Keywords
3Rs, Daily torpor, Food restriction, Refinement, Mice
This article is included in the NC3Rs gateway.
Many behavioural, pharmacological, and metabolic studies in mice require fasting, yet the possibility of fasting-induced torpor affecting data is rarely considered. Torpor is a state characterised by depressed metabolism and profound alterations in physiology and behaviour. In this study, we aimed to determine whether a chronic food restriction paradigm, common in behavioural studies, was sufficient to induce torpor in mice.
Mice were food restricted to ~85-90% of their bodyweight, as is typically done, and monitored using continuous thermal imaging.
We observed that body temperature significantly decreased over days of food restriction, and it was significantly related to the drop in bodyweight (r2=0.8989, p<0.0001). All mice reliably entered torpor daily from day 8 of food restriction which coincided with bodyweight stabilisation at ~85%. We found a strong positive relationship between the magnitude of the decrease of bodyweight and the proportion of mice entering torpor each day (r2=0.8715, p<0.0001).
Overall, we found that torpor is readily induced in response to food restriction. Considering that hunger is frequently used as a motivational drive in behavioural tasks, it is likely that torpor occurrence is common in such studies, while remaining undetected and unaccounted for. Due to the profound effect of torpor on physiology, it is possible that torpor induction may be confounding subsequent data and represents an important source of variation. We recommend that body temperature is always monitored noninvasively in studies where food restriction is employed, to determine when torpor is occurring, and that torpor history is appropriately controlled for within and across experimental groups.
3Rs, Daily torpor, Food restriction, Refinement, Mice
Scientific benefit:
• Identification of torpor induction in response to food restriction in laboratory mice
• Raising awareness of torpor induction as a result of food restriction (an unwanted side effect which may impact study outcome measures)
3Rs benefit:
• Identifying and controlling for potential sources of variation in experimental variables due to torpor occurrence
• Welfare implications of food restriction in behavioural studies - inducing torpor as an unwanted side effect
• Thermal imaging, providing a non-invasive measuring of torpor
Current applications:
Potential applications:
The laboratory mouse is a widely used model organism for scientific research, accounting for 54% (934,200) of experimental procedures performed on animals in the UK in 2021 (Annual Statistics of Scientific Procedures on Living Animals Great Britain 2021). As part of many experimental procedures, fasting and food restriction are frequently used, for example in behavioural and sensory neuroscience (Padamsey et al., 2021; Pioli et al., 2014), metabolic studies (Ayala et al., 2010; Berglund et al., 2008), pharmacological studies (Chen et al., 2018; Huang et al., 2015), and circadian phenotyping (Greenwell et al., 2019; Northeast et al., 2019), amongst others.
The potential for fasting or food restriction to be a confound for experimental data is rarely considered within studies that utilise this technique, despite fasting being known to significantly alter physiology (Jensen et al., 2013). Food restriction is especially prevalent in behavioural neuroscience as a method for incentivising engagement with a task. Frequently, mice undergo chronic food restriction in which bodyweight is maintained at ~85-90%. However, behavioural data is subject to high levels of inter- and intra-individual variation, therefore requiring large sample sizes, although reproducibility is often limited (Baker & Penny, 2016; Begley & Ioannidis, 2015; Mandillo et al., 2008).
We hypothesised that torpor, a naturally occurring state of metabolic suppression and hypothermia in response to limited food availability, may be an unaddressed confounding factor in behavioural studies. It has been reported that laboratory mice enter torpor in response to fasting (Ambler et al., 2021; Hudson & Scott, 1979; Jensen et al., 2013), resulting in profound changes to central and peripheral physiology. For example, torpor has been associated with sleep disruption and altered brain activity (Huang et al., 2021; Northeast et al., 2019; Vyazovskiy et al., 2017), and altered levels of circulating hormones such as thyroid hormones (Bank et al., 2015), leptin (Gavrilova et al., 1999), and ghrelin (Gluck et al., 2006).
Here, we aimed to determine the likelihood of torpor induction in response to a food restriction paradigm common in behavioural studies. We focused on the influence of bodyweight due to previous studies demonstrating a link between bodyweight and torpor (Kato et al., 2018; Solymár et al., 2015), and due to weight loss being a key component of food restriction paradigms.
Procedures were performed in compliance with the United Kingdom Animals (Scientific Procedures) Act of 1986 (Project Licence Number: P828B64BC), and the University of Oxford Policy on the Use of Animals in Scientific Research. All experiments performed under this project licence were approved by the University of Oxford Animal Welfare and Ethical Review Board (AWERB) on 28th February 2017 (reference: DPAGEP (17) 9, Item 5a). A retrospective review of the project licence was considered by the AWERB on 19th September 2019 as part of the in-house assurance process. The retrospective review report was approved by the AWERB, and ongoing research supported by the committee.
All efforts were made to ameliorate suffering of animals throughout. To this end, the health and behaviour of all animals was checked at least twice daily, and animals were monitored remotely via webcams. Bodyweight was monitored daily. Humane endpoints included weight loss of >20% and adverse behavioural changes, such as signs of grimace (Langford et al., 2010), which did not improve following a 6h observation period.
Adult, male C57BL/6J mice were obtained from an in-house colony maintained by the University of Oxford Biomedical Services (N=8, aged ~11 weeks at the start of the study). C57BL/6J was chosen as they are a common strain used for behavioural studies, or as a background for genetic mutants. Power calculations were not used for this study, as it was an exploratory study, and we expected that the effect size would be large due to a torpor bout typically involving a considerable (>5°C) drop in body temperature that is easily detected (Huang et al., 2021; van der Vinne et al., 2020). Furthermore, there are few existing studies of a similar nature which limits the ability to accurately perform power calculations.
A single sex was used to maximise the applicability of the data to existing data, as most research to date, unfortunately, has been only conducted in males, especially within the behaviour field. Although we have not performed this study in females to date, we would expect similar outcomes due to torpor being readily induced in female mice elsewhere (Zhang et al., 2020). Mice were individually housed in wire-top cages (48×15×13 cm) on a 12:12 h light-dark cycle for the duration of the experiment. Cages were housed in custom light-tight chambers (LTCs; Figure 1A), with four cages per chamber (Fisher et al., 2012; Tam et al., 2021). LTCs were illuminated by cool white LED strips (Maplin, UK; 200 lux at the cage floor) during the light phase. Ambient room temperature and relative humidity were maintained at 21±1°C and 60±1%, respectively. Water was provided ad libitum throughout.
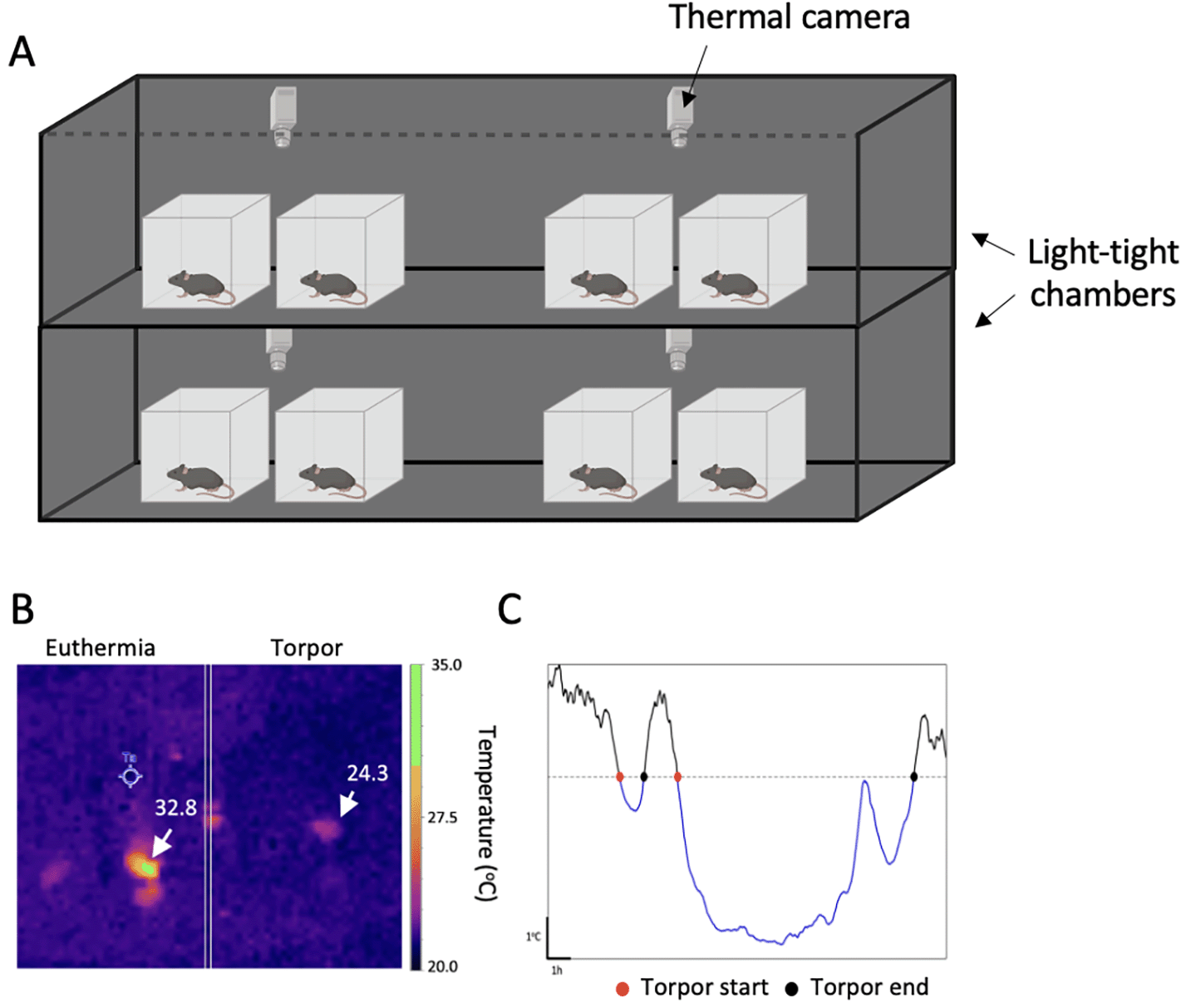
(A) Mice were individually housed in standard M3 wire-top cages and placed under thermal imaging cameras (two cages per camera) which were used to continuously monitor environmental and skin temperature. Cameras were placed ~20 cm above the cage floor and positioned to ensure mice were in view. During food restriction, mice were fed at the same time each day and weighed ~2 hours after feeding. Mice were maintained at ~85% of free feeding bodyweight. (B) Thermal images of a mouse during euthermia (left), and a mouse during torpor (right). Skin temperature (Tskin) was measured by recording the warmest pixel in view every 1 s (1 Hz). Ta represents measurement of the ambient temperature (coldest pixel every 1 s). (C) Representative Tskin trace of a torpor bout during food restriction. Torpor bouts were detected by determining when Tskin had decreased by >3 standard deviation below the median euthermic temperature, for >1 h, for each mouse. Red circles indicate where torpor was determined to begin, and black circles indicate where torpor was determined to end using these criteria.
The change in peripheral skin temperature (Tskin) in response to food restriction was recorded using continuous thermal imaging cameras, mounted ~20 cm above the cage base (Optris® Xi 80 compact spot finder thermal imaging camera with 80° wide angle lens, Optris GmbH, Berlin, Germany; one camera per two animals). Custom Perspex blocks (23×12×3.2 cm, Aquarius Plastics, Surrey, UK) were used to block access to the area beneath the food hopper to minimise mice going out of view of the camera. Additionally, bedding material (sizzle nest) was kept to a minimum, and enrichment items, such as tunnels, were removed to prevent mice from being obscured from view of the thermal cameras, although ~10 g of nesting material was provided to align with the thermal preference of mice (Gaskill et al., 2011).
Tskin was determined by recording the hottest pixel every 1s for the duration of the study using the manufacturer’s software (Optris® PIX Connect, Optris GmbH). Data were processed by applying a high-pass filter 18°C and a low-pass filter at 35°C to remove artefacts that occurred due to movement and changes in animal posture. The filtered data were binned into 1-minute intervals, and a 20-minute moving average applied to smooth the data and further remove noise ready for analysis (Huang et al., 2021; van der Vinne et al., 2020).
During this study, the effect of time of feeding on torpor characteristics were also assessed. As such, mice were assigned to one of two experimental groups: the morning-fed group and the night-fed group (n=4 for each). However, these data are beyond the scope of this report and will be described in an accompanying publication (Wilcox et al., 2024d). The analysis presented here are from all mice (n=8) combined. No criteria were set for including or excluding animals other than the humane endpoints, which was not reached by any animals.
Following single housing, cages were placed in the LTCs, and mice left undisturbed for 2 weeks under ad libitum conditions to habituate to the new environment. Baseline measurements of bodyweight and Tskin were taken at the end of the habituation period prior to the start of food restriction. Food restriction was initiated by removing all food. A single ration of food (2.0-2.5g; 2016 Teklad Global Rodent Diet®, 16% protein, Envigo, Blackthorne, UK) was provided at the same time each day; the initial amount was determined by calculating 70% of individual average ad libitum daily intake, and then titrated based on bodyweight (van der Vinne et al., 2018). Mice were maintained at ~85% of their free feeding bodyweight, determined by taking a daily bodyweight measurement ~2 hours after food was provided. Additional food was provided the following day if bodyweight had dropped below 85%. Conversely, the following day’s food ration was reduced if bodyweight was >85%.
Torpor induction was determined using non-invasive Tskin measurements. Previously we have shown that thermal imaging is able to reliably detect bouts of hypothermia associated with torpor (van der Vinne et al., 2020). However, Tskin cannot be used to calculate core body temperature (Tcore), making previously used torpor definitions of a Tcore <32°C inappropriate (Brown & Staples, 2010; Solymár et al., 2015; Swoap & Gutilla, 2009). Instead, we developed a custom algorithm, based on the criteria described by Huang et al. (2021), to define torpor as when Tskin dropped by >3 standard deviations below the median euthermic Tskin for >1h for each animal. The start and end of torpor were determined as when Tskin crossed this threshold (Figure 1C). These measurements were used to determine the effect of food restriction on torpor propensity and the amount of time spent in torpor.
Data were processed using MATLAB (MathWorks®, USA, v2023a) and analysed using Prism (GraphPad, version 9). Data are presented as mean values ± standard error of the mean (SEM) unless otherwise stated. Data were tested for normality using a Shapiro-Wilk test prior to analysis. One-way ANOVAs with repeated measures were used to determine how variables changed over time, followed by a post-hoc multiple comparisons test (Tukey’s) with a Bonferroni correction for multiple comparisons. A Geisser-Greenhouse correction was applied in all cases. Simple linear regression analysis was used to investigate the relationship between variables of interest. Data was determined to be significant when p<0.05.
As expected, food restriction resulted in a significant decrease in bodyweight over time (F(4.11, 28.8)=105.9, p<0.0001). Bodyweight dropped until day 8 of food restriction when weight stabilised between 85-90%, as was our aim in line with most behavioural food restriction paradigms (Figure 2A). To determine the effect of food restriction on body temperature, the change in mean Tskin from baseline (day 0, the last day of free feeding) was calculated. This method was chosen due to inter-individual variability in absolute Tskin measurements (van der Vinne et al., 2020). Tskin significantly decreased over days of food restriction, with the mean daily temperature dropping by ~3°C towards the end of the study (F(3.43,24.0)=34.9, p<0.0001; Figure 2B). The drop in Tskin is likely due to the induction of torpor, and mice spending more time in torpor. This is supported by an increasing proportion of mice entering torpor between days 1–7 of food restriction, and the increasing amount of time spent in torpor each day (Figure 2C, 2F). From day 8 onwards, all mice were entering at least one torpor bout per day. Further, the distribution of Tskin measurements prior to food restriction was unimodal, indicating that body temperature was being maintained with little deviation from a setpoint, as expected for endothermic species. However, after ~1 week of food restriction the distribution shifted to bimodal, with a second peak at a much lower Tskin, corresponding to heterothermy (Figure 2G).
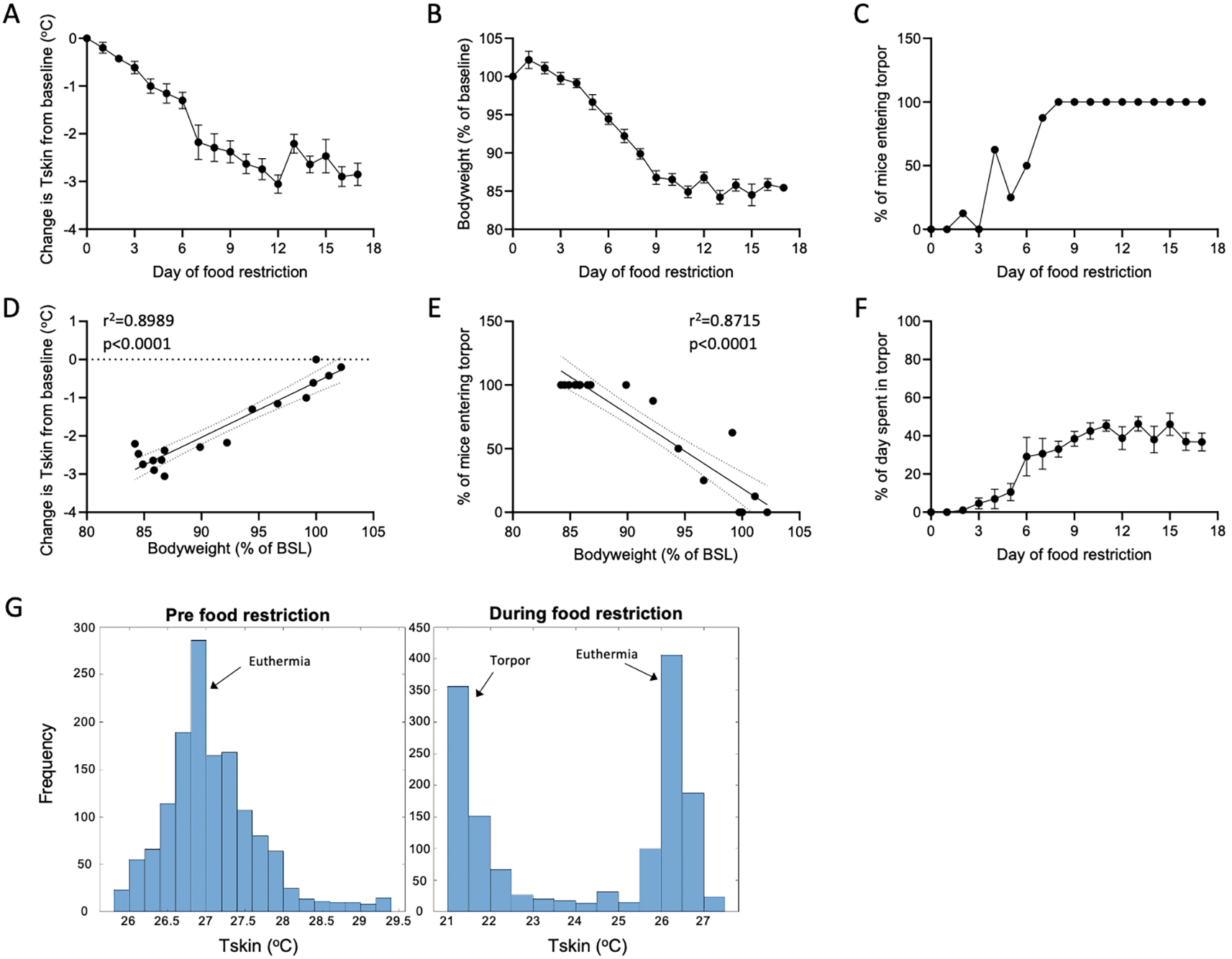
(A) Change in mean daily Tskin from baseline (day 0) over days of food restriction. Tskin was found to significantly decrease over days of food restriction (F(3.43,24.0)=34.9, p<0.0001; one-way ANOVA with repeated measures) with a maximal decrease of 3°C. Data are represented as a mean value ± SEM (n=8). (B) Bodyweight, expressed as a percentage of baseline (day 0), significantly decreased over days of food restriction, as expected (F(4.11,28.8)=105.9, p<0.0001; one-way ANOVA with repeated measures). Bodyweight stabilised at 85-90% on day 8 of food restriction. Data are represented as a mean value ± SEM (n=8). (C) Time course of the percentage of mice entering torpor on each day of food restriction. All mice reliably entered torpor each day from day 8 onwards (n=8) (D) Simple linear regression analysis of bodyweight (as a % of baseline) and change in body temperature compared to baseline (day 0). A strong positive relationship was found between the two parameters (r2=0.8989, p<0.0001). Each data point represents a day of recording. (E) A significant negative relationship was observed between the proportion of mice entering torpor and decrease of bodyweight (as a % of baseline). Note that all mice were entering torpor at 85% and 90% bodyweight, and almost all animals were entering torpor at slightly above 90% bodyweight. Each data point represents a day of recording. (F) The amount of time spent in torpor each day, expressed as a percentage, over days of food restriction. (G) Frequency distribution or recorded Tskin values from a representative mouse before food restriction (left), and during food restriction (right). A unimodal distribution of temperature is observed pre-food restriction, indicating maintenance around a set point. Two peaks are observed during food restriction, indicating a deviation from the euthermic set point, as is typical for heterothermy.
Next, we investigated whether our observations were related to decreasing body weight. A strong positive relationship was found between bodyweight and the change in Tskin, with Tskin decreasing as bodyweight dropped (r2=0.8989, p<0.0001; Figure 2D). Moreover, a strong negative relationship was found between bodyweight and the proportion of mice entering torpor on each day of food restriction (r2=0.8725, p<0.0001). Notably, a high proportion of mice were entering torpor when bodyweight was at ~90% of free feeding bodyweight, indicating that even a weight loss of 10% is sufficient to induce torpor in laboratory mice (Figure 2E).
Our results show that torpor is readily induced in response to food restriction, typically occurring in all mice from day 8, although a high proportion of mice began entering torpor before this point. Notably, day 8 coincides with when bodyweight first began to stabilise at 85% bodyweight, which is often the target bodyweight required before behavioural testing commences (Pioli et al., 2014). Although bodyweight in this study was titrated to 85%, we still observed a high occurrence of torpor at bodyweights of 90%. Bodyweight can often increase to 90% during chronic food restriction paradigms; however, the data presented here suggest that even this degree of weight loss would be sufficient to induce torpor. As such, it is likely that torpor will be occurring regularly during the period of behavioural testing. Although it is unlikely that testing would be performed in torpid animals, as they are lethargic and show reduced behavioural responsiveness at low body temperatures, the short-term and delayed effects of torpor induction on brain function and behaviour are largely unknown. More generally, due to the profound changes in physiology associated with torpor, it is possible that variables of interest will be confounded due to previous torpor history. Although limited, some behavioural data gathered from post-torpid mice found that behavioural performance was significantly altered (Nowakowski et al., 2009). Moreover, a study in ground squirrels reported high levels of variability between individuals in memory retention when performing a previously learned task following torpor (Hensleigh et al., 2022).
Here, we focused on a chronic food restriction paradigm at ~85% of free-feeding bodyweight; however, previous work has shown that torpor can be induced after only 7 hours of total food removal (Brown & Staples, 2010; Swoap et al., 2006; Swoap & Weinshenker, 2008). Acute fasting is commonplace as part of metabolic and pharmacological studies (Ayala et al., 2010; Beumer et al., 2006), therefore, based on previous research, we anticipate that our findings are also applicable to studies where acute fasting is used.
Due to technical limitations of the thermal cameras, mice were singly housed throughout this study. Further, single housing allowed for more precise titration of the amount of food provided each day to maintain individual bodyweight at ~85%. Single housing is common practice during torpor studies in mice due to the use of thermal cameras (Hitrec et al., 2019), or due to surgery being required to implant temperature-sensitive telemeters (Oelkrug et al., 2011). However, group housing is often used during behavioural testing to reduce cage costs and for welfare benefits. There is some evidence in other species that torpor is still employed in group settings and can result in increased entries into torpor due to reduced energy savings because of social thermoregulation via huddling (Geiser, 2010; Jefimow et al., 2011). Moreover, a study in group housed sugar gliders found that food restriction resulted in synchronisation of torpor bouts (Nowack & Geiser, 2016). To our knowledge, social torpor has not been investigated in laboratory mice; however, evidence from other species suggests that the results presented here would be applicable to group housed mice.
Based on our data, we recommend that studies using both acute and long-term food restriction in mice are closely monitored for torpor bouts, for example, using continuous non-invasive thermal imaging. Even in group housed settings, thermal imaging could be used to detect sustained drops in body temperature corresponding to mice entering torpor. Doing so will enable data to be compared from cages with similar torpor histories before running a task or taking a sample. Moreover, knowing prior torpor history will allow for additional controls to be implemented and may help to explain unexpected differences between experimental units. From a 3Rs perspective, our recommendations can be used to refine experimental paradigms requiring food restriction, therefore helping to reduce variability.
SLW, SNP, DMB and VVV designed experiments. SLW performed measurements. SLW and VVV performed analysis. SLW wrote the manuscript with input from all other authors.
Figshare: Induction of torpor in response to a common chronic food restriction paradigm: implications for behavioural research using mice.
- Bodyweight measurements: https://doi.org/10.6084/m9.figshare.25722546 (Wilcox et al., 2024a).
- Temperature recordings: https://doi.org/10.6084/m9.figshare.25722426 (Wilcox et al., 2024b).
This project contains the following underlying data:
- Bodyweight.xlsx
- 230920_1.dat
- 230920_2.dat
- 230920_3.dat
- 230920_4.dat
- 240920_1.dat
- 240920_2.dat
- 240920_3.dat
- 240920_4.dat
- 250920_1.dat
- 250920_2.dat
- 250920_3.dat
- 250920_4.dat
- 260920_1.dat
- 260920_2.dat
- 260920_3.dat
- 260920_4.dat
- 270920_1.dat
- 270920_2.dat
- 270920_3.dat
- 270920_4.dat
- 280920_1.dat
- 280920_2.dat
- 280920_3.dat
- 280920_4.dat
- 290920_1.dat
- 290920_2.dat
- 290920_3.dat
- 290920_4.dat
- 300920_1.dat
- 300920_2.dat
- 300920_3.dat
- 300920_4.dat
- 011020_1.dat
- 011020_2.dat
- 011020_3.dat
- 011020_4.dat
- 021020_1.dat
- 021020_2.dat
- 021020_3.dat
- 021020_4.dat
- 031020_1.dat
- 031020_2.dat
- 031020_3.dat
- 031020_4.dat
- 041020_1.dat
- 041020_2.dat
- 041020_3.dat
- 041020_4.dat
- 051020_1.dat
- 051020_2.dat
- 051020_3.dat
- 051020_4.dat
- 061020_1.dat
- 061020_2.dat
- 061020_3.dat
- 061020_4.dat
- 071020_1.dat
- 071020_2.dat
- 071020_3.dat
- 071020_4.dat
- 081020_1.dat
- 081020_2.dat
- 081020_3.dat
- 081020_4.dat
- 091020_1.dat
- 091020_2.dat
- 091020_3.dat
- 091020_4.dat
- 101020_1.dat
- 101020_2.dat
- 101020_3.dat
- 101020_4.dat
- 121020_1.dat
- 121020_2.dat
- 121020_3.dat
- 121020_4.dat
- 131020_1.dat
- 131020_2.dat
- 131020_3.dat
- 131020_4.dat
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: ARRIVE author checklist: https://doi.org/10.6084/m9.figshare.26213432 (Wilcox, 2024c)
- ARRIVE Author Checklist - BW and torpor.pdf
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The following software was used for processing and analysing the data presented here:
- MATLAB, available at: https://uk.mathworks.com/products/matlab.html
- GraphPad Prism, available at: https://www.graphpad.com
These software programmes used for data analysis require a licence for use. All data processing and statistical analysis performed can be conducted in the open access software, R, available at https://www.r-project.org/.
We thank Laura E. McKillop and Vincent van der Vinne for assistance during the planning and running of experiments. We thank Sara Wells for her comments on this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Mitchell SE, Delville C, Konstantopedos P, Derous D, et al.: The effects of graded levels of calorie restriction: III. Impact of short term calorie and protein restriction on mean daily body temperature and torpor use in the C57BL/6 mouse.Oncotarget. 2015; 6 (21): 18314-37 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Nutritional interventions, ageing, metabolism
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hibernation, torpor, physiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 24 Jul 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
Please enter your details to receive updates from the NC3Rs
We'll update you about the NC3Rs and the NC3Rs Gateway.
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)