundation for Statistical Computing, Vienna, Austria). The numerical data were described as mean ± standard deviation (SD), and the categorical data were reported as frequency (percentage).
The Shapiro-Wilk normality test for normally distributed continuous variables and the Wilcoxon Rank-Sum test for non-normally distributed continuous variables were used before using the Student’s t-test. Univariate analysis with a Chi-square or Fisher exact test for categorical variables and a Student’s t-test for continuous variables was performed to evaluate whether study variables were significantly different between groups regarding the recurrence of the tumor. To identify independent predictors of recurrent tumors, the factors from the univariate analysis with a P-value < 0.05 were included in the multivariable analysis. A systematic screening process was employed to select variables with a P-value of less than 0.05.
Receiver operating characteristics (ROC) curve analysis was used to identify the suitable cutoff point for the statistically significant factors, predicting tumor recurrence.
From January 1, 2013 to November 30, 2023, there were 39 patients with 63 target tumors who achieved the criteria and were included in this research. There were 32 (82.1%) men and 7 (17.9%) women with a mean age of 62±9 years (range 47-85). All had cirrhosis, and 24 (61.5%) patients had evidence of varices. Hepatitis B was the most frequent cause of cirrhosis (16/39, 41.0%). Approximately half of the patients (21/39, 53.8%) had BCLC stage B. Patient characteristics are summarized in Table 1.
In total, 63 target tumors (mean per patient 1.6, range 1 to 6; mean diameter 3.0±1.4 cm; range 1.1-6.0) were analyzed. The tumors were in the right hepatic lobe (38/63, 60.3%), left hepatic lobe (17/63, 27.0%), and caudate lobe (8/63, 12.7%). Forty-five (71.4%) tumors are located in the subcapsular region. Most had tumor capsules (46/63, 73.0%).
Most of the tumors were treated with only one session of cTACE (53/63, 84.1%). Segmental, subsegmental, and subsubsegmental selectivity were performed in 19/63 (30.2%), 26/63 (41.3%), and 18/63 (28.5%), respectively. Grade 0, 1, and 2 portal vein refluxes were observed in 41/63 (65.1%), 16/63 (25.4%), and 6/63 (9.5%), respectively. CBCT guidance was used only in 13/63 (20.6%).
The lipiodol staining pattern was considered to be homogeneous in 42/63 (66.7%) and inhomogeneous in 21/63 (33.3%). The median values of the mean HU, maximum HU, and minimum HU of lipiodol staining were 645 HU, 1894 HU, and 95 HU, respectively. The median lipiodol washout rate was 7.7 HU/month. The median decreased diameter and decreased area were 0 cm/month and 10 mm2/month, respectively.
After a mean follow-up of 25.9 months (range 6.0–104.0), tumor recurrence was detected in 18/63 (28.6%) tumors at a mean of 27.8 months (range 6.0–54.0). The mean follow-up time of the nonrecurrent group was 25.2 months (range 6.0–104.0).
Tumoral, cTACE procedural, and lipiodol staining variables were studied for potential risk factors for tumor recurrence. On univariate analysis, the incidence of tumor recurrence significantly increased with increased tumor size (p = 0.007), inhomogeneous lipiodol staining pattern (p<0.001), low minimum lipiodol radiodensity (p = 0.012), and high lipiodol washout rate (p = 0.046) (Table 2). On multivariate analysis, significant independent risk factors for tumor recurrence were lipiodol staining pattern (odd ratio, 13.78; 95%CI, 3.36–56.48; p<0.001) and lipiodol washout rate (odd ratio, 6.39; 95%CI, 1.3–31.54; p=0.012) (Table 3).
| Variables | Total (n = 63) | Recurrence (n = 18) | No recurrence (n = 45) | P-value |
|---|---|---|---|---|
| Target tumor | ||||
| Tumor size (cm) | 2.6 [1.7-3.7] | 3.5 [2.5-4] | 2.1 [1.6-3.2] | 0.007* |
| Tumor location | 0.201 | |||
| Right lobe | 38 (60.3) | 13 (72.2) | 25 (55.6) | |
| Left lobe | 17 (27.0) | 2 (11.1) | 15 (33.3) | |
| Caudate lobe | 8 (12.7) | 3 (16.7) | 5 (11.1) | |
| Tumor capsule | 1 | |||
| Yes | 46 (73.0) | 13 (72.2) | 33 (73.3) | |
| No | 17 (27.0) | 5 (27.8) | 12 (26.7) | |
| Tumor region | 1 | |||
| Subcapsular | 45 (71.4) | 13 (72.2) | 32 (71.1) | |
| Non-subcapsular | 18 (28.6) | 5 (27.8) | 13 (28.9) | |
| cTACE procedure | ||||
| Selectivity | 0.648 | |||
| Segmental | 19 (30.2) | 5 (27.8) | 14 (31.1) | |
| Subsegmental | 26 (41.3) | 9 (50) | 17 (37.8) | |
| Subsubsegmental | 18 (28.5) | 4 (22.2) | 14 (31.1) | |
| Portal vein reflux | 0.515 | |||
| Grade 0 | 41 (65.1) | 14 (77.8) | 27 (60) | |
| Grade 1 | 16 (25.4) | 3 (16.7) | 13 (28.9) | |
| Grade 2 | 6 (9.5) | 1 (5.5) | 5 (11.1) | |
| Total cTACE | 0.452 | |||
| 1 time | 53 (84.1) | 14 (77.8) | 39 (86.7) | |
| >1 times | 10 (15.9) | 4 (22.2) | 6 (13.3) | |
| CBCT guidance | 0.741 | |||
| Yes | 13 (20.6) | 3 (16.7) | 10 (22.2) | |
| No | 50 (79.4) | 15 (83.3) | 35 (77.8) | |
| Lipiodol staining data | ||||
| Lipiodol staining pattern | <0.001* | |||
| Homogeneous | 42 (66.7) | 5 (27.8) | 37 (82.2) | |
| Inhomogeneous | 21 (33.3) | 13 (72.2) | 8 (17.8) | |
| Mean lipiodol radiodensity (HU) | 645 [466.5-842.5] | 515 [371.8-784] | 665 [481-877] | 0.169 |
| Maximum lipiodol radiodensity (HU) | 1894 [1190.5-2919] | 1771 [951.8-2718.8] | 2002 [1209-3042] | 0.431 |
| Minimum lipiodol radiodensity (HU) | 95 [57.5-171] | 70.5 [45.8-92.5] | 114 [70-185] | 0.012* |
| Lipiodol washout rate (HU/month) | 7.7 [2.3-15.4] | 9.7 [7.2-20.9] | 6.2 [0.2-13.8] | 0.046* |
| Decreased diameter (cm/month) | 0 [0-0.1] | 0 [0-0.1] | 0 [0-0.1] | 0.623 |
| Decreased area (mm2/month) | 10 [4.9-22.2] | 20.3 [6.1-28.5] | 9.2 [4.7-18.8] | 0.13 |
| Predicting factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Tumor size (cm) | 1.94 (1.19-3.14) | 0.007* | 1.5 (0.76-2.93) | 0.229 |
| Lipiodol staining pattern (homogeneous, inhomogeneous) | 12.02 (3.33-43.41) | < 0.001* | 13.78 (3.36-56.48) | < 0.001* |
| Lipiodol washout rate (HU/month) | 5.23 (1.33-20.58) | 0.046* | 6.39 (1.3-31.54) | 0.012* |
| Minimum lipiodol radiodensity (HU) | 3.63 (1.14-11.5) | 0.012* | 1.26 (0.29-5.53) | 0.761 |
The area under the ROC curve (AUC) for minimum lipiodol radiodensity on ROC analysis for tumor recurrence prediction was 0.704, indicating a 70.4% probability of accurately differentiating between recurrence and nonrecurrence. The estimated threshold value of minimum lipiodol radiodensity for predicting tumor recurrence was 94 HU. Below, there was a risk for tumor recurrence with a sensitivity of 78% and a specificity of 64% (Figure 1).
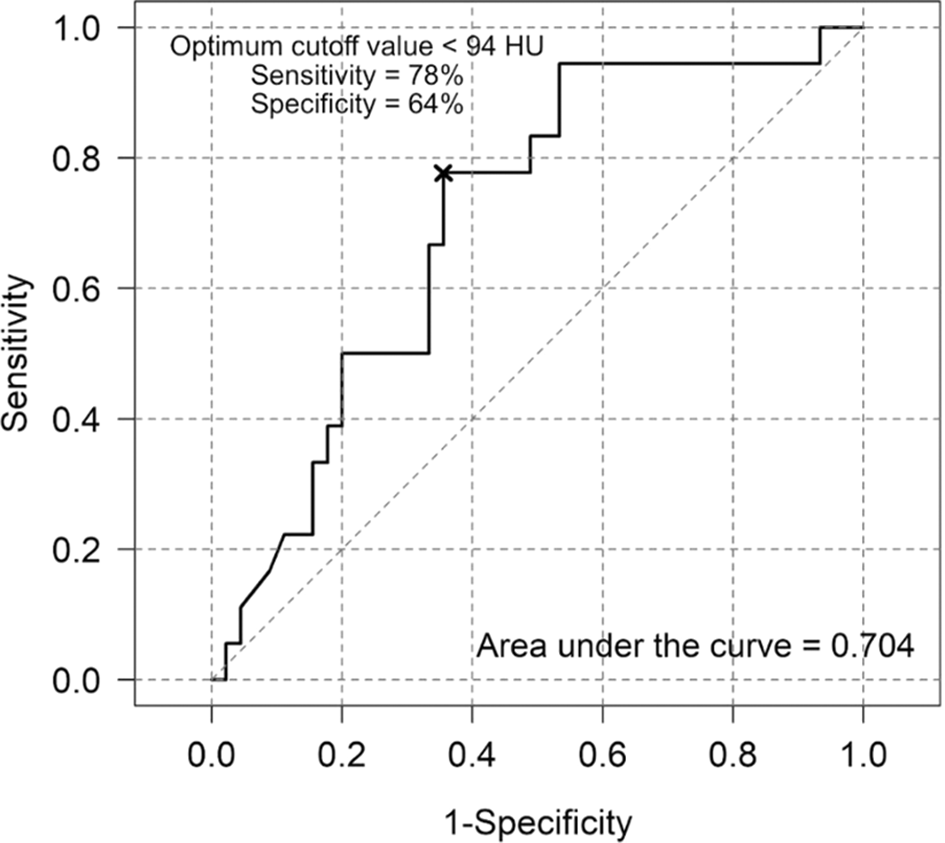
The AUC for lipiodol washout rate in tumor recurrence prediction was 0.662. The lipiodol washout rate's ROC curve analysis indicates an estimated threshold value of 6.44 HU/month, above which was the risk for tumor recurrence, with a sensitivity of 83% and a specificity of 51% (Figure 2). The example cases were shown in Figures 3 and 4.

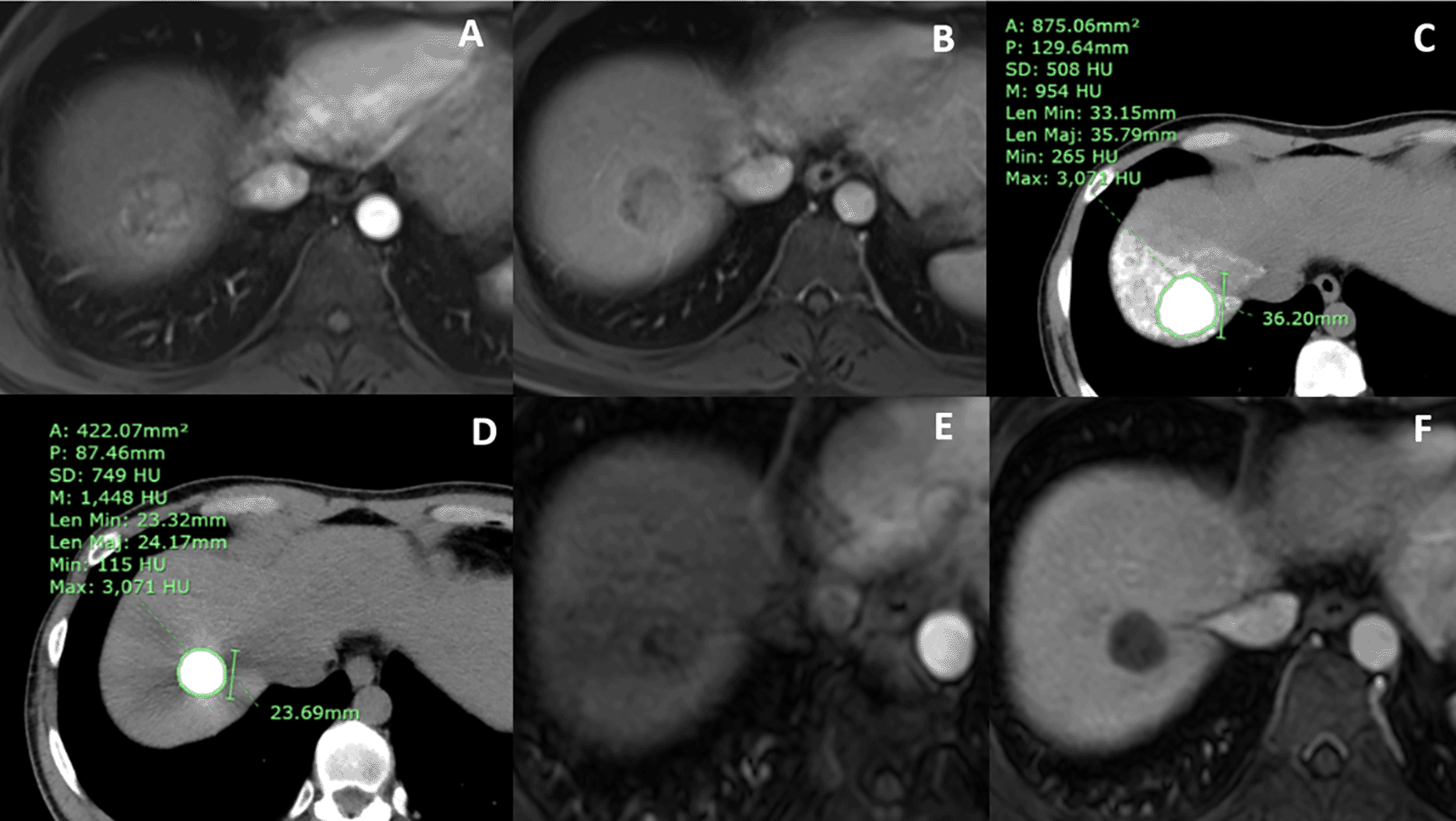
(A) arterial and (B) portovenous phases MR showed an arterial-enhancing nodule with portovenous washout in hepatic segment 7, which was typical for HCC. (C) Baseline CT at 1 week after cTACE showed no residual tumor and homogeneous lipiodol staining with a mean lipiodol radiodensity of 954 HU and a minimum lipiodol radiodensity of 265 HU. (D) Follow-up CT at 7 months after baseline CT showed a mean lipiodol radiodensity of 1,448 HU. The lipiodol washout of the tumor was recorded as 1.0 HU. The calculated lipiodol washout rate of the tumor was 0.14 HU/month. (E) arterial and (F) portovenous phase final MR was done 24 months after cTACE, showing no local recurrence.
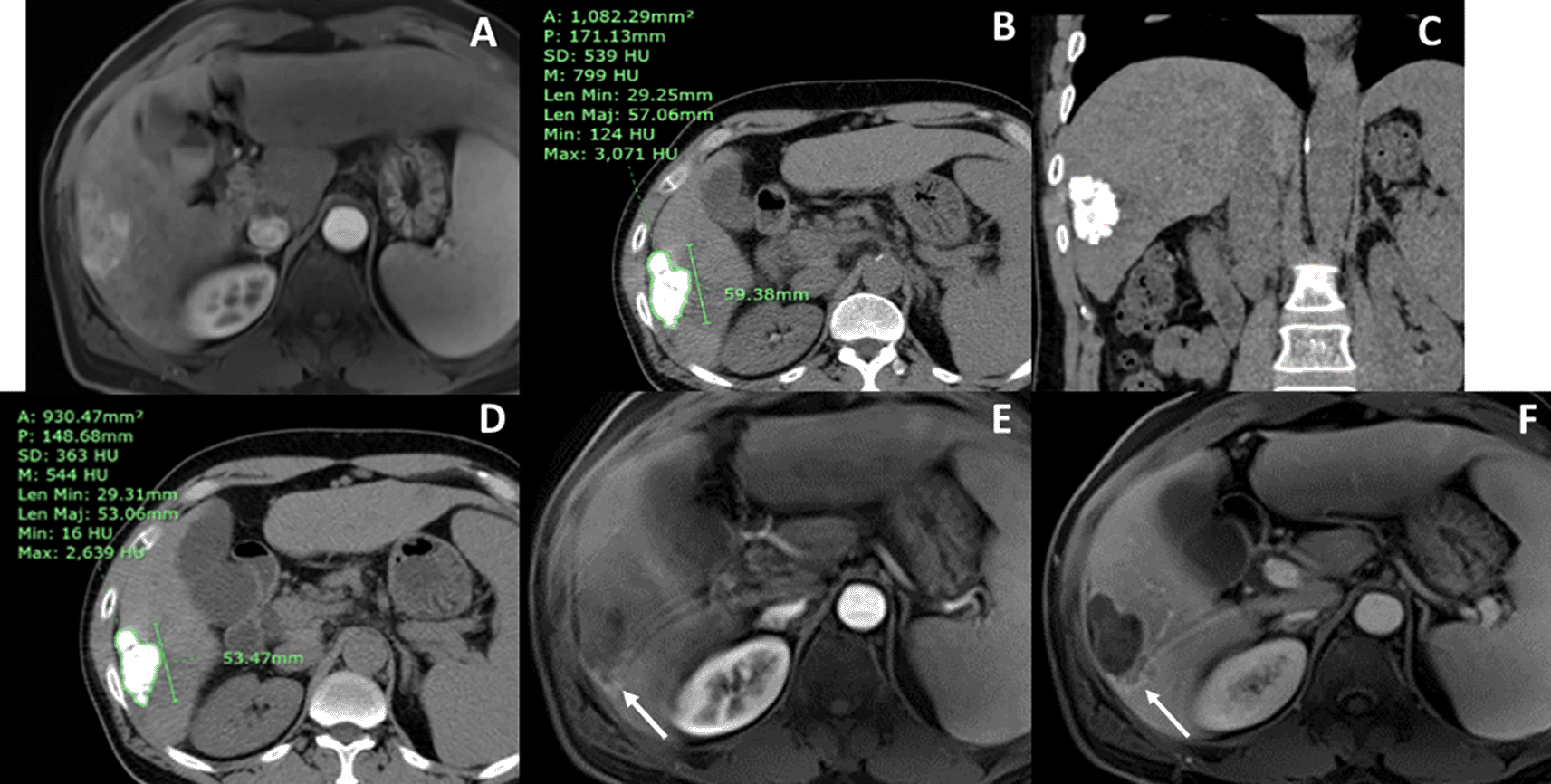
(A) arterial phase MR showed an arterial-enhancing nodule in hepatic segment 6, which had portovenous washout (not shown). (B) axial and (C) coronal views of baseline CT at 4 weeks after cTACE showed no residual tumor and inhomogeneous lipiodol staining with a mean lipiodol radiodensity of 799 HU and a minimum lipiodol radiodensity of 124 HU. (D) Follow-up CT at 4 months after baseline CT showed a mean lipiodol radiodensity of 544 HU. The calculated lipiodol washout rate of the tumor was 63.75 HU/month. (E) arterial and (F) portovenous phase final MR were done 6 months after cTACE, showing local recurrence at the posterior aspect of the tumor (arrow).
In this study, we found eighteen (28.6%) of 63 tumors had local tumor recurrence after achieving complete response from cTACE at a mean time to recurrence of 27.8 months (range 6.0–54.0 months). The recurrent rate was rather low as compared to the previous studies, in which the reported rates of tumor recurrence were 41.3-54.3% at a median time to recurrence of 3.6–12 months (range 3.0–31.0 months).4,8,15
We found that the inhomogeneous lipiodol staining pattern was significantly correlated with tumor recurrence. Moreover, among the recurrent group, tumors with homogeneous lipiodol staining had a longer time to recurrence than tumors with inhomogeneous lipiodol staining (mean 33.0±23.5 vs. 22.6±11.8 months, respectively). This result is analogous to the previous studies. Dioguardi Burgio et al.8 similarly reported that HCCs with incomplete lipiodol accumulation after cTACE had an increased risk of local progression even when there was an initial complete response. However, they showed no significance of time to local progression between the complete and incomplete lipiodol staining groups (mean 13.4±3 vs. 11.1±2 months, respectively). Also, one study by Kinugasa et al.7 showed that heterogeneous lipiodol uptake was one of the risk factors for local recurrence in patients with early-stage HCC treated with TACE. These could be explained by the reports of the previous studies that discovered inhomogeneous lipiodol uptake correlated with incomplete pathologic tumor necrosis.5,9,12 We speculated that inhomogeneous lipiodol staining may occur due to incomplete catheterization, suboptimal delivery of lipiodol emulsion, or the incapacity of lipiodol particles to enter the tiniest tumoral arteries. Interestingly, we found that among 21 tumors with inhomogeneous lipiodol staining, 8 (38.1%) tumors showed no recurrence. We presumed that embolization of more than one vascular territory may result in watershed regions and cause inhomogeneous lipiodol staining. Some tumoral features might also cause nonuniform lipiodol retention, such as fatty metamorphosis, hemorrhagic necrosis, or fibrotic portions. Park et al.5 reported a case of HCC with fatty metamorphosis that had pathologic complete tumor necrosis even though there was inhomogeneous lipiodol accumulation after cTACE. Conversely, in our study, among 42 tumors with homogeneous lipiodol staining, 5 (27.8%) tumors had tumor recurrence. This finding was consistent with the previous studies that showed a complete response on CT according to mRECIST, which was overestimated by 34.3–37.6%.5,16 This overestimation is frequently explained by intratumoral lipiodol deposition's notable attenuation feature, which makes it difficult to determine the viable enhancing part.
Regarding HU values of lipiodol retention, we found that only lipiodol washout rate was the significant risk factor for tumor recurrence on both univariate and multivariate analysis (OR, 6.39; 95%CI, 1.3–31.54; p = 0.012). Likewise, Matsui et al.4 reported that tumor recurrence might be predicted independently by baseline lipiodol uptake and washout rate. They stated that the washout rate of more than 37.8 HU/month (sensitivity 78% and specificity 74%) had a high potential for early tumor recurrence. In our study, we discovered a threshold washout rate of 6.44 HU/month (sensitivity 83% and specificity 51%), which is much less than that found in the aforementioned study. This could be because of a different study design. In our study, we selected the latest CT study of the patient as the follow-up CT for calculation, in contrast to Matsui Y's, in which the value was calculated according to the first CT study that showed lipiodol washout. The longer follow-up time could result in a higher divisor, resulting in a lower value for our outcome.
We found that mean lipiodol radiodensity was not a predictor for tumor recurrence. This result was different from the previous two studies that showed the clinical significance of the mean HU. One study by Matsui et al.4 showed that mean HU was a significant predictor for tumor recurrence after a complete response on imaging. They analyzed the cutoff value of mean HU at baseline CT within 1 week after cTACE of 270.2 HU (sensitivity 95%, specificity 93%). The other study by Park et al.7 showed that pathologic complete necrosis was significantly predicted by the mean HU of lipiodol retention. A cut-off value of 460 HU (mean 54.5 days after cTACE) was correlated with complete necrosis (sensitivity 67.4%, specificity 75.0%). We supposed that our study did not show statistical significance due to the high variation of the time interval between cTACE and baseline CT, which ranged from 1 to 8 weeks (mean 4.4±1.9 weeks). Nevertheless, we found that the minimum HU of lipiodol staining in the recurrent group was noticeably greater than the no recurrence tumor group on univariate analysis (p = 0.012), with a threshold value for predicting tumor recurrence of 94 HU (sensitivity 78%, specificity 64%). There were a few limitations in our study. Firstly, due to its small population and retrospective observational design, this study may have been biased in an unexpected way. The cTACE protocol and the imaging surveillance guidelines could not be arranged. The techniques of drug delivery, types and volumes of chemotherapeutic agents, and volume of lipiodol were based on the operators’ considerations, which could have an impact on the treatment outcome. Secondly, hand drawing only one selected slice on the axial plane could not represent the overall tumor. However, we thought that this method was practical and comfortable for real-life practice. Thirdly, since our goal was to learn more about the local response of tumors treated with cTACE rather than long-term outcomes, we were not focusing on intrahepatic metastasis or overall survival in this study. We think that additional prospective research using a 3D volumetric measurement and a standard follow-up approach could yield more precise and reliable results.
Inhomogeneous lipiodol staining pattern and lipiodol washout rate of >6.44 HU/month were predictors for recurrence of HCC after a complete response after TACE. These correlations may provide useful guidance for subsequent imaging surveillance and treatment approaches.
The Human Research Ethics Committee of Thammasat University (Medicine) approved the study with the certificate project number MTU-EC-RA-0-162/65 and approved on August 30, 2022. The informed consent was waived because the research involved no more than minimal risk to subjects.
Zenodo: Quantitative assessment of the lipiodol staining after transarterial chemoembolization for hepatocellular carcinoma: can it predict tumor recurrence?
Doi: https://doi.org/10.5281/zenodo.10592696
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0) (https://creativecommons.org/licenses/by/4.0/).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)