Keywords
exercise, blood pressure, quality of life, Pilates, movement therapy
This article is included in the Manipal Academy of Higher Education gateway.
Systemic hypertension is a global non-communicable disease that creates an essential need for alternate forms of lifestyle modifications, including exercise, to lower elevated blood pressure. Mat Pilates, a feasible form of mind-body coordinated exercise, may provide benefits with limited resources.
This randomized controlled trial aimed to assess the efficacy of Mat Pilates on resting blood pressure and health-related quality of life in individuals with systemic hypertension.
A two-arm, single-blinded, block randomized controlled trial will be recruited with120 participants into control and experimental groups (1:1 ratio). Mat Pilates will be administered to the experimental group and standard care to the control group for 12 weeks. Resting and central blood pressure, health-related quality of life, and echocardiographic parameters will be measured before and after the trial. We hypothesized that Pilates may not be beneficial in lowering resting blood pressure in hypertensive patients. A repeated-measures analysis of variance (ANOVA) will test the within- and between-group effects of Mat Pilates on various outcomes.
The trial was approved by the Institutional Research Committee, Manipal College of Health Professions, Manipal Academy of Higher Education, Kasturba Medical College, and Kasturba Hospital Institutional Ethics Committee, Manipal. Written informed consent will be obtained from all the participants. All stakeholders and committees will communicate key findings regarding the implementation of mind-body association exercises as a measure of lifestyle modification in individuals with systemic hypertension.
Clinical Trials Registry of India: CTRI/2021/07/035002. Registered on July 20, 2021, http://ctri.nic.in.
exercise, blood pressure, quality of life, Pilates, movement therapy
Strengths and Limitations of this study
• This single-blinded, randomized controlled trial will enable awareness of a feasible, cost-effective form of Mat Pilates exercise for participants diagnosed with hypertension that can be easily implemented in low-resource settings.
• As a mind-body association exercise, individuals with systemic hypertension would benefit from relaxed breathing maneuvers, which will in turn aid in the relaxation of various muscle groups and peripheral vasculature.
• As this is a single-center trial, this study may not be accessible to participants outside the study setting. In addition, Mat Pilates needs supervision for a minimum of 2 weeks to ensure the correct learning of exercise patterns.
Systemic arterial hypertension is a significant contributor to the non-communicable disease spectrum. The World Health Organization (WHO) reports that systemic hypertension accounts for almost 45% and 51% of mortality from heart disease and stroke worldwide, respectively.1 The Global Action Plan for the control and prevention of non-communicable diseases (NCDs) aims to reduce the prevalence of systemic hypertension by the year 2025.2 The South Asian subcontinent is undergoing extensive economic growth, leading to inimical urban lifestyle changes. A mortality rate of 1.5 million deaths per year has been reported due to cardiovascular disease (CVD) among Indians, and by 2020, CVD will be the leading cause of mortality and morbidity.3,4 Therapeutic modes adopted for systemic hypertension include antihypertensive drug therapy, lifestyle modifications such as diet, and regular physical activity or exercise. Dietary Approaches to Stop Hypertension (DASH) and regular aerobic exercise for 30 minutes per day are mandated determinants to lower elevated blood pressure.5–7 Meta-analyses of various randomized controlled trials report dual benefits of aerobic and resistance exercise in lowering blood pressure.8,9 However, these studies sparsely include alternate approaches of mind-body exercises such as Pilates, Tai Chi, Yoga, etc.
The incorporation of the Pilates method of exercise differs from routine structured physical exercise protocols, as it is based on a mind-body association. A thought process used positively to execute a particular movement with specific breathing patterns, generating kinesthetic awareness aids in reducing mental stress, anxiety, enhancing sleep, and a sense of well-being. Changes in body movement as a whole with concentration, synchronized breathing, and precise control of the body part to be moved with precision in a rhythmic manner creates an environment of the movement pattern and ability to identify the muscle being recruited to perform the desired movement.10–14 Pilates can be performed either on the mat or using Pilates equipment using one’s body weight.15,16 It has been effectively reviewed on various health-related parameters such as body composition,17 stress urinary incontinence,18 and breast cancer survivors’ health-related quality of life,19 falls, and physical fitness in the elderly.20–22
Following the principles of control, breathing, concentration, flow of movement, centering, and Pilates have often been used to improve physical function and muscle conditioning. The benefits of Mat Pilates being a feasible, novel, and recreational mode of exercise implementing cost-effective resources of mats has been reported in different populations such as multiple sclerosis, Parkinson’s disease, older adults, and low back pain.23–26 Energy expenditure (EE), blood lactate levels, oxygen uptake (VO2 uptake), and low cardiovascular stress (including heart rate and blood pressure) have been observed with the implementation of Mat Pilates when compared to the Reformer Pilates method (which utilizes larger equipment) in healthy young female adults.27 Although the above studies report beneficial effects of Mat Pilates on a spectrum of disease conditions, there is a scarcity of studies reporting the effects of mat Pilates on variations in peripheral and central blood pressure parameters in individuals with systemic hypertension. In addition, structural and hemodynamic changes within the cardiac chambers have rarely been reported in relation to this exercise method in hypertensive individuals.
The primary and secondary objectives of this trial were to study the effects of mat Pilates on.
Primary outcomes:
1. Resting blood pressure (systolic and diastolic blood pressure) (mm of Hg)
2. Central systolic blood pressure and pulse pressure (mm of Hg)
3. Mean arterial pressure (MAP) (mm of Hg)
Secondary outcomes:
4. Health related quality of life (HrQoL)
5. Two-dimensional (2D) echocardiographic parameters included the left ventricular ejection fraction (LVEF) and end-diastolic volume.
Hypotheses:
Null hypothesis: This trial hypothesizes that mat Pilates will not have a beneficial reduction in arterial blood pressure and will not improve health-related quality of life in participants with systemic hypertension.
Alternate hypothesis: Mat Pilates has a positive effect on reducing resting blood pressure, including central pulse pressure and central systolic pressure. In addition, the application of mat Pilates will improve health-related quality of life in individuals with systemic hypertension.
Considering the fewer studies reported, this trial will be a two-arm, single-blinded, prospective randomized controlled trial (RCT) aimed at evaluating the efficacy of a 12 weeks, mat Pilates program on changes in resting and central blood pressures (central pulse pressure and central systolic pressure) in a total of 120 participants.
Participants will be screened in the outpatient departments of General Medicine and Cardiology, Kasturba Hospital, Manipal.
Mat Pilates exercises will be administered and supervised in the outpatient department of the Physiotherapy and Health Performance Laboratory, Kasturba Hospital Manipal.
Participants aged between 30 and 60 years diagnosed with systemic arterial hypertension with optimal blood pressure control on regular antihypertensive therapy will be recruited for this clinical trial.
Inclusion criteria:
1. Stage 1 (systolic blood pressure > 130–139 mmHg or diastolic blood pressure > 80–89 mmHg) and Stage 2 (systolic blood pressure >140–159 mmHg or diastolic blood pressure > 90–99 mmHg) hypertensive individuals.
Exclusion criteria:
1. Stage 2 hypertension: systolic blood pressure >160 mmHg and diastolic blood pressure >100 mmHg.
2. Accelerated hypertension/Hypertensive crisis.
3. Any discontinuation or change in the antihypertensive drug regime during the study period.
4. Diagnosed cases of acute myocardial infarction, valvular heart disease (aortic, mitral, tricuspid, pulmonic), arterial aneurysms with impending cardiac arrhythmias (on external or internal pacing devices, implanted cardioverter defibrillator (ICD).
5. Pregnancy and lactating hypertensive women.
6. Individuals with cancer on previous or current chemotherapy, radiation therapy.
7. Acute or chronic renal insufficiency on peritoneal or hemodialysis.
8. Individuals with neurological disease causing impaired cognition and incoordination of movements.
9. Musculoskeletal trauma with disability restricting movement patterns.
10. Individuals with vertigo
11. Individuals unable to comprehend instructions for the exercise program.
The study protocol was approved by the Kasturba Medical College and Kasturba Hospital Institutional Ethics Committee, Kasturba Hospital, Manipal (an associate hospital of the Manipal Academy of Higher Education, Manipal, Karnataka, India) (registration number: ECR/146/Inst/KA/2013/RR-19) to recruit participants (IEC: 596/2019. Date of approval September 11, 2019). The trial has been registered under the Clinical Trials Registry of India (CTRI/2021/07/035002. Registered on July 20, 2021, http://ctri.nic.in.) This clinical trial adheres to the guidelines and ethical principles for medical research of the Declaration of Helsinki involving human participants.
Any protocol-related amendments during the trial tenure will be communicated to the Institutional Research Committee, Institutional Ethics Committee, Directorate of Research, Manipal Academy of Higher Education, and Clinical Trial Registry of India. Written informed consent will be obtained from all the participants. Stakeholders and committees will receive key findings regarding the implementation of mind-body association exercises as a measure of lifestyle modification in individuals with systemic hypertension. Post-trial ancillary care will be provided to participants in the control group after completion of the clinical trial period if they wish to undergo intervention group exercises.
The calculated sample size was based on the primary outcome of resting BP considering a level of significance (α) of 0.05, with a power of 80%. With an anticipated standardized difference of four standard deviations of the primary outcome, the estimated minimal clinically important difference (MCID) at 2 mmHg after a period of 12 weeks (at baseline and after 12th week) in both the control and experimental groups, a sample of 44 participants in each group was calculated. Considering an attrition of 20%, the sample size was calculated to be 55 participants and rounded up to 60 participants per group owing to block randomization.
A random number sequence will be generated using computerized random number generator. Participants will be randomized to the control and intervention groups with 1:1 allocation and a block size of 6 consisting of ten participants in each block per group. The allocation sequence will be generated using computer-generated randomized allocation software. Using the sequentially numbered opaque envelope (SNOSE) method, allocation concealment is performed. Of the 120 participants, 60 will be allocated to the control or experimental group.
This RCT will be a single-blind study. Random allocation to both groups will be performed by a health professional who is not a member of the investigator team. At the completion of twelve weeks, a blinded outcome assessor will assess set outcomes of the respective groups.
Prior permission was sought from the copyright publishers for the administration of the World Health Organization (WHO) BREF Health-Related Quality of Life Questionnaire in two languages. Participants will be screened for eligibility based on their diagnosis and stage of systemic hypertension. Eligible participants will then be familiarized with the study using the participant information sheet, and willing participants will be recruited after obtaining their written informed consent. Preliminary screening will be implemented and written informed consent will be obtained at the outpatient clinic of the Department of Medicine, Kasturba Hospital, Manipal, Karnataka, India.
Baseline assessment of three resting blood pressure measurements in supine lying position with a rest period of one minute in between measurements will be recorded at the same time of the day (10 am). Participants will be instructed to abstain from consumption of alcohol, caffeine, or any caffeine-containing beverage 10 hours prior to the exercise testing session. The values of central systolic blood pressure, central pulse pressure, and mean arterial pressure will be measured by the principal investigator. Left ventricular ejection fraction (LVEF) and left ventricular end-diastolic volume will be measured by a professional expert trained in echocardiography. Including demographic data, the baseline scores of the WHO BREF HRQOL, anthropometric measurements, and exercise stress testing will be measured. This time point will be denoted as T0. Baseline assessment of eligible participants will be performed at the Health Performance Laboratory, Department of Physiotherapy, Clinical Teaching Center, and echocardiographic measurements will be recorded at the Department of Cardiology, Kasturba Hospital, Manipal, India. The participants will then be sequentially block randomized into two groups by a blinded investigator.
Participants in the experimental group will undergo a two-week supervised Mat Pilates program, after which exercises will be performed at home for 10 weeks. An exercise instruction manual for the mat Pilates program will be provided to every participant at the end of 2 weeks. A weekly telephonic, supervised monitoring call will be implemented to address and record any issues related to compliance, adherence, or adverse events. In addition, they will be advised to follow standard care with the recommended salt intake of 2.4 g) or sodium chloride (6 g) per day and regular physical activity of 3-5 days per week.28
The control group will receive standard care and a prescribed antihypertensive drug regimen. After 12 weeks, the outcomes will be reassessed and recorded (T1) by a blinded investigator. Adherence will be set at 75% of the total number of sessions (27 of 36 sessions). A flowchart of the procedure is shown in Figure 1. The schedule of participant enrolment, interventions, and assessments has been provided (Figure 2) according to the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) guidelines.29
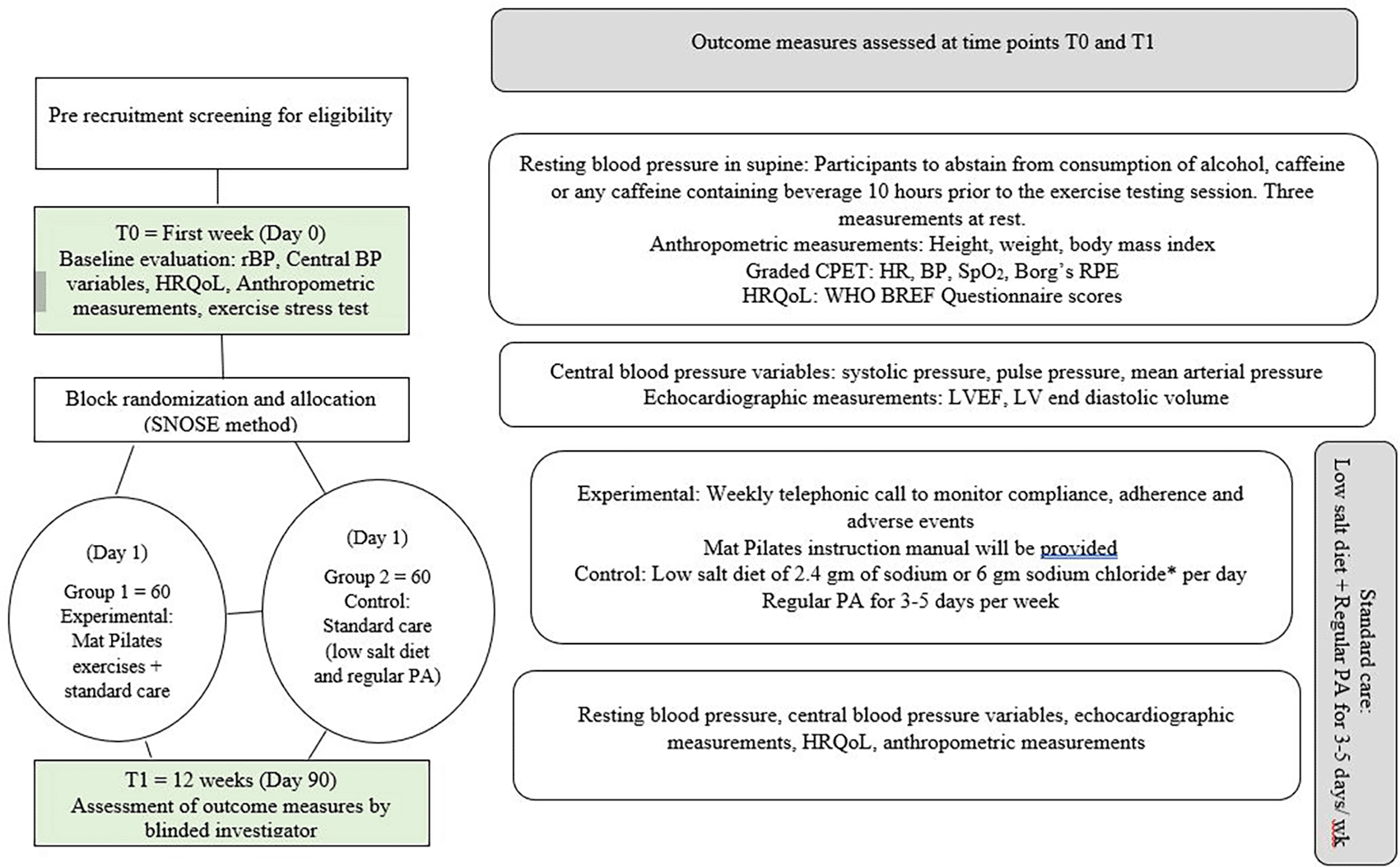
T0, T1 Time points at baseline (T0) and after 12 weeks (T1); BP, blood pressure; CPET, cardiopulmonary exercise stress test; HrQoL, health related quality of life; LV, left ventricle; LVEF, left ventricular ejection fraction; RPE, rating of perceived exertion; SNOSE, sequentially numbered opaque sealed envelope; WHOQOL-BREF, World Health Organization Quality of Life Brief version.

T0, T1, Time points at baseline (T0) and after 12 weeks (90 days) (T1); 2D Echocardiography, Two dimensional echocardiography; LV, Left ventricular; HRQoL, health related quality of life.
The Mat Pilates exercise program will consist of 11 different exercises with a warm-up of 15 min and cool down for 10 min. Three sessions per week of 60 minutes each will be implemented (Table 1). The exercises will be familiarized with, taught, and monitored by a certified mat Pilates instructor.
Adverse events will be recorded and reported every week during telephonic monitoring. Participants will be questioned regarding any major or minor events that may have occurred during the course of their exercise program tenure. The reporting of events, if any, will be implemented by the Institutional Ethics committee for further proceedings.
The measuring equipment used will be standardized and calibrated periodically every 3 months. The Diamond® Aneroid (BPDL-250 Dial Deluxe) pressure monitor with field calibration feature will be used to measure the resting blood pressure. Mats of six feet by four feet with one and a half inch thickness will be used to perform mat Pilates exercises. The Microlife® WatchBP Office Central, which is an oscillometric calibrated blood pressure device, will measure central blood pressure parameters non-invasively using an arm cuff. Additionally, this device could detect any ongoing atrial fibrillation in the participant. The balance protocol for exercise stress testing will be performed on a Stayfit® testing treadmill.
Primary
Resting blood pressure values (systolic/diastolic) in mmHg will be assessed at T0 and T1. Central blood pressure variables (systolic/diastolic/pulse) will be assessed non-invasively pre- and post-intervention, indicating target organ perfusion and perfusion pressure in the central organs (cerebrum, myocardium, kidneys, and liver). Changes observed in central blood pressure with regular exercise would aid in identifying any progression or reduction in target organ health in systemic hypertension.
Secondary
Health-related quality of life (HrQOL) in the mental, physical, and psychosocial domains will be assessed at T0 and T1, indicating any variation in the function of daily living in individuals with systemic hypertension. Two-dimensional echocardiographic findings (LVEF, left ventricular end diastolic volume) as secondary outcomes will aid in identifying any structural, ventricular wall stress-related changes in the myocardial wall before and after 12 weeks of the mat Pilates program versus standard care.
Data will be statistically analyzed using the Jamovi software. Descriptive analysis will be used to interpret demographics, intention-to-treat analysis will be used for missing data, and subgroup analysis based on age will be used to interpret age-wise changes in blood pressure. The Kolmogorov–Smirnov test will be used as a test of normality, and repeated-measures analysis of variance (ANOVA) will be used to analyze the within-group and between-group differences and pre- and post-differences at T0 and T1. Analysis of covariance (ANCOVA) will be used to analyze the contribution of covariates in the study. All analyses of recorded data will comply with the standards of data analysis for randomized controlled trials that include the analysis of missing data due to attrition.
This randomized controlled trial study protocol highlights the effect of a 12 weeks mat Pilates exercise program on various blood pressure variables. This is one of the first studies to report the efficacy of Pilates exercises and changes in central blood pressures. The strengths of this study include changes in central and peripheral blood pressures, which may reduce cardiovascular stress induced in individuals with systemic hypertension. Changes in left ventricular volumes and myocardial wall thickness could be determined, thereby aiding in a cost-effective method to exercise in hypertensive individuals. Increased central blood pressure causes target organ hypoperfusion, leading to early end organ failure.
As this trial is a prospective, single-blinded study, the probability of selection bias would be reduced. An added advantage of a home-based program after a two week supervised mat Pilates exercise program would support participants in comprehending the need to exercise better, making this program cost-effective, feasible, and reduce frequent center-based visits. Although home-based, lack of supervision during the exercise regime may be a limitation of this trial. Secondary limitations would include communication between participants in both groups, which could alter exercise behavior, and alternate options for training at other exercise centers. However, if this RCT proves to be effective, it will be the one of the first studies to assess the efficacy of Mat Pilates on central blood pressure variables and ventricular remodeling related to probable changes in ventricular mass-to-volume ratios.
The study protocol was approved by the Kasturba Medical College and Kasturba Hospital Institutional Ethics Committee, Kasturba Hospital, Manipal (an associate hospital of the Manipal Academy of Higher Education, Manipal, Karnataka, India) (registration number: ECR/146/Inst/KA/2013/RR-19) to recruit participants (IEC: 596/2019. Date of approval September 11, 2019). The trial has been registered under the Clinical Trials Registry of India (CTRI/2021/07/035002. Registered on July 20, 2021, http://ctri.nic.in.) This clinical trial adheres to the guidelines and ethical principles for medical research of the Declaration of Helsinki involving human participants.
Any protocol-related amendments during the trial tenure will be communicated to the Institutional Research Committee, Institutional Ethics Committee, Directorate of Research, Manipal Academy of Higher Education, and Clinical Trial Registry of India. Written informed consent will be obtained from all the participants. Stakeholders and committees will receive key findings regarding the implementation of mind-body association exercises as a measure of lifestyle modification in individuals with systemic hypertension. Post-trial ancillary care will be provided to participants in the control group after completion of the clinical trial period if they wish to undergo intervention group exercises.
Patients or the public were not involved in designing, conducting, or disseminating methods in our research.
NSP conceptualized the study concept, objectives, design, performed a preliminary and ongoing literature review, and drafted the manuscript. SKN is a clinical expert in the field of systemic hypertension who performed a literature review and scrutinized the final draft of the manuscript. GAM and VK aided in the study conception, scrutinizing, and preliminary drafting of the manuscript.
NSP is currently an Assistant Professor pursuing her PhD in the Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education with more than 12 years of teaching and research experience in the field of cardiac and pulmonary sciences. SKN is currently Professor in the Department of Medicine, Kasturba Medical College, Manipal Academy of Higher Education and has over 25 years of research and clinical expertise in the area of non-communicable diseases such as hypertension and diabetes mellitus. AGM is currently Professor and Dean, Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education with more than 30 years of research experience in the field of diabetes mellitus and rehabilitation. VK is currently Professor, Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, with over 25 years of experience in the areas of geriatrics and pulmonary rehabilitation.
The World Health Organization Quality of Life BREF Questionnaire (English Version) 30 will be used for this study for participants fluent in English and Kannada version will be used for participants fluent in Kannada, a regional language from the state of Karnataka. 31
Dataverse: SPIRIT Guidelines checklist: Checklist for Efficacy of Mat Pilates on the resting blood pressure and health related quality of life in individuals with systemic hypertension versus standard care: study protocol for a single centered single blinded randomized controlled trial, DOI: https://doi.org/10.7910/DVN/GLMROL.
The authors acknowledge the research and technical expertise provided by the clinical experts of the departments of Cardiology and Cardiovascular Technology, Kasturba Hospital, Manipal, Karnataka, India for 2D echocardiography measurements and interpretation, and the Department of Data Sciences, Prasanna School of Public Health, Manipal Academy of Higher Education for providing statistical analysis, sample size, and attrition rate calculation. The authors acknowledge the valuable contribution and guidance regarding the statistical analysis provided by Dr. Ravishankar N from the Department of Biostatistics, Vallabhbhai Patel Chest Institute, Delhi University, Delhi.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pilates, hypertension, cardiac rehabilitation
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pulmonary rehabilitation, Respiratory neuroplasticity, Cardiovascular Rehabilitation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Jul 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)