Keywords
Recurrent laryngeal nerve, external laryngeal nerve, internal laryngeal nerve, thyroid surgery, neck surgery
This article is included in the Manipal Academy of Higher Education gateway.
Because of its close relationship with vessels that supply the thyroid gland, it is necessary to understand and document the course of the laryngeal nerves to prevent accidental damage. Otherwise it can lead to weakness in vocal cord function if ligated along with the arteries during thyroidectomy or other surgical procedures. The aim of our study was to examine and document the origin, relationship, and branching patterns of the laryngeal nerves on the side of the neck.
The present cross sectional anatomical study was conducted on 64 dissected necks of formalin-fixed cadavers that were utilized for undergraduate teaching purposes. Different parameters were measured in the neck along with the relationship between the laryngeal nerves and their accompanying arteries.
We observed usual and unusual course of laryngeal nerves along with some variations. Some of the novel findings were an internal laryngeal nerve piercing the thyroid cartilage lamina and an external laryngeal nerve communicating with the superior cervical ganglion.
The present study observed anatomical variations in the course of the laryngeal nerves, which are relevant for clinical practice. The potential risks of damage to these laryngeal nerves during various surgical procedures make it necessary to review and compile its relationship with the surrounding structures.
Recurrent laryngeal nerve, external laryngeal nerve, internal laryngeal nerve, thyroid surgery, neck surgery
Innervation of the larynx plays an important role in the respiration, vocalization, coughing, deglutition, and vomiting reflexes. It is by superior (SLN) and recurrent laryngeal (RLN) branches of Vagus nerve (VN) (Standring, 2014).1 The SLN is further divided into the external and internal laryngeal nerves (ELN and ILN, respectively) before supplying the larynx. The ILN is essentially sensory and supplies the mucosa of the supraglottic part of the larynx. Along its course, it pierces the thyrohyoid membrane (TM) and is accompanied by superior laryngeal artery (SLA). The ELN, which mainly contains the motor component, descends along the superior thyroid artery (STA) to supply the cricothyroid muscle (CTM). RLN containing motor and sensory components supply all muscles of the larynx, except the CTM, and innervate the laryngeal mucosa below the vocal cord. In the neck it lies in the groove between the trachea and the esophagus before entering the larynx (Standring, 2014).1 Owing to their proximity to the vessels supplying the thyroid gland, these laryngeal nerves are vulnerable to injuries, generally during thyroid surgeries. SLN injury is subtle, usually unnoticed, and challenging to detect (Teitelbaum et al., 1995).2 Generally, SLN injury is confused with subluxation of the arytenoid, even after thorough evaluation (Schroeder et al., 2003).3 Consequently, laryngeal endoscopy and electromyography are required to diagnose SLN injuries (Teitelbaum et al., 1995).2 The ILN is vulnerable to injury during neck surgeries using an anterior or anterolateral approach (AbuRahma et al., 2000, Melamed et al., 2002).4,5 Iatrogenic injuries to the ELN occur during ligation of the STA for thyroidectomy and cause postoperative complications such as hoarse and weak voice (Sakorafas et al., 2012; Cernea et al., 1992).6,7 The variable relationship between the RLN and inferior thyroid artery (ITA) near the base of the thyroid gland makes it vulnerable to accidental injuries during thyroidectomy. The risk of permanent RLN palsy is 0.3% -3% and transient palsy is 3%-8% (Hayward et al., 2013).8 RLN injury may lead to laryngeal muscle paralysis, with symptoms ranging from hoarseness of voice in unilateral lesions to stridor and laryngeal obstruction in bilateral damage (Erbil et al., 2007; Jeannon et al., 2009; Kahky et al., 1993; Tang et al., 2012).9–12 Even if intraoperative neuromonitoring using the RLN technique is used to prevent RLN injury, evidence indicates that it is as effective as visualization of the RLN during dissection and is not greater than it (Hayward NJ et al., 2013; Calo et al., 2013; Phelan et al., 2013).8,13,14 The potential risks of damage to these laryngeal nerves during various surgical procedures necessitate precise documentation of its topographic relations.
The literature review did not reveal enough studies with respect to the laryngeal nerves in cadaveric samples, particularly from our sample population. This was a rationale to perform this research and the aim of our study was to examine and document the origin, relationship, and branching patterns of the laryngeal nerves on the side of the neck. The specific objective was to document the anatomical variations in the course of laryngeal nerves. It is hypothesized that the Vagus nerve and branches are vulnerable injury because of its wandering course and complex branching pattern.
This is an observational cross-sectional, institution based study conducted on 64 dissected neck of formalin fixed cadavers, which were utilized for undergraduate teaching purpose, in the department of Anatomy of our institution from 2019 to 2023. The convenient sampling method was considered based on the availability of specimens at our department in this study period. The source of cadavers were from donated bodies, the body donor had given the written consent before donating the body. The study protocol is approved by Institutional Ethics Committee of Kasturba Medical College, Mangalore (ECR/541/Inst/KA/2014/RR-17) on 21st August 2019 (IEC KMC MLR 08-19/355).
Only adult cadavers were included for the study and if there were any pathological changes, the same were excluded. Since there were very few female cadavers in our department, the gender based comparison was not performed.
All measurements were in millimeters, measured with the help of a scale, thread, and caliper. The measurements were performed by the same person to prevent inter-observer bias. The measurements were also performed thrice, and the average was taken to prevent intra-observer bias. The following parameters were observed bilaterally in these specimens:
1. Length of ILN from its origin till it pierces the TM
2. Vertical distance between ILN and the upper border of thyroid lamina
3. Relation between ILN and SLA
4. Branches of ILN before piercing the TM
5. Length of ELN from its origin to its termination on CTM
6. Relation of ELN with inferior constrictor muscle (ICM)
7. Vertical distance between ELN and apex of thyroid gland (AT)
8. Branches of ELN to AT
9. Relations of RLN with trachea and oesophagus
10. Relations of RLN with ITA
11. Distance between the base of thyroid lobe and crossing of RLN and ITA
12. Number of branches of RLN before it enters the larynx
13. Distance between entry of RLN into larynx and CTJ
The length of the ILN and ELN were measured using a measuring scale and thread. The other distances in the study were measured using a digital Vernier caliper. Since this is a descriptive study, the variations in the course and relation of the ELN and RLN are expressed as percentages.
The protocol of this study is available online at https://www.protocols.io/file/piy5cbrd7.docx.
Measurement of the length of the ILN, distance between the ILN from the upper border of the thyroid lamina, Length of ELN, distance between the ELN and AT, distance between the base of the thyroid lobe and crossing of the RLN and ITA, and the distance between the entry of the RLN into the larynx and the CTJ are compiled in Table 1.
Relation of ILN & superior laryngeal artery (SLA): In the majority of cases, ILN was found posterior to the SLA, followed by cranial and caudal relations, as depicted in Table 2 (Fig. 1a,b, and c). Branches of ILN: in the majority of the specimens, ILN was single and did not give any branch before piercing the TM, as shown in Table 3 (Fig. 2a, b, c & d).
| Position of ILN with respect to SLA | Number of specimens | % of specimen | Figure | |||
|---|---|---|---|---|---|---|
| Right | Left | Bilateral | Total | |||
| Posterior | 18 | 15 | - | 33 | 51.56 | 1a |
| Cranial | 13 | 16 | - | 29 | 45.31 | 1b |
| Caudal | 1 | 2 | 3.1 | 1c | ||
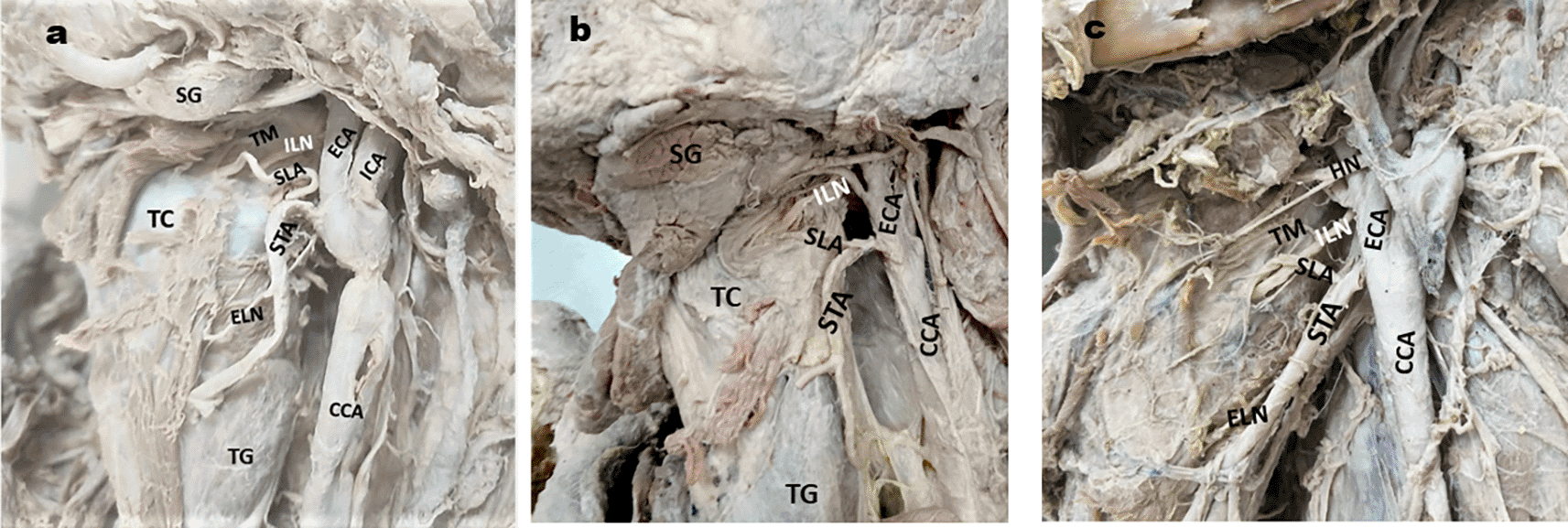
1a. ILN is posterior to the SLA: 1b. ILN is cranial to the SLA: 1c. SLA is caudal to the ILN.
ELN- external laryngeal nerve; CCA- common carotid artery; ICA- Internal carotid artery; ECA- external carotid artery. STA- superior thyroid artery; SG- Submandibular gland; TG- Thyroid gland; TM- Thyrohyoid membrane; TC- Thyroid cartilage, HN-hypoglossal nerve.
| Number of branches | Number of specimens | % of specimen | Figure | ||
|---|---|---|---|---|---|
| Right | Left | Total | |||
| Single without branches | 26 | 28 | 54 | 84.37 | 2a |
| Two | 5 | 2 | 7 | 10.93 | 2b |
| Three | - | 2 | 2 | 3.1 | 2c |
| Multiple | 1 | - | 1 | 1.5 | 2d |
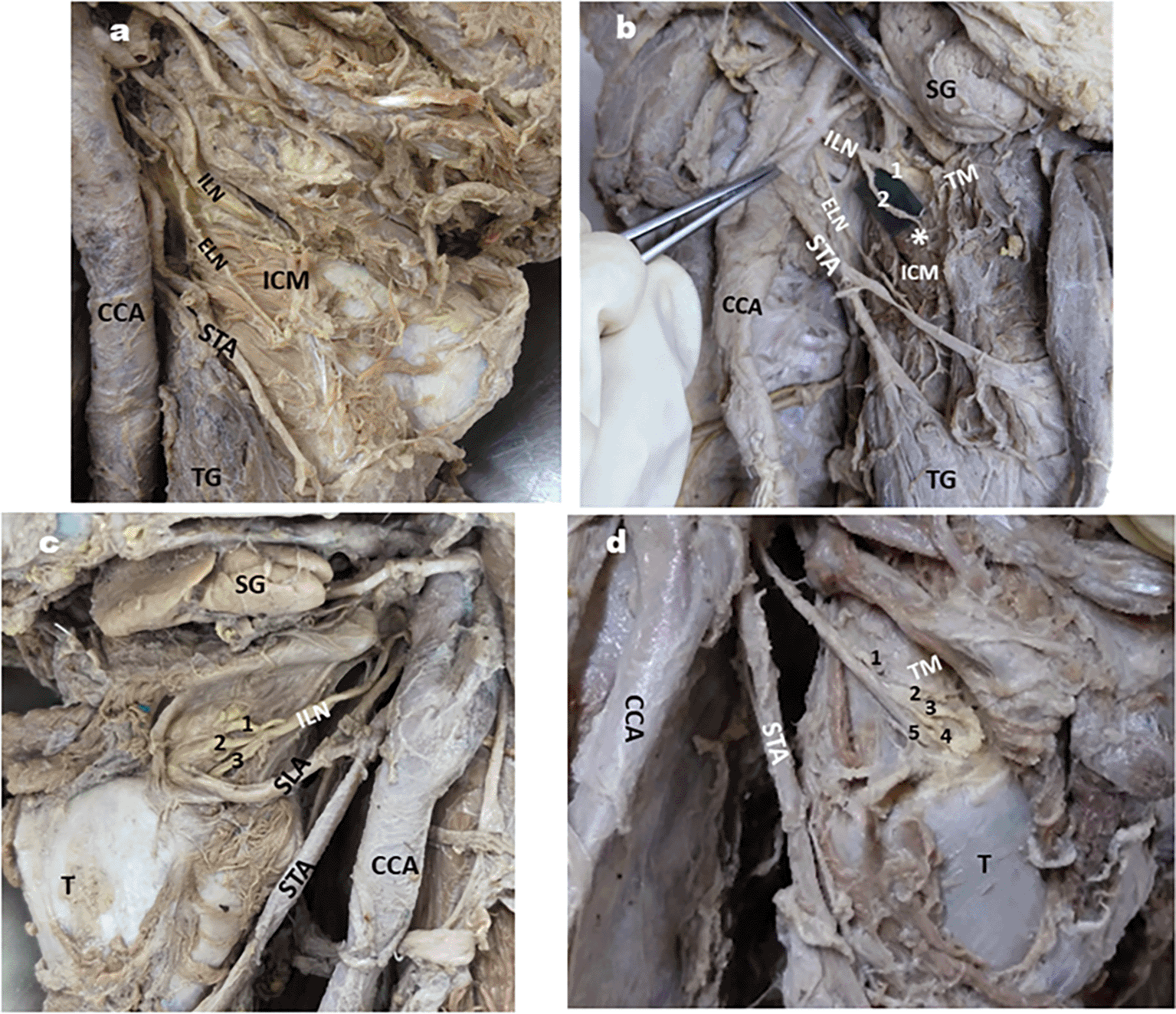
2a: ILN without branches; 2b: ILN giving two branches one of the branch (*) supplying ICM;
2c: ILN giving three branches; 2d: ILN giving multiple branches.
1,2-2 branches of ILN, ELN- External laryngeal nerve; CCA- common carotid artery; ECA- external carotid artery; STA- Superior thyroid artery; SG- Submandibular gland; ICM- Inferior constrictor muscle; TG- Thyroid gland; 1,2,3- 3 branches of ILN; T-thyroid cartilage; TM- Thyrohyoid membrane; 1,2,3,4,5-5 branches of ILN.
On the right side of one cadaver, the ILN pierced the lamina of the thyroid cartilage, 4 mm below its upper border (Fig. 3).
Branches of ELN: In all specimens, it supplied the cricothyroid muscle (CTM). However, in one left-sided specimen, the ELN communicated with a branch of the superior cervical sympathetic ganglion. (Fig. 4)

ILN- internal laryngeal nerve; C- Communication of ELN with S, CCA- Common carotid artery (cut and reflected up); V- Vagus nerve.
Relation between ELN & ICM:
• In 90.6% of specimens, ELN was superficial to ICM,
• In 6.25% it was piercing the ICM near its lower border,
• In 2 specimens it pierced the ICM near its middle part
Distance between ELN & apex of thyroid gland: Fig. 5a & b.
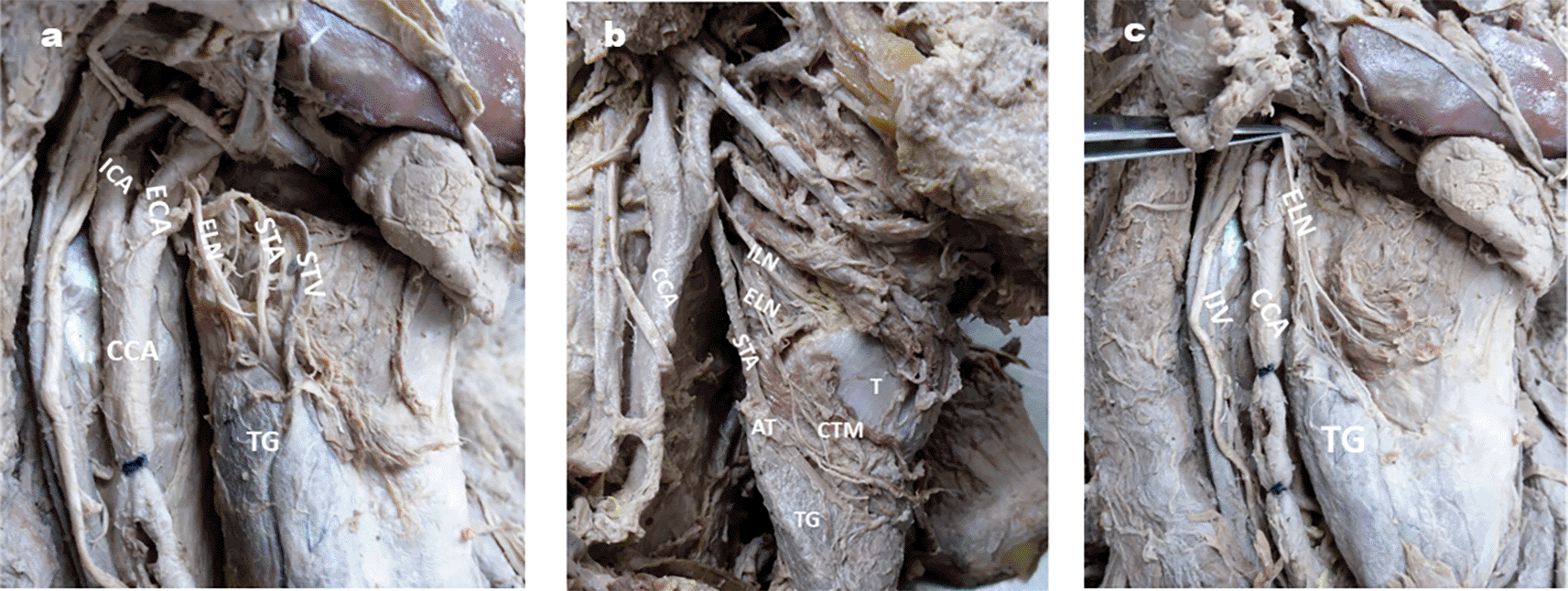
5a: ELN is close to STA at apex of TG on right side of neck (< 1cm);
5b: ELN is very close to STA at apex of TG on right side of neck ( >1cm);
5c: ELN giving branches to apex of thyroid gland lobe on right side of neck.
STA- superior thyroid artery, STV- Superior thyroid vein, TG- Thyroid gland, CCA- Common carotid artery, ECA- External carotid artery, ICA- Internal carotid artery, SG- Submandibular gland, V- vagus nerve, IJV- Internal jugular vein.
In the majority of specimens (90.6%), the distance between the ELN and AT was less than 1 cm (average 5.22 mm). These distances are tabulated in Table 4.
In 68.75% of specimens, the RLN was in the tracheoesophageal groove, whereas in 31.25%, it was lateral to the trachea.
Relation between RLN & inferior thyroid artery (ITA):
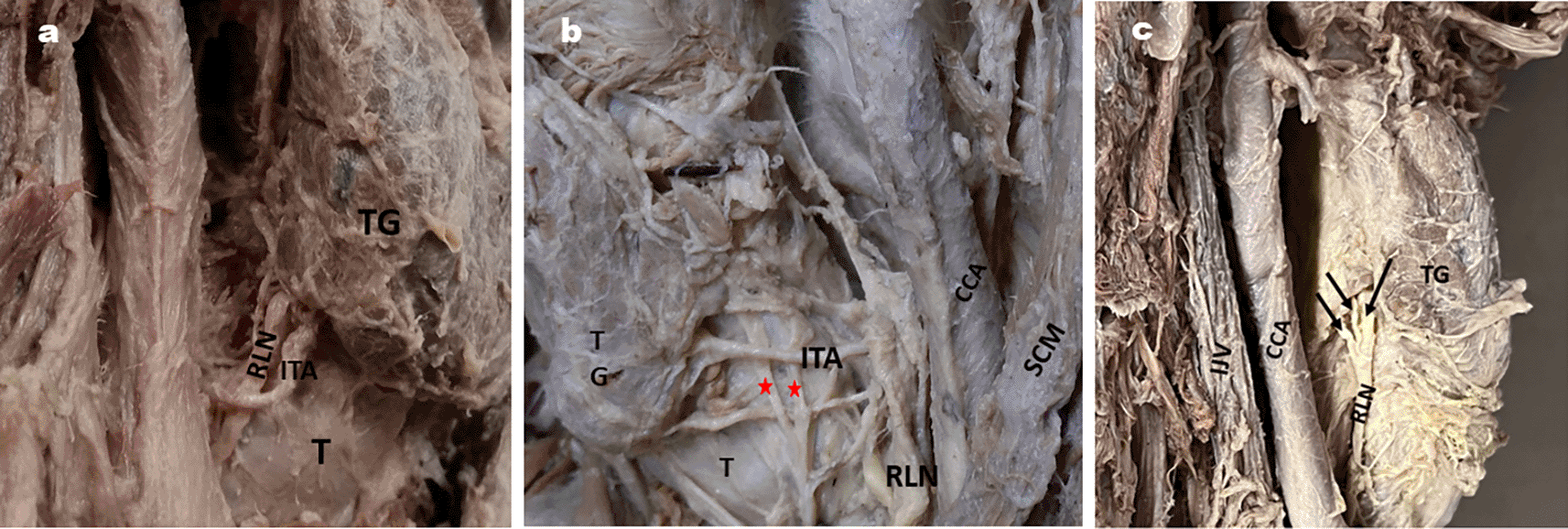
In the majority of specimens, the RLN was posterior to the loop of the ITA, as shown in Table 5 (Fig. 6a, b, c and d).
| RLN position with respect to ITA | Number of specimens | % of specimen | Figure | ||
|---|---|---|---|---|---|
| Right | Left | Total | |||
| Posterior to the loop | 19 | 13 | 33 | 50 | 6a |
| Anterior to the loop | 5 | 16 | 29 | 32.81 | 6b |
| Between the branches of ITA | 6 | 2 | 8 | 12.5 | 6c |
| ITA between the branches of RLN | 2 | 1 | 3 | 4.68 | 6d |
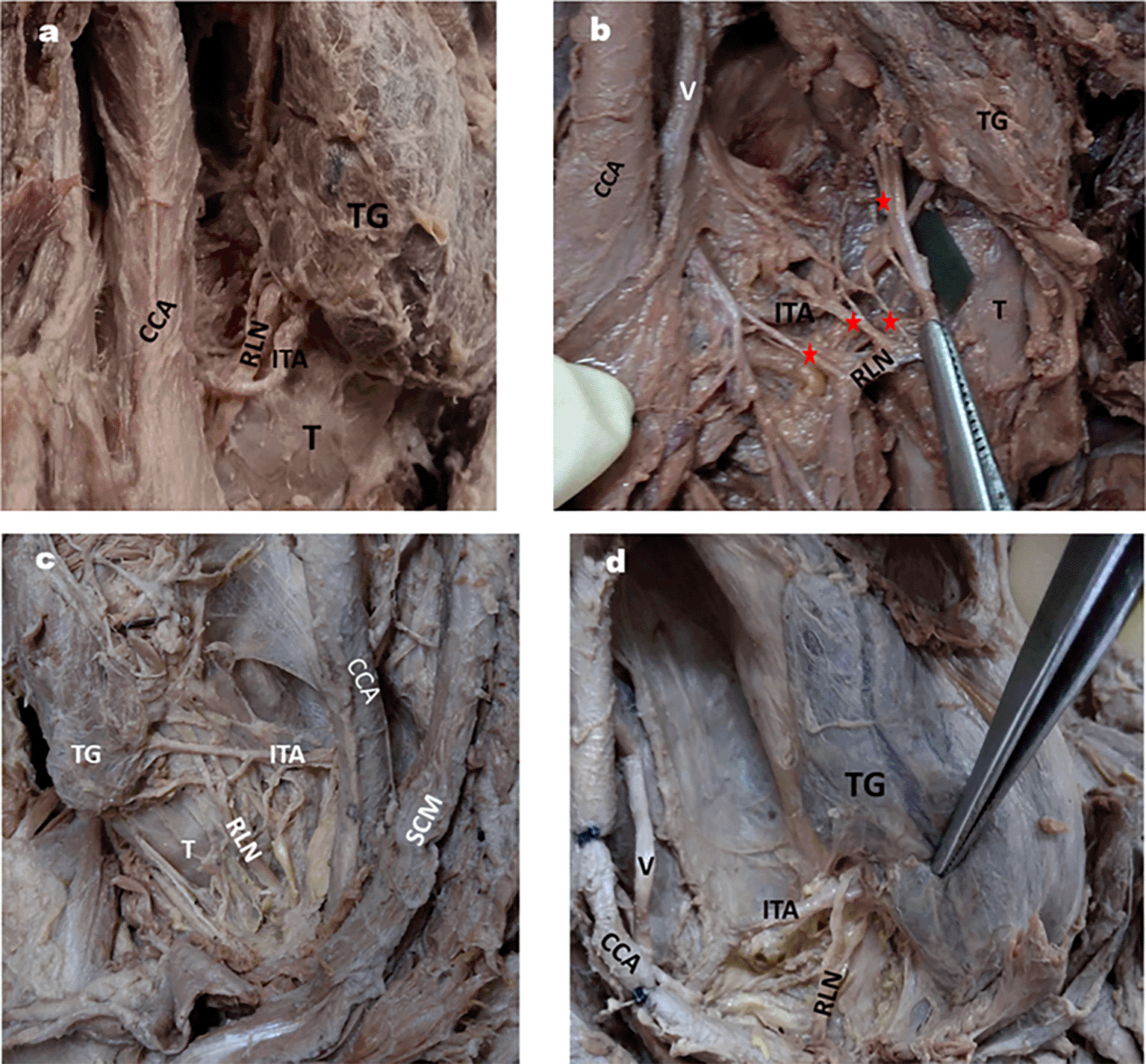
6a: RLN posterior to Inferior thyroid artery.
6b: RLN anterior to inferior thyroid artery and also shows 4 branches of RLN by red stars.
6c: RLN passing in between the branches of inferior thyroid artery on left side of neck.
6d: ITA passing in between the branches of RLN on right side of neck.
TG- thyroid gland; T- Trachea, CCA- Common carotid artery; V-vagus nerve, TG- Thyroid gland, SCM- Sternocleidomastoid.
Branches of RLN: In the majority of specimens, RLN provided three branches, as tabulated in Table 6 (Fig. 7a, b and c).
| Number of branches | Number of specimens | % of specimens | Figure | ||
|---|---|---|---|---|---|
| Right | Left | Total | |||
| Single without branches | 1 | 1 | 2 | 3.12 | 7a |
| Two | 9 | 11 | 20 | 31.25 | 7b |
| Three | 22 | 19 | 41 | 64 | 7c |
| Four | 1 | - | 1 | 1.5 | 6b |
7a: RLN without any branches on right side of neck.
7b: RLN giving 2 branches on the left side of neck indicated by red stars.
7c: RLN giving 3 branches on the right side of neck indicated by black arrows.
ITA- inferior thyroid artery; TG- Thyroid gland; T- Trachea, CCA- Common carotid artery; V-Vagus nerve; SCM- Sternocleidomastoid muscle.
Avoiding nerve or vascular injury is the basic principle behind the success of any surgery. Familiarity with the usual length of the laryngeal nerves, their relationship with neighboring structures, and accompanying arteries will facilitate successful surgeries such as thyroidectomy, carotid endarterectomy, or cervical spine surgery.
The knowledge of anatomical localization of ILN is a prerequisite for surgeries involving sensory denervation of the larynx for odynophagia or neuralgia of superior larynx, supraglottic laryngectomy and laryngeal transplantation (Cernea et al., 1992; Yanagisawa et al., 1970; Schmidt et al., 1981; Strome et al., 2001)7,15–17 The average length of ILN in previous studies was 44.9 mm (Furlan et al., 2003)18 and 57.2 mm (Kiray et al., 2006).19 The mean length of ILN was 33.8 mm in our study (Table 1).
ILN and its relation with Thyrohyoid membrane
The identification of the area of the TM pierced by the ILN is crucial in the dissection of the neck for hypopharyngeal squamous cell carcinoma because any injury to this nerve may prove to be fatal due to loss of cough reflex (Sun et al., 2022).20 We observed a distance of 2 mm to 12 mm (0.2-1.2 cm), between the ILN from the point of penetrating the TM to the upper border of the thyroid lamina, with an average of 6.33 mm (0.63 cm). Paraskevas et al. (2012) noticed that this distance ranged between 0-1.8 cm, but in 87.5% of cases, it ranged from 0.1 to 1.2 cm.21 They described the 0-1.8 cm range as the ‘wide zone’ and 0.1 to 1.2 cm as the ‘danger zone’ between TM penetration of ILN and thyroid cartilage. Familiarity with the ‘danger zone’ and any deviation from the usual pattern can be helpful in preserving ILN during surgical procedures in this region. Guven et al., 2021 have observed this distance ranging from 6-16 mm (0.6-1.6 cm) with the mean of 12 ±2.61 mm (1.2±0.26 cm) in patients undergoing laryngectomy.22
Relation between SLA and ILN
Although a varied relationship between ILN and SLA was observed in our study, in majority of the specimens, the ILN was posterior to the SLA before piercing the TM (51.56%) ((Table 2; Fig. 1a). It was above (cranial) the SLA in 29 specimens (45.31%) (Fig. 1b) and below (caudal) the SLA in two specimens (3.1%) (Fig. 1c). In contrast, Kiray et al. (2006) observed SLA crossing the ILN and piercing the TM above the ILN in 75% and below it in 25% of cases.19 Furlan et al. (2003) reported an ILN medial to the SLA in 89% and distal to the SLA in 11%.18 Paraskevas et al. (2012 have observed SLA parallel caudal and anterior to the ILN in 88.89% and superior and anterior to the ILN in 11.11% of cases.21
Branching pattern of ILN (Table 3)
The branching pattern of the ILN before it pierces the TM has been studied by several authors. Stephen et al. (1999) reported that ILN branched before piercing TM in 16% and after penetration in 84% of cases,23 whereas according to Kiray et al., it was 37.5% and 62.5%, respectively.19 In a study by Guven et al. (2021), ILN was divided into three branches in 58.62% of cases and two branches in 41.37% of cases.22 Paraskevas et al. (2012) reported three branches of ILN before it pierces the TM in 72.22% of cases and two branches in 27.78% of cases.21 In our study, ILN did not give any branch before piercing the TM in 84.37% (Fig. 2a), in 10.93% it was divided into two branches (Fig. 2b), in 3.1% of the three branches (Fig. 2c), and in only one right-side specimen, it was divided into multiple branches (Fig. 2d). Awareness of the branching pattern of the ILN is important during surgical procedures of the larynx, such as laryngectomy. Even if a single branch of the ILN is removed during these procedures, it may cause significant postoperative complications; therefore, re-innervation of these branches is crucial in restoring the reflex function of the larynx. Additionally, we observed a rare variation in ILN piercing the lamina of the thyroid cartilage in one right-sided specimen (Fig. 3). This variant course may result in some unexplained complaints due to nerve compression, leading to misinterpretation by the clinician if he/she is unaware of such a variation.
The principles of thyroidectomy are based on the identification and preservation of the ELN, as injury to the ELN is detrimental to the patient by producing a weak and hoarse voice. The close relationship between the ELN and the upper pole of the thyroid gland makes nerves vulnerable to injury during thyroid surgery. The lower the deviation of the nerve from the STA, the higher is the risk of injury during dissection (Potenza et al., 2017).24
In our study, the mean length of the ELN was 58 mm (Table 1), whereas Furlan et al. (2003) reported a mean length of 62.6 mm,18 Thomassin et al., (1982)25 as 65 mm, and Kambic et al., (1984) as 80 mm.26 The course of the ELN has been studied by several authors (Cernea et al., 1995; Kierner et al., 1998; Cha et al., 2017; Friedman et al., 2002).27–30 Cernea et al. (1995) were the first to describe a classification of ELN with respect to its relationship with the STA.27 Accordingly, they classified 3 types of ELN wherein, type 1 crosses STA more than 1 cm above the apex of the thyroid gland (AT), type 2a crosses STA less than 1 cm above the AT and Type 2b crosses STA under AT cover. They mentioned that type 2b has a higher risk of damage than type 1 because, in this type, ELN is very close to STA. Kierner et al., (1998) revised nomenclature of Cernea et al., (1995) and described four types of ELN, where type 1 (42%), type 2 (30%) and type 3 (14%) are the same as type 1, type 2a and type 2b, respectively of Cernea et al., classification.28 In type 4, the ELN descends posterior to the STA and crosses its branches just above the AT. In our study commonest finding of ELN was type 2 (< 1 cm) of Kierner et al, in 82.81% (Fig 5a) followed by type 1 (> 1 cm) in 9.37% and type 3 in 7.81% (Table 4). We did not encounter Kierner et al. type 4 in our study. Cha et al. (2017) found ELN <1 cm distance from AT in 51.7%, >1 cm distance from AT in 27.6%, and under AT in 20.7%.29
ELN and inferior constrictor (ICM) & cricothyroid muscle (CTM)
Friedman et al. (2002) described three types of ELN in relation to ICM, CTM and STA.30 In type 1, ELN runs superficial to ICM throughout, descending with STA until its termination in CTM. In type 2, the ELN penetrates the ICM in its lower part, whereas in type 3, the ELN penetrates the ICM in its upper part. Type 2 and type 3 ELN of Friedman et al. (2002) are difficult to identify during thyroid surgery as they are deep to the ICM but are safer than type 1, as type 1 is more prone to injury. We observed type 1 of Friedman et al., in 90.6% of the specimen, type 2 in 6.26%, and type 3 in 3.1% of the specimens in our study. Few authors have reported these as unidentified ELN (Jonas et al., 2000; Lore et al., 1998).31,32 In such situations, the ELN can be identified by nerve stimulation at the junction of the ICM and CTM (Thomassin JM., 1982).25 Additionally, we observed a rare variation wherein the ELN communicates with the superior cervical ganglion (Fig. 4). Such variation might lead to disturbances in sympathetic function if the ELN is injured during thyroid or other neck surgeries.
The RLN is closely related to the ITA near the base of the thyroid gland, and inadvertent injury to the RLN may lead to hoarseness of voice and cough if unilateral. Bilateral injury may cause dyspnea or life-threatening laryngeal obstruction (Jiang et al., 2014).33
RLN and trachea-oesophageal groove
The course of RLN makes it vulnerable to injuries during certain surgical interventions in the neck (Culp et al., 2023).34 This can include surgeries involving the thyroid or parathyroid gland (Joliat et al., 2017),35 laryngeal malignancies (Benninger et al., 1998)36 or endotracheal intubation (Norris et al., 2011).37 We found RLN in the tracheoesophageal groove in 68.75% and lateral to the trachea in 31.25% of the patients. Sailaja (2016) reported RLN lateral to the trachea on 27 (15 right, 12 left) sides and in the tracheoesophageal groove on 23 sides (10 right, 13 left).38 Shao et al., (2016) have also reported that the right RLN is more anterolateral in position than the left RLN.39 Asgharpour et al., (2012) have seen the RLN anterior to the tracheoesophageal groove in 41.6%, in the groove in 33%, and posterior to the groove in 24.5%.40 They also found that the right RLN is more anterior than the left side. They opine that the right RLN is more exposed than the left. This may be due to differences in the embryological origin.
RLN and relation with inferior thyroid artery (ITA):
Surgical injuries are the most common cause of RLN palsy, accounting for 11 to 32% (Titche, 1976).41 Surgeries which can cause injury to the RLN are thyroidectomy, parathyroidectomy, esophagectomy, tracheoplasty, mediastinoscopy, correction of patent ductus arteriosus, etc. (Titche, 1976; Friedrich et al., 1998).41,42 Thyroidectomy is the most common cause of RLN injury, accounting for approximately 5 to 14%, according to various studies (Hayward et al., 2013; Friedrich et al., 1998, Zakaria et al., 2011).8,42,43 This can occur when a branch of the ITA is accidentally injured during the procedure. In most specimens (50%), the RLN was posterior to the ITA compared to other positions (Table 5; Fig. 6a, b, c, d) in our study. This is in accordance with the results of Tang et al. (2012) and Sailaja (2016) (86.25% and 56%, respectively).12,38 On the contrary, Campos et al. (2000) observed RLN behind the ITA in a comparatively lesser number of specimens.44 However, they observed RLN passing between the branches of ITA in the majority of the cases they studied (≈50%). When the RLN passes between the branches of the ITA, as shown in Fig. 6c, it makes it more vulnerable to injury during dissection for surgical procedures. Few researchers have opinioned that RLN injury is more common when the nerve is anterior or between the branches of the ITA than posterior to it (Skandalakis et al., 1976; Steurer et al., 2002).45,46 Surgeons should be aware of these types of variable relations between the RLN and ITA to avoid nerve damage.
Branching pattern of RLN
The number of branches from the RLN before it pierces the muscles of the larynx is clinically significant because the branches may entangle the ITA, place the nerve at risk, and paralyze the laryngeal muscles (Shao et al., 2016).39 In our study, a varied number of branches of the RLN before piercing the larynx was found, with a maximum of 64% (Table 6; Fig. 7a, b & c). Sailaja (2016) revealed that the number of branches of the RLN was 2 (75%), 3 (9%), more than 3 in 8%, and 0 in 8%.38 Common pattern of extralaryngeal branching of the RLN is bifurcation with an incidence of 51%, and trifurcation or multiple branches have been reported by a few authors (Shao et al., 2016; Cakir et al., 2006; Henry et al., 2016).39,47,48 This type of branching of RLN before its entry into the larynx is the common cause for surgical morbidity in thyroid surgery (Shao et al., 2016).39 Other than ITA, other reliable landmarks used for the identification of the RLN are distance between the entrance of RLN to the larynx and inferior cornu of the thyroid cartilage or inferior tubercle of the thyroid cartilage or anterior part of cricoid cartilage (Cakir et al., 2006).47 We have found the mean distance between the cricothyroid joint and the entrance of RLN into the larynx as 19.5 mm with the range of 13 mm and 30 mm. The present study has few limitations like effect of formalin on the dimensions of the nerves and gender based comparison, which was not performed.
In the present study, we recorded the usual course of laryngeal nerves along with some rare variations, such as ILN piercing the thyroid cartilage lamina or multiple branches of the RLN that lie in close proximity to the branch of the ITA. The compiled data may aid in the success of surgeries in the neck, in and around the laryngeal nerves by minimizing the risk of their accidental damage. The findings of this study can be considered as a morphological data base of the laryngeal nerves, for our sample population.
This study was conducted on 64 dissected neck of formalin fixed cadavers, which were utilized for undergraduate teaching purpose, in the department of Anatomy of our institution. The study protocol is approved by Institutional Ethics Committee of Kasturba Medical College, Mangalore (ECR/541/Inst/KA/2014/RR-17) on 21st August 2019 (IEC KMC MLR 08-19/355).
Consent form was waived by the Institutional Ethics committee, as it is a cadaveric study. However the body donors had given written consent before donating the body to our department.
Figshare: Morphometry of laryngeal nerves in cadavers, https://doi.org/10.6084/m9.figshare.25002848. 49
This project contains the following underlying data:
Data is under license CC0
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Anatomy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 08 Jul 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)