Keywords
Colorectal Cancer (CRC), Metastasis, tumor progression, Autophagy, Krϋpple-like factor 8 (KLF8), MMR-Deficient, chemoresistance.
This article is included in the Bioinformatics gateway.
Colorectal cancer (CRC) is a widespread malignancy globally, yet effective therapeutic approaches for advanced, metastatic, and chemo-resistant cases remain limited. In this study, we knocked out CRC cell line HCT 116 for two autophagy genes (ATG5 and ATG7), then we conducted a transcriptomic analysis on those isogenic cell lines. which revealed an upregulation of Krϋppel-like factor 3 (KLF3) expression, that was biologically validated.
In this study, we performed CRISPR/Cas9 gene editing on HCT 116 followed with transcriptomics analysis on HCT 116 KO cells for ATG5 and ATG7. Various bioinformatics analyses were performed to investigate the KLF3/8 with autophagy and affected functional pathways, and immune genes related to the different types. Validation of expression in different cell lines were done using qPCR and Western blot.
To further investigate the role of autophagy genes in CRC, we utilized publicly available data and web-based tools. Our analysis showed a marked correlation between KLF3/KLF8 and the expression of autophagy genes in CRC, denoting that its upregulation is likely to be a compensatory mechanism. We also examined the co-expression of autophagy genes and KLF3/KLF8 with multiple markers of epithelial-to-mesenchymal transition (EMT), and significant positive correlations were observed. Moreover, KLF8 expression was upregulated at the mRNA level in the metastatic cell lines LoVo and SK-CO-1, compared to HCT 116. Interestingly, KLF3/KLF8 expression was high in MSS molecular subtype of CRC as shown in HCT 116 cell line knocked in with MLH gene as well as they were negatively correlated with crucial immune-infiltrating cells such as CD8+ cells, indicating their potential as a negative biomarker for response to immunotherapy.
Our study proposes that a synergistic approach involving the inhibition of KLF8 and autophagy holds a potential therapeutic target for effectively tackling metastatic CRC cells, especially in cases characterized by deficient mismatch repair (MMR).
Colorectal Cancer (CRC), Metastasis, tumor progression, Autophagy, Krϋpple-like factor 8 (KLF8), MMR-Deficient, chemoresistance.
Colorectal cancer (CRC) is a prevalent and deadly tumor affecting both men and women. It ranks second among the leading causes of cancer-related deaths worldwide, accounting for 9.2% of all such deaths, and is the third most prevalent cancer, representing 6.1% of new cases.1 Projections indicate a worrisome rise in deaths from colon and rectal cancers, expected to increase by 71.5% and 60%, respectively, by 2035.2 CRC is a complex disease with diverse clinical manifestations, molecular indicators, and prognosis.
Autophagy, a cellular process, plays a significant role in the development of CRC and renders cancer cells less susceptible to chemotherapy. By promoting autophagy, cancer cells gain energy and essential metabolites.3 Autophagy ensures cellular homeostasis by facilitating the turnover of defective proteins and organelles, and its activation increases under cellular stress and nutritional scarcity.4–6 Autophagy may act as a tumor suppressor in the early stages of tumor formation, but it appears to promote tumor growth once tumors have already developed. Moreover, autophagy helps tumor cells overcome metabolic stress caused by rapid growth, hypoxia, and limited nutrient supply, which are characteristic of malignant tumors.7,8 Particularly under hypoxic and metabolic stress conditions, autophagy provides additional metabolites and energy to cancer cells.9,10 Importantly, autophagy has also been implicated in cancer cell metastasis, with Epithelial-Mesenchymal Transition (EMT) being a critical process for cancer cell invasiveness and metastasis.11 Regulation of autophagy is mediated via several transcription factors, or a transcriptional gene network.12 Add that autophagy inhibition did not show a marked suppressing effect on cancer cell proliferation, however, compelling evidence supports the notion that autophagy actively contributes to the migration and invasion of cancer cells.13
Recent evidence suggests that Krüppel-like factors (KLFs) are key players in tumor development, growth, and metastasis. Several KLF members, including KLF4, KLF5, and KLF11, have been associated with the oncogenesis of various human cancers. KLFs belong to a family of zinc finger-containing transcription factors closely related to the general transcription factor Sp1. With over 26 known members, this family is characterized by a conserved C-terminus comprising three zinc finger DNA-binding domains. Dysregulation of several KLF family members has been observed in different human cancers, where they can function as either tumor suppressors or oncogenes, depending on the unique cellular context of cancer.14 Furthermore, KLFs are involved in various cellular processes, including differentiation, cell death, and cellular proliferation,15 modulation of autophagy and longevity (in C. elegans), with a potential role in vascular aging in mammals.16
Krüppel-like factor 8 (KLF8) has been the focus of recent studies due to its role in regulating genes associated with various biological functions and pathological processes, such as proliferation, migration, invasion, and inflammation. Widespread expression of KLF8 has been observed in several cancer types, where it has been found to be essential for DNA repair and resistance to apoptosis.17–21 In gastric cell formation and progression, downregulation of KLF8 expression has been shown to decrease cell proliferation, migration, and invasion by reducing the expression of key factors involved in these processes.22
In this study, we utilized CRISPR/Cas9 to target ATG5 and ATG7; both are key genes involved in the autophagy machinery. Transcriptomics analysis revealed differential expression of the KLF3 transcription factor. It is worth noting that KLF8 features a distinctive DNA-binding domain at its C-terminus composed of three zinc fingers. Still, its N-terminal region also shares similarities with KLF3. Most significantly, it has a Pro-Val-Asp-Leu-Ser/Thr motif, shared with KLF3.23–25 Since KLF8 and KLF3 appeared to be co-expressed in multiple tissues and bind the identical DNA sequences and the protein partner CtBP, this suggests a potential functional overlap between these two transcription factors. However, the interplay between autophagy and KLF3/KLF8-mediated malignant phenotypes in CRC remains poorly characterized. Interestingly, autophagy is required for the degradation of KLF3, which might explain the high deferential expression of KLF3 in the autophagy knockout cell lines.26 Additionally, the association of autophagy with KLF family members, particularly group 1 (including KLF3 and KLF8), has not been previously investigated in tumors and may represent a novel therapeutic approach in CRC.
Hence, the primary objective of this study is to elucidate the roles of various autophagy-related genes and the involvement of KLF3/KLF8 in CRC carcinogenesis, focusing on evaluating their potential as prognostic markers and therapeutic targets for CRC.
A diverse panel of cell lines representing various stages of colorectal cancer was utilized. The panel included the HCT 116 cell line as the parental cell line and LoVo and SK-CO-1 as metastatic cell lines. HCT 116 (CCL-247), LoVo (CCL-229) and SK-CO-1 (HTB-39) cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Additionally, two panels of cell lines were employed, consisting of the parent HCT 116 cell line (MLH1−/−) (RRID: CVCL_0291) and its isogenic counterpart, HCT 116 MLH1+/− (RRID: CVCL_HD84), were obtained from Horizon Discovery, gene editing company (Cat. No. HD104-006, Waterbeach, UK). The HCT 116 MLH1+/− cell line was obtained through CRISPR-Cas9 gene editing, where a single allele of the MLH1 gene was inserted. All cell lines were cultured under standard conditions at 37 °C in a humidified atmosphere with 95% air and 5% CO2. Specifically, the CRC cell lines were maintained in RPMI culture media (Sigma-Aldrich, Cat # R8758, Germany).
1. CRISPR/Cas-9 gene editing
We used the ribonucleotide protein27 delivery system for CRISPR technique to knock out autophagy-related gene 5 (ATG-5) and ATG-7 in the HCT 116 cells. All the reagents were purchased from Thermo scientific company, USA. Two guide RNA (gRNA) were individually used to target ATG5/7 genes. Lipofectamine™ CRISPRMAX™ Cas9 Transfection Reagent was used to transfect the cells with the gRNA and Cas9. The genomic cleavage efficiency was assessed by immunoblotting against the desired genes. TrueGuide sgRNA Positive Control, CDK428 was used in all steps as a positive control, while a scrambled gRNA, Trueguide gRNA Negative control, was used as a negative control in all the steps to ensure the specificity of the target. The pool of cells with the highest transfection efficiency was considered for the downstream validation. To generate isolated clones with a complete knockout, the mixed pool of cells was diluted 1 cell/200 μl liquid medium and then dispersed into 96 wells plates. The individual cells were left to form clones. The clones were tested for the expression of ATGF5/7 via immunoblotting.
Whole transcriptome RNA sequencing was carried out on the HCT 116 control and ATG5 and ATG7 knockout using CRISPR/Cas9 system. About 100ng of total RNA was used for library preparation using Ion AmpliSeq™ Transcriptome Human Gene Expression Kit (Thermo Scientific). About 100 pM purified library was taken and pooled equally with four individual samples per pool and were amplified using emulsion PCR on Ion OneTouch 2 instrument (OT2) (Thermo Fisher, RRID:SCR_023289, Cat # 4474778, USA) followed by enrichment using Ion OneTouch ES. Thus, prepared template libraries were then sequenced with Ion S5 XL Semiconductor sequencer using the Ion 540 Chip (Life Technologies, Cat # A27765, California, USA).
RNA-seq data were analyzed using Ion Torrent Software Suite version 5.4 (Thermo Fisher, RRID:SCR_023289, Cat # 0017145, USA) and the alignment was carried out using the Torrent Mapping Alignment Program (TMAP).29 TMAP is optimized for aligning the raw sequencing reads against reference sequence derived from hg19 (GRCh37) assembly and the specificity and sensitivity were maintained by implementing a two-stage mapping approach by employing BWA-short, BWA-long, SSAHA,30 Super-maximal Exact Matching31 and Smith-Waterman algorithm32 for optimal mapping. Raw read counts of the targeted genes were performed using Samtools (Samtools view –c –F 4 –L bed_file bam_file) and the number of expressed transcripts was confirmed after Fragments Per Kilobase Million (FPKM) normalization.29
A script was used (written in R programming language) was used to analyze the differentially expressed genes,10 with function calls to DESeq2 package from Bioconductor library sets. Raw read counts from RNASeq were normalized using quantile normalization. All counts ranked “0” were excluded. Differentially expressed genes between every two set of cell lines [ATG5-KO vs wild HCT 116 (NC), and ATG7-KO, vs wild HCT 116 (NC)] were assessed using 2 tailed t-test. Differentially expressed genes with p-value of <0.01, adjusted p-value <0.5 were included for analysis of pathways and transcription factors using Enrichr.
RIPA lysis buffer (Cat. No. ab156034, Abcam) was used for protein extraction (30-80 μl according to pellet size), followed by centrifugation at 14,000 RPM for 15 min at 4°C. The protein concentration in the cell lysate was measured using a BCA kit (Cat. No. 23225, Thermo Scientific Pierce following the manufacturer’s instructions (Cat. No. 23227, Thermo Scientific Pierce, Waltham, MA, USA). A volume equivalent to 35μg of total protein was separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) that was then electrophoretically transferred to PVDF membrane (Bio-Rad, USA). The membrane was blocked for one hour at room temperature using 5% Bovine Serum Albumin (BSA) powder (Sigma Aldrich).
Following blocking, the membrane was washed with Tris-buffered saline with 0.1% Tween® 20 Detergent (TBST) and incubated overnight at 4 °C with primary antibodies, including anti-ATG5 (Cat. No. 12994S), anti-ATG7 (Cat. No. 8558S), anti-β-actin (Cat. No. 4970S) (all from Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C. Secondary antibodies (Cell Signaling Technology, Danvers, MA, USA) were incubated with the membrane for 1 hour at room temperature at a ratio of 1:1000. The ECL kit (Thermo Scientific Pierce, Waltham, MA, USA) was used to identify chemiluminescence to identify protein bands, Bio-Rad Image Lab software (www.bio-rad.com, ChemiDocTM Touch Gel and Western Blot Imaging System; Bio-Rad, Hercules, CA, USA) was used.
Total RNA was extracted from cancer cells using Qiagen RNA Mini Kit (Cat. No. 12183018A, Thermo Scientific). The RNA quality and quantity were measured by a nanodrop2000 spectrophotometer (Thermo Scientific, USA). cDNA was synthesized from RNA using SensiFAST™ cDNA Synthesis Kit (Bioline Reagents Ltd., London, UK). Real-Time PCR was done for 2 genes encoding KLF8 and KLF3. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Quant Studio 3 (Thermo Fisher) was used (using SensiFAST™ SYBR Hi-ROX kit). The experiment was done in triplicates. Each reaction will yield the average threshold cycle (Ct) values for the genes, and the 2(−ΔΔC(T)) relative approach was used to quantify the expression (Table 1).
TIMER2
The TIMER 2 web application (http://timer.cistrome.org) analyzes the co-expression of autophagy genes and KLF3/KLF8 in COAD and their expression with multiple EMT markers. TIMER 2 analyzes and quantifies the tumor-infiltrating immune cells from the TCGA dataset using a variety of algorithms. This instrument assesses the relationship between the abundance of immune-infiltrating cells and the gene of interest’s transcript. With the aid of this instrument, we looked into the relationship between autophagy genes and KLF3/KLF8 in CRC together and with different EMT markers.33 When choosing the TIMER algorithm and Spearman correlation, we first picked immune association to each gene and each type of immune cell.
Gene Expression Database of Normal and Tumor Tissues 2 (GENT2)
We used the GENT2 database http://gent2.appex.kr. to assess the expression of KLF3/KLF8 in various CRC subtypes.34 Microarray platforms (Affymetrix U133A or U133Plus2) are used in this tool’s NCBI GEO library.35 We used this tool to look into the expression of KLF3/KLF8 in CRC molecular subtypes, querying each gene individually across the molecular subtypes.
Gene Expression Profiling Interactive Analysis2 (GEPIA2)
To measure the autophagy genes and KLF3/KLF8 expression levels in different CRC stages, we used GEPIA2 web-based server. The data originates from the TCGA RNA Sequence Dataset (http://gepia2.cancer pku.cn/#index).36 By looking up each gene under the stages tab individually.
UALCAN
UALCAN is a web-based tool (http://ualcan.path.uab.edu) that utilizes OMICS data from cancer data sets like TCGA, MET500, and CPTAC. Studying the promotor methylation level in different stages of CRC and in different stages of lymph node metastasis and determining significance using the Student t-test between two applications.37 The Beta value indicates level of DNA methylation ranging from 0 (unmethylated) to 1 (fully methylated). Different beta value cut-off has been considered to indicate hyper-methylation [Beta value: 0.7-0.5] or hypo-methylation [Beta-value: 0.3-0.25].
Gene Set Cancer Analysis (GSCALite), a web-based server, is used to look into the state of genes in datasets from cancer patients (http://bioinfo.life.hust.edu.cn/web/GSCALite/).38 Interestingly, we used this tool to examine the function of autophagy genes and KLF3/KLF8 in cancer-related pathways in the COAD data set from TCGA, as it can predict gene activity in cancer-associated pathways.
The GeneMANIA database (http://genemania.org)39 was utilized to generate hypothesis regarding the interactions between KLF3/KLF8 with a total of twenty proteins that surround it. Protein-protein interactions were examined based on several networks, including as co-expression and common pathways, using KLF3/KLF8 as a research tool.
The data were analyzed using various statistical tests, as described in the figure legends. The mean values are presented from at least three independent experiments. The differences between different cell lines in KLF3 and KLF8 were assessed using the Student’s T-test for the qPCR experiments. All statistical analyses were performed using GraphPad Prism software (version 8.0.0 www.graphpad.com) (San Diego, CA, USA). P-values less than 0.05 were considered significant.
We evaluated the expression of ATG5/7 in multiple CRC cell lines in which we found high expression levels in HCT 116, LoVo and SK-CO-1 while HT29 cell line showed relatively low expression (Figure 1A). However, previous literature showed that HCT 116 showed a higher controlled autophagy flux compared to other CRC cell lines.40 Thus, HCT 116 has been used to knockout ATG5/7 via CRISPR/Cas9 editing techniques.
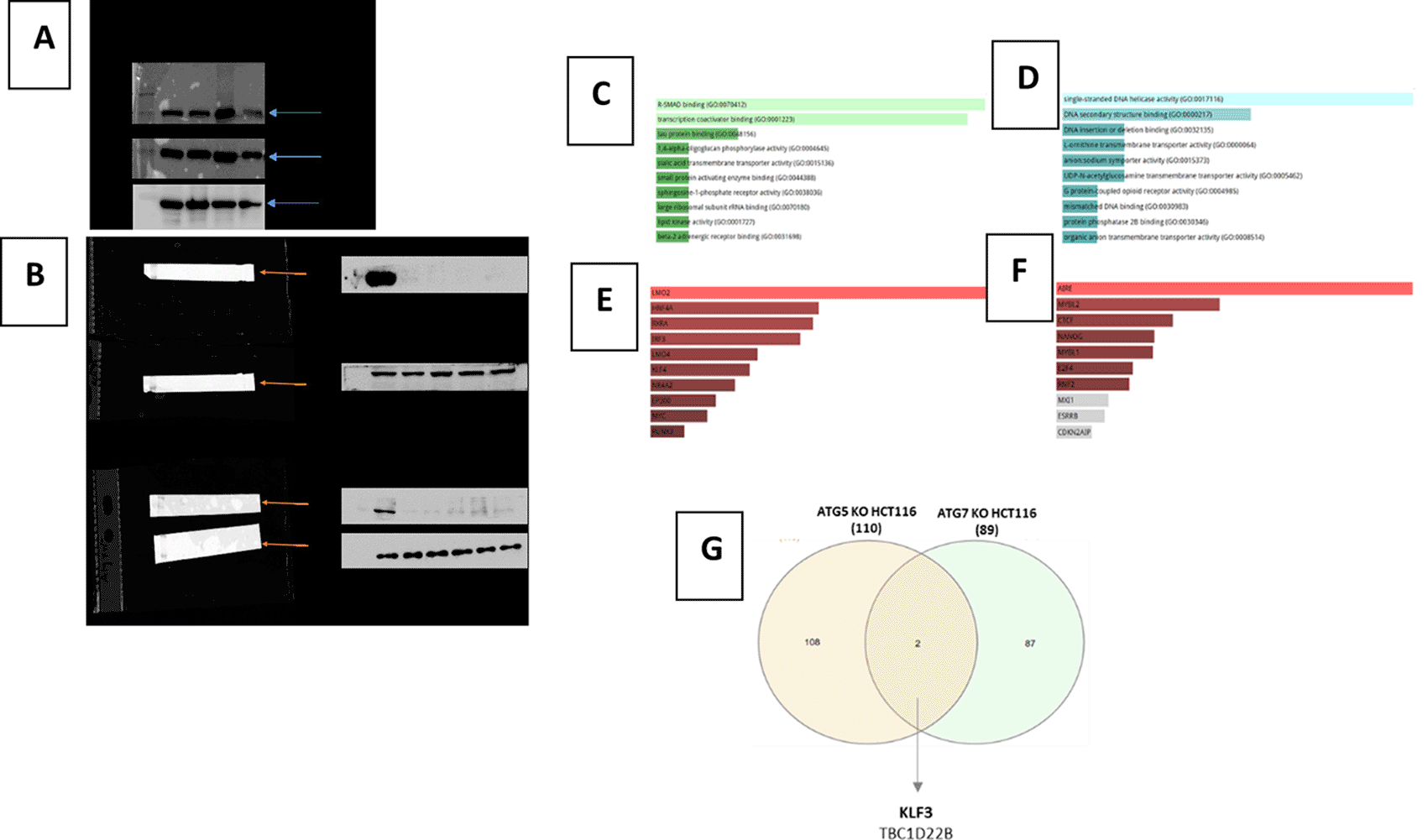
(A) Representative Western blot of ATG5, ATG7 levels in a panel of CRC cell lines including: HCT 116, HT29, LoVo, SK-CO-1. β-actin was used as the loading control.
(B) Representative Western blot of ATG5 and ATG7 Knocking out of the HCT116 cell line using CRISPR/Cas9 gene editing. Colony selection was carried out to ensure the purity of the cell population knocked out for ATG5 and ATG7.
(C) Whole transcriptomic analysis in ATG5 KO cell lines captured multiple differentially upregulated pathways.
(D) Whole transcriptomic analysis in ATG7 KO cell lines captured multiple differentially upregulated pathways.
(E) Lower panel shows top differentially expressed transcription factors predicted to be upregulated in HCT 116 KO for ATG5.
(F) Lower panel shows top differentially expressed transcription factors predicted to be upregulated in HCT 116 KO for ATG7.
(G) The Venn chart shows the shared genes among top differentially upregulated pathways in both ATG5-KO and ATG7- KO HCT 116 cell lines, showing KLF3 transcriptional factor as the top upregulated gene in both.
The GSEA relies on protein-protein interactions for transcription factors extracted from several literature databases (Transcription Factor PPI). The analysis was performed using Enricher web tool. The Gene Ontology (GO) pathways were ranked according to the lowest adjusted p-value representing statistical significance between the KO cell line and NC.
We applied CRISPR/Cas9 gene editing to target ATG5 and ATG7 to get HCT 116-knockout ATG5 and HCT 116-knockout ATG7 (Figure 1B).41 Whole transcriptomic analysis in those KO cell lines captured multiple differentially upregulated pathways in ATG5 KO and ATG7 KO as shown in Figure 1C, 1D, respectively. The top shared genes among the upregulated pathways are shown in Figure 1E, 1F. Remarkably, KLF3 transcriptional factor was found to be the top upregulated gene in both KO cell lines (Figure 1G).41
We utilized GEPIA2 to investigate the expression levels of KLF3/KLF8 in different stages of colorectal cancer (CRC). As illustrated in Figure 2A, KLF3 expression was significantly increased in stages II and III compared to stage I. KLF8 expression significantly increased in the late tumor stages specifically stage IV (Figure 2B). Subsequently, we examined the methylation status of the promoter regions of KLF3 and KLF8 genes in various stages of CRC tumors and different stages of nodal metastasis using the UALCAN web tool. Our analysis revealed that the methylation level of the KLF3 promoter was significantly decreased in stage 4 of CRC tumors (Figure 2C). It showed significant change across N0 and N2 stages of lymph node invasion (Figure 2D). The methylation level of the KLF8 promoter was significantly decreased in stage 4 of CRC tumors and in late stages of lymph node invasion (Figure 2E, F). Overall, these findings suggest that KLF3 and KLF8 are associated with the progression of CRC, specifically in the later tumor stages.
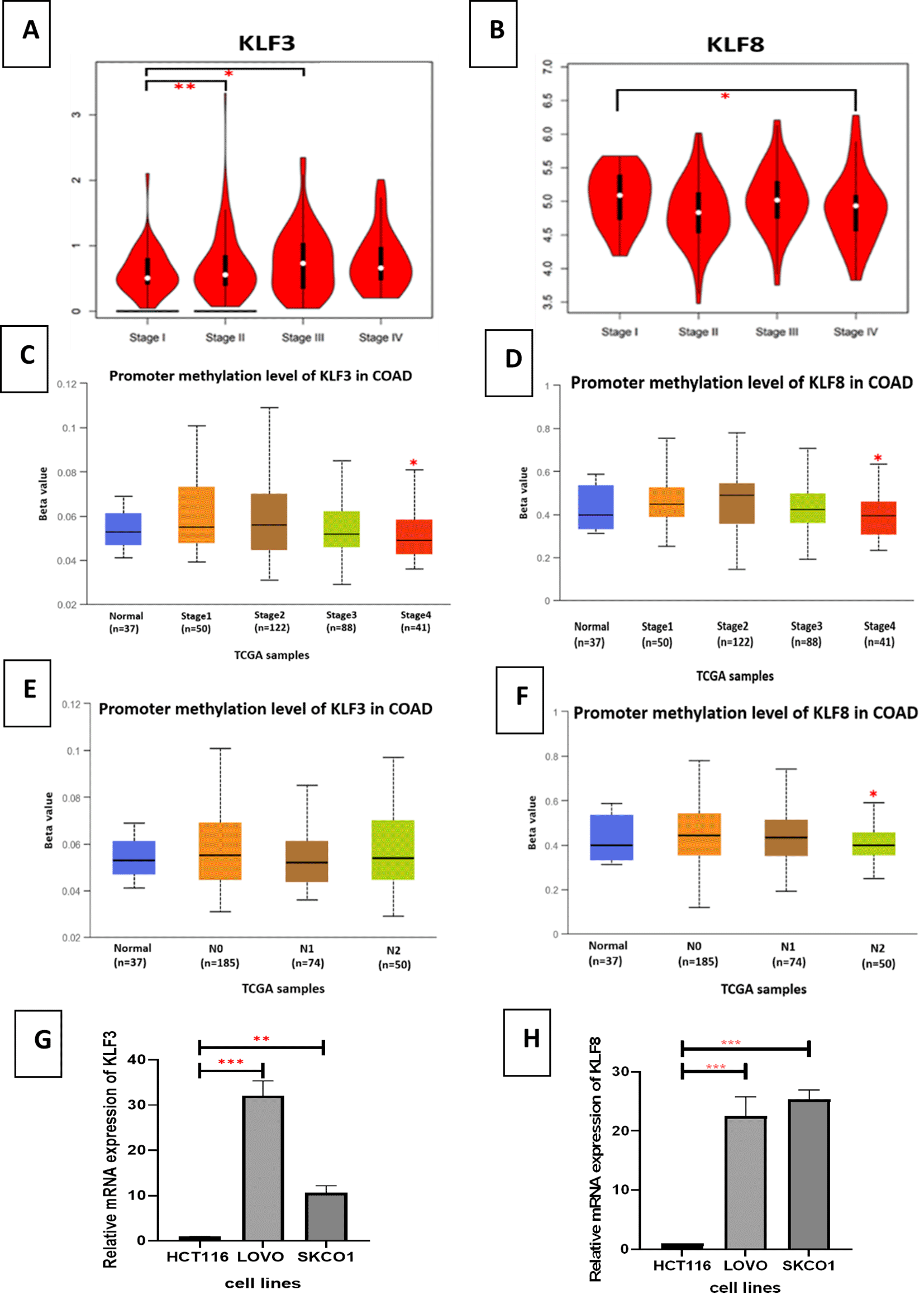
(A) KLF3 expression level in CRC stages: using GEPIA2 web server that provide RNA-seq from TCGA data set. (B) KLF8 expression level in CRC stages: using GEPIA2 web server that provide RNA-seq from TCGA data set. Y-axis represents the expression level of each gene as (log2(TPM+1)).TPM: Transcript per million.
(C) KLF3 promotor methylation level in different stages COAD, (D) KLF8 promotor methylation level in different stages COAD, (E) KLF3 promotor methylation level in different stages of the lymph node metastasis, (F) KLF8 promotor methylation level in different stages of the lymph node metastasis. This is done using UALCAN web tool.
(G) KLF3 gene expression comparison in different CRC cell lines: HCT 116, LoVo and SK-CO-1, (H) KLF8 gene expression comparison in different CRC cell lines: HCT 116, LoVo and SK-CO-1. Gene expression was analyzed using the Comparative Ct (ΔΔCt). Results are presented as mean (± SEM) relative to unstimulated control. The values were compared across the different groups using ANOVA test. ∗∗p < 0.01, ∗∗∗p < 0.001.
To validate the expression of KLF3 and KLF8 in CRC cells specifically in different tumor stages, we utilized metastatic cell lines LoVo and SK-CO-1 to detect KLF8 and KLF3 expression compared to the parental HCT 116 cell line via qPCR. We found a significant increase in the expression of KLF3 and KLF8 in both cell lines, compared to the HCT 116 cell line (Figure 2G, H).
To further investigate the relationship between autophagy genes and KLF3/KLF8 in colorectal cancer (CRC), we employed the TIMER2 web tool. Given our observation of distinct expression patterns of KLF3 in ATG5-/- and ATG7-/- knockout cells, our objective was to investigate the possible correlation and co-expression between autophagy genes and KLF3/KLF8 in CRC. The results, shown in Figures 3A, revealed significant positive correlations between KLF8 and majority of the autophagy genes, including ULK1, ULK2, beclin1, LC3B, ATG5, ATG7, AMBRA1, and most notably, UV radiation resistance associated gene (UVRAG) in both colon adenocarcinoma (COAD) (Figure 3A).41 Similarly, KLF3 exhibited significant positive correlations with ULK1, ULK2, BECN1, MAPLC3B, ATG5, ATG7, AMBRA1, and UVRAG in COAD (Figure 3B). These findings suggest a strong correlation between KLF3/KLF8 and the expression of autophagy genes in CRC.
Co-expression of autophagy genes and EMT genes
Previous studies have indicated that autophagy promotes tumor progression and overcoming metabolic stress caused by rapid proliferation, hypoxia, and limited nutrient supply.7,8 Moreover, autophagy has implications in the multistep process of epithelial-to-mesenchymal transition (EMT).42,43 Therefore, we utilized the TIMER2 web tool to explore the potential relationships and co-expression patterns between autophagy genes, KLF3/KLF8, and EMT-related genes such as CDH1, CDH2, CTNNB1, SNAI1, SNAI2, VIM, and ZEB1, which represent E-cadherin, N-cadherin, Beta-Catenin, SNAIL1, SLUG, Vimentin, and ZEB1 markers, respectively.
As depicted in Figure 4, both ULK1 and ULK2 exhibited significant positive correlations with Beta-Catenin, SNAIL1, SLUG, Vimentin, and ZEB1 and showed relatively lower positive correlations with E-cadherin. Furthermore, beclin1 and LC3B were significantly positively correlated with ZEB1, Beta-Catenin, and SNAIL1. ATG7 and ATG5 showed significant positive correlations with ZEB1, Beta-Catenin, SLUG, Vimentin, and E-cadherin, while ATG10 exhibited a significant positive correlation with Beta-Catenin alone. Notably, UVRAG displayed substantial positive correlations with Beta-Catenin, SLUG, Vimentin, SNAIL1, and notably the highest positive correlation with the crucial EMT marker, ZEB1. These findings align with the idea that autophagy strongly correlates with the EMT process in CRC metastasis. Importantly, UVRAG, which exhibits the most significant expression correlation with KLF8, also displays the highest positive correlation with the key EMT marker, ZEB1. This indicates a substantial correlation between UVRAG, KLF8, and the EMT process.
Similarly, significant correlations were observed between KLF3, KLF8, and multiple EMT genes. As depicted in Figure 5A, KLF3 exhibited a significant positive correlation with Beta-catenin and ZEB1. At the same time, KLF8 displayed significant positive correlations with Beta-catenin, SLUG, N-cadherin, Vimentin, and notably, the highest positive correlation was observed with ZEB1 (Figure 5B). Interestingly, KLF8 was negatively associated with CDH1 gene coding for E-cadherin. These findings suggest that both autophagy and the Krüppel-like family, including KLF3 and KLF8, play a significant role in the Epithelial-Mesenchymal Transition (EMT) process in colorectal cancer (CRC). The correlations between these genes and EMT-related markers provide further evidence of their involvement in the EMT pathway, which is crucial for CRC metastasis and progression.
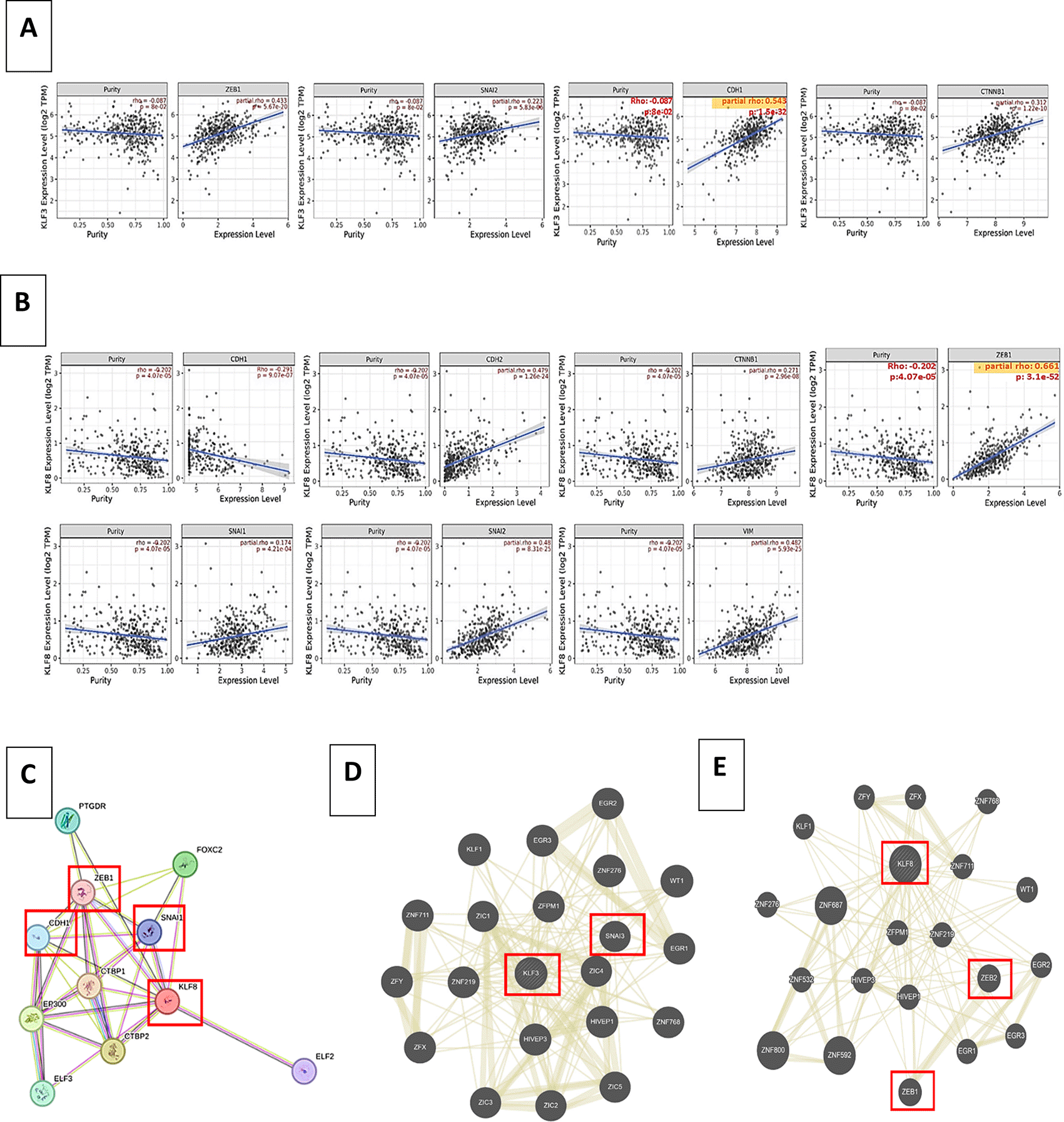
(A) Spearman’s rank correlation coefficient obtained from TIMER 2 web tool of KLF3 and EMT markers co-expression in COAD, (B) Spearman’s rank correlation coefficient obtained from TIMER 2 web tool of KLF8 and EMT markers co-expression in COAD, (C) KLF8-protein interaction using STRING, (D) Shared protein domains associated with KLF3 using GeneMANIA, (E) Shared protein domains associated with KLF8 using GeneMANIA.
Similarly, using the web-based tool STRING, we performed 8-protein interactions. Interestingly, we found that KLF8 expression is positively associated with multiple EMT markers, including zeb1 and snai1 (Snail) (Figure 5C). Moreover, Using GeneMANIA, a protein-protein interaction network further confirmed the direct correlation of KLF3 and KLF8 EMT markers as illustrated in Figure 5D and 5E, respectively.
After investigating the status of KLF3/KLF8 co-expression with autophagy genes as well as EMT markers, we utilized GSCALite to determine the molecular functions of these genes in COAD (Figure 6A, B) and across all cancer types (Figure 6C, D). We found significant correlations in COAD between specific autophagy genes and essential molecular functions. The AMBRA1 gene showed a significant correlation with apoptosis inhibition. ULK2 and UVRAG genes were significantly correlated with activating the epithelial-mesenchymal transition (EMT). ATG7 gene displayed a significant correlation with activation of the RAS-MAPK pathway. Interestingly, ATG5 was correlated with EMT inhibition. Furthermore, in other cancer-related pathways across all cancer types, UVRAG exhibited positive correlations with the activation of DNA damage, estrogen hormone pathway, and receptor tyrosine kinase (RTK) pathway. These results, based on gene correlations with major regulators of cancer-related pathways, suggest that autophagy genes may play important roles in multiple cancer-related pathways.
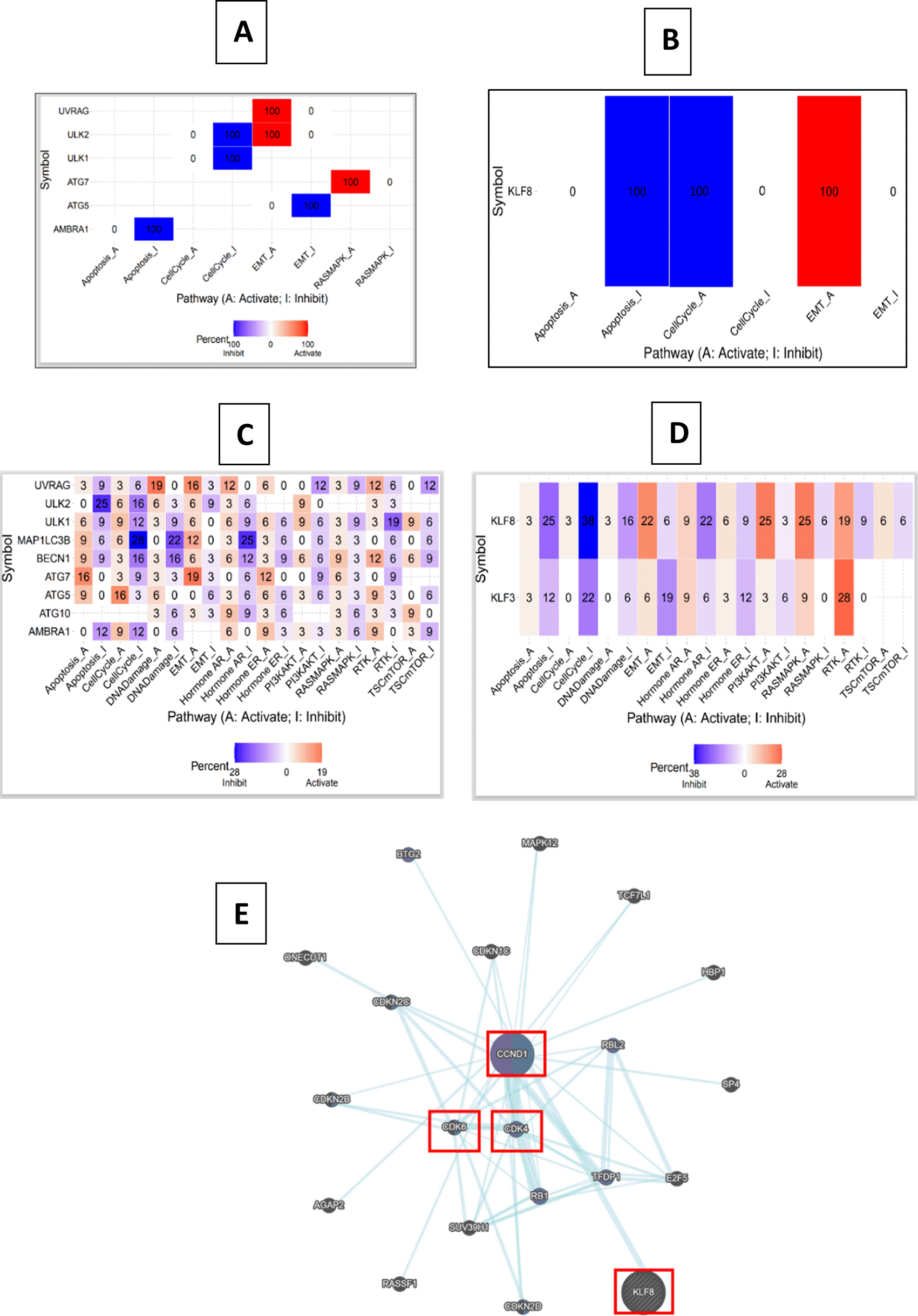
(A) COAD's predicted pathways of autophagy genes, (B) COAD's predicted pathways of KLF8, (C) Predicted pathways of autophagy genes in cancers, (D) Predicted pathways of KLF3/8 in cancers The color scale represents the percentage of activation and inhibition of different pathways in colorectal cancer, (E) Pathway-related genes associated with KLF8 using GeneMANIA.
In COAD, KLF8 gene exhibited significant correlations with apoptosis inhibition, cell cycle activation and EMT activation. Both KLF3 and KLF8 genes showed correlations with several cancer-related pathways. Specifically, KLF8 gene displayed positive correlations with the PI3-AKT, RAS-MAPK, and RTK pathways. Moreover, using GeneMANIA web tool (Figure 6E), pathways interactions in KLF8 showed enrichment of translational initiation of genes related to cell-cycle progression such as CDK4 and CDK6. Further, KLF8 was associated with CCND1which involved in promoting cell-cycle, invasion and metastasis.
These findings shed light on the molecular functions of autophagy genes and KLF3/KLF8 in COAD and across various cancer types. The correlations observed provide insights into the potential involvement of these genes in cancer-related pathways, suggesting their roles in key biological processes and signaling pathways associated with cancer development and progression.
CRC is divided into key pathogenic molecular subtypes. The first group is microsatellite instable (MSI) due to mismatch repair (MMR) gene abnormalities (16%) or DNA polymerase epsilon proof reading (3%). The second subtype is microsatellite stable (MSS), which accounts for 84% of CRC cases. MSS CRC is distinguished by a high rate of somatic mutations in multiple genes, including APC, TP53, PIK3CA, KRAS, and SMAD4. We used the web-based program GENT2 to investigate the gene expression of KLF3/KLF8 in various tumor subtypes (Figure 7A, B). Comparing MSI and MSS subtypes, we found that KLF3 showed relatively high expression in MSS molecular subtypes of CRC, whereas KLF8 showed significantly high in MSS.
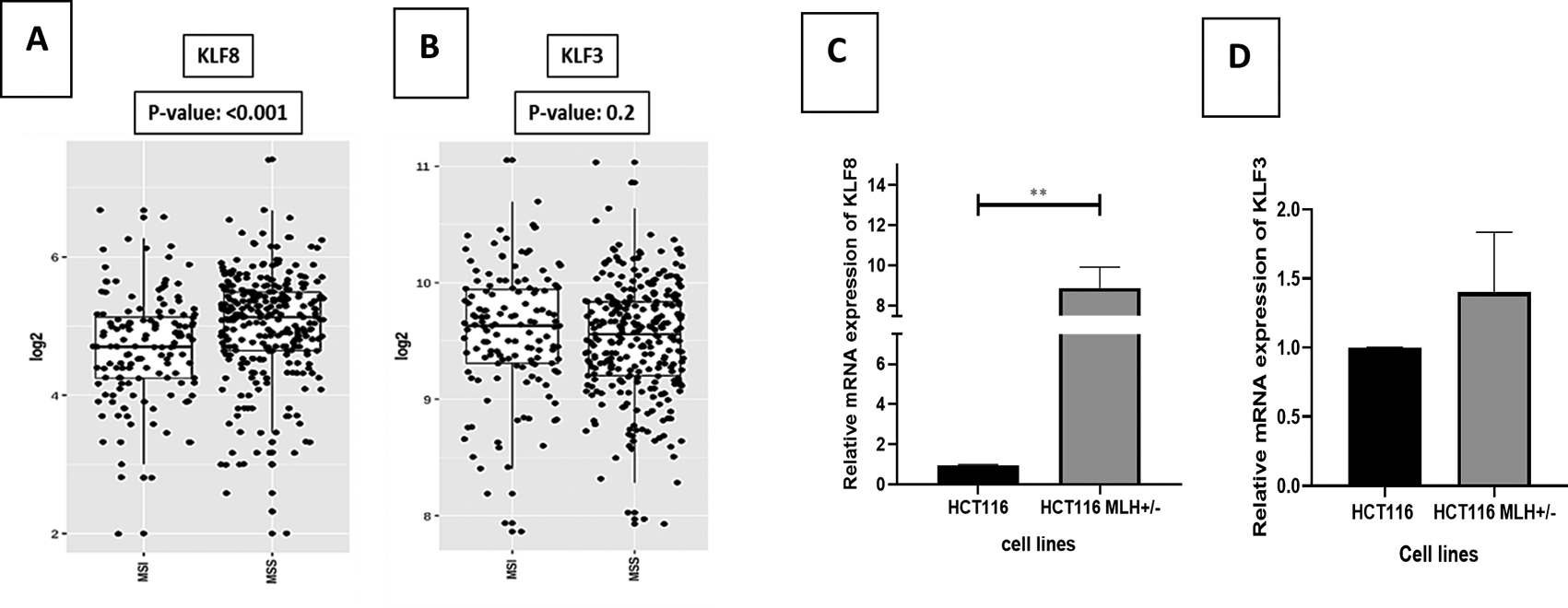
(A) KLF3 expression level in colon cancer molecular subtypes, (B) KLF8 expression level in colon cancer molecular subtypes. Using GENT2 web- based tool to denote data from NCBI GEO database, we enquire COAD and choose subtype as the targeted analysis. Two sample T tests were conducted by the GENT2 web tool, p < 0.005 was considered as significant. (C) KLF8 gene expression in HCT116 and its isogenic cell line HCT116 MLH+/-, (D) KLF3 gene expression in HCT116 and its isogenic cell line HCT116 MLH+/-. The values were compared across the different groups using unpaired t test. ∗∗p < 0.01. Results are presented as mean (± SEM) of mRNA expression.
Moreover, we included an isogenic cell line HCT 116 MLH1+/− with its parental HCT 116 cell line (MLH1−/−). We observed that MLH1 proficient cells were associated with high KLF8 expression but decreased KLF3 expression, compared to the wild-type MLH1 deficient (Figure 7C, D).
We conducted bioinformatics analysis using TIMER2 to assess the correlation between the expression of KLF3 and KLF8 genes and the cellular composition of the tumor microenvironment.44
KLF3 displayed significant positive correlations with CD8+ T cells, CD4+ T cells, neutrophils, Tregs, and NK cells. However, a significant negative correlation existed between KLF3 expression and M2 MCQ infiltration. KLF3 showed no significant correlation with MDSC (Figure 8A). Similarly, KLF8 exhibited significant positive correlations with CD4+ T cells, Tregs, endothelial cells, neutrophils, and M2-type macrophage quantification (MCQ) immune infiltration. However, KLF8 sowed significant negative correlations with the infiltration of CD8+ T cells, NK cells, and myeloid-derived suppressor cells (MDSC) (Figure 8B).
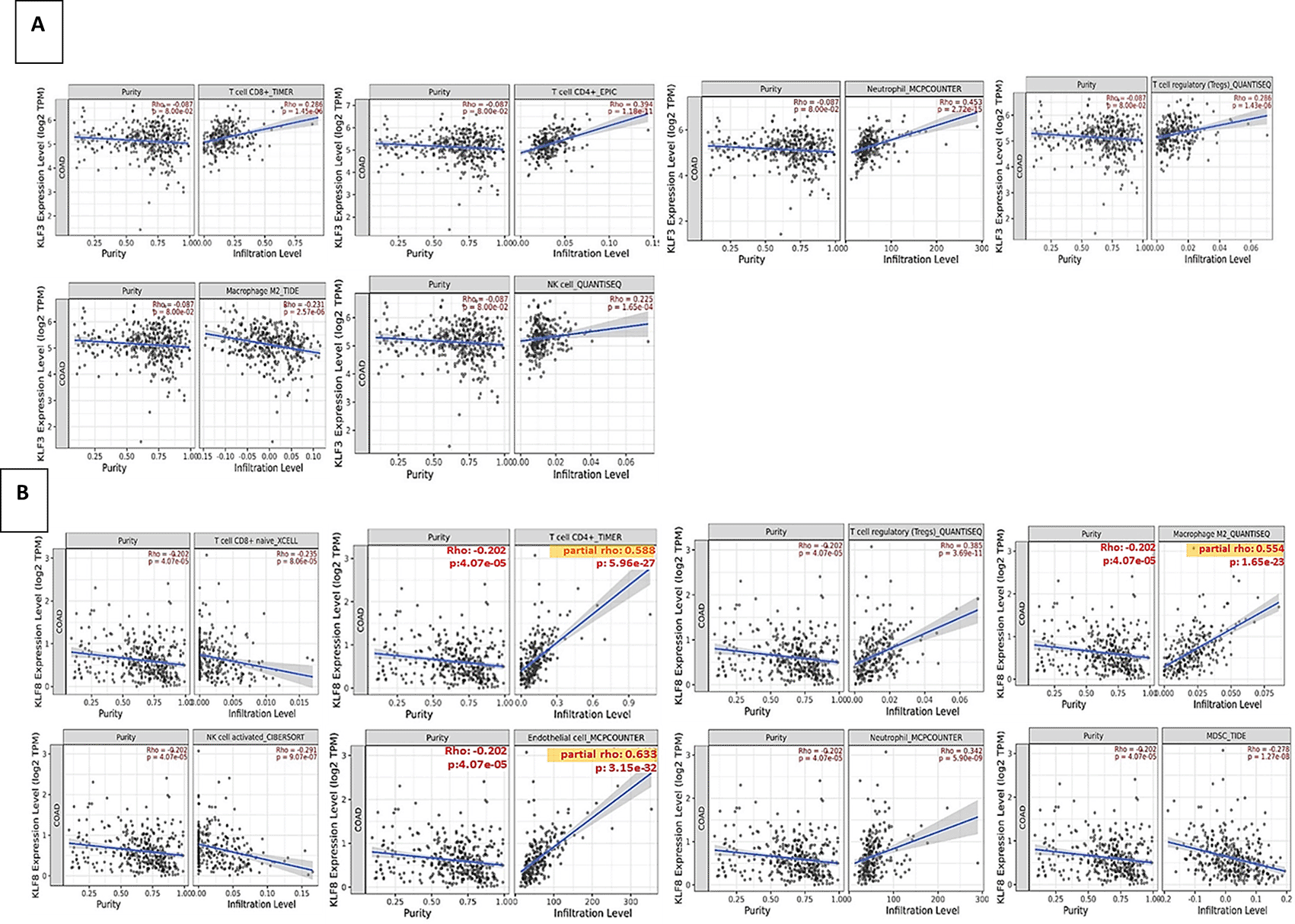
(A) KLF3 expression and immune infiltrating cells abundance of CD4+ T cells, CD4+ T cells, neutrophils, Tregs, M2 MCQ and NK cells.
(B) KLF8 expression and immune infiltrating cells abundance of CD4+ T cells, CD4+ T cells, neutrophils, Tregs, M2 MCQ, NK cells, endothelial cells and myeloid-derived suppressor cells (MDSC).
These findings provide insights into the relationships between autophagy genes, KLF3, KLF8, and various immune cell populations in the tumor microenvironment of CRC. The correlations observed highlight the potential roles of these genes in immune cell infiltration and the modulation of the tumor immune response.
CRC invasion and metastasis are major contributors to high mortality rates and tumor recurrence.45 Therefore, understanding the underlying processes involved in these malignant phenotypes is crucial to developing therapeutic targets and overcoming chemotherapy resistance.
Most CRC cell line were found to have a strong baseline autophagic flux. However, previous literature showed that HCT 116 showed a higher controlled autophagic flux compared to other CRC cell lines.40 Thus, we implemented our CRISPR/Cas9 experiments on HCT 116 cell lines specifically to study the effect of autophagy inhibition efficiently. Moreover, targeting ATG5/7, which are specific and crucial autophagy genes, is essential to block the conventional autophagy pathway. Specifically, in HCT 116, was associated with a dramatic decrease in cell survival when targeting ATG5 or ATG7.46 All gene editing techniques are predicted to have an off-target effect. From our side, we used the ribonucleoprotein-CRISPR Cas9 method, which has been proven to have a lower off-target effect compared to the plasmid delivery system since it limits the activity time for Cas9.47 The sgRNAs used were selected based on the top scores or high scores according to the Thermoscientific Fischer system of sgRNA designing. Using this system allowed us to produce a few knockout (KO) clones; however, one clone was taken further for downstream experimentation.
The relationship between autophagy and its role and interaction with KLF3 and KLF8 in CRC remains poorly characterized.48 Furthermore, the correlation between the KLF family, specifically KLF3 and KLF8, and autophagy has not been previously studied in tumors, presenting an opportunity for novel therapeutic approaches in CRC. In this study, we aimed to investigate the expression levels of multiple autophagy genes in relation to KLF3 and KLF8 in CRC, as well as their clinicopathological features, utilizing various web tools and large RNA sequence datasets obtained from cancer patients.
Our experimental investigation found that KLF8 and KLF3 expression was significantly higher in the late stages of CRC, indicating its strong association with enhanced EMT and metastasis. Specifically, the mRNA expression level of KLF8 was significantly higher in metastatic cell lines such as LoVo and SK-CO-1 compared to HCT 116 parental cell line, however, KLF3 expression was significantly higher in LoVo cell line. KLF8 has been found to play a role in modulating metabolism in gastric cancer by targeting GLUT4, thereby increasing glucose uptake crucial for cancer cell proliferation and metastasis.49 Likewise, KLF3 has been recognized as an oncogenic transcription factor in CRC with a significant impact on the regulation of tumorigenesis.50
Based on our transcriptomic analysis and the findings from our bioinformatics analysis, we have identified a strong correlation between the transcriptional factors KLF3 and KLF8 and the expression of multiple autophagy genes in CRC. This correlation has been addressed in cancer cells for the first time, providing valuable insights into the potential crosstalk between KLF3/KLF8 and autophagy in CRC. Interestingly, our investigation revealed that the inhibition of autophagy in ATG5-/- and ATG7-/- cell lines resulted in differential expression of KLF3, suggesting that it may act as a compensatory mechanism in response to autophagy suppression. It is worth noting that KLF3 and KLF8 are highly related transcriptional regulators that bind to similar DNA sequences and exhibit overlapping roles.51 Because autophagy is required for KLF3 degradation, its expression was elevated in our transcriptomic analysis in the KO cell lines. The 3-MA treatment was found to impede the turnover of KLF2 and KLF3.26 Moreover, according to PubChem database,; the two proteins have a high homology.52 Notably, KLF8 transcriptional repression activity is triggered by its interaction with CtBP.53 KLF8 performs transcriptional activation in addition to transcriptional repression. KLF8’s glutamine residues Q118 and Q248 are crucial for the protein’s transcriptional activation function.54
Autophagy has been implicated in the invasion and metastasis processes, prompting us to investigate the association between autophagy genes and different epithelial-mesenchymal transition (EMT) markers, which play a crucial role in the metastasis of colorectal cancer (CRC). Our analysis revealed a strong correlation between autophagy genes and various EMT markers, underscoring their interconnectedness in CRC progression. Furthermore, both KLF3 and KLF8 showed significant positive correlations with multiple EMT markers. Notably, KLF8 exhibited the highest association with the ZEB1 gene (p = 3.1e-52). This finding is particularly interesting, considering that UVRAG, an autophagy gene, displayed the strongest positive correlation with the critical EMT marker ZEB1. Remarkably, KLF8 is associated with decreased E-cadherin. Overexpression of KLF8 decreases E-cadherin expression, promoting invasion and serving as a possible EMT inducer. In non-small cell lung cancer, decreased KLF8 expression prevents malignancy through preventing cell invasion and EMT.55 Previous studies have shown that KLF8 induces FHL2-mediated invasion, EMT, and metastasis in CRC.56 In gastric cancer, KLF8 has been implicated in promoting invasion and metastasis via the EMT process by targeting the hypoxia-induced factor (HIF-1) gene.57 The observed correlations provide insights into the potential mechanisms underlying the role of autophagy and KLF8 in the invasion, metastasis, and EMT, offering opportunities for further exploration and potential therapeutic interventions targeting these pathways.
Consistent with our findings, previous studies have demonstrated the necessity of autophagy for EMT activation and metastasis in various cancer types. For example, autophagy is required for EMT activation and metastasis in hepatoblastoma cells58 as well as in TGF-β1-mediated EMT in non-small-cell lung carcinoma cells.59 Thus, despite the contextual relationship between the complex autophagy machinery and cancer, it is still a promising target, as there are numerous common regulatory pathways between autophagy and cancer. Tumor samples with high ULK2 and UVRAG were found to be significantly associated with mucinous colorectal adenocarcinoma, indicating unfavorable prognosis.
TME plays a crucial role in modulating the rate of invasion and metastasis in colorectal cancer (CRC). The interaction between CRC cells and their microenvironment is influenced by various factors, with the tumor microsatellite status (MSI vs. MSS) being one of the key determinants. Patients with MSS tumors generally have a worse prognosis compared to those with MSI tumors.60 Using the GENT2 web tool,34 our study observed that KLF8 expression was significantly higher in MSS tumors, while KLF3 expression was higher in MSI tumors. This finding was further validated through qPCR analysis, where we confirmed that the MLH1+/- cell line, representing the MSS subtype, exhibited significantly higher KLF8 expression than the HCT 116 cell line, representing the MSI phenotype. Importantly, KLF8 has been identified as a novel regulator of DNA damage response pathways in breast cancer.61 Ineffective DNA repair can lead to genomic instability, which impacts cellular functions and contributes to cancer development. KLF8 interacts with PARP-1 and other DNA damage regulators, forming a complex that influences cellular functions and alters DNA repair, thereby affecting genomic stability and contributing to enhanced cancer progression.61,62 However, further investigations are warranted to elucidate the specific role of KLF8 in the context of MSS phenotype in CRC. Understanding the involvement of KLF8 in regulating DNA repair pathways and its impact on genomic instability could provide valuable insights into the mechanisms underlying MSS CRC progression and potential therapeutic strategies.
The MSI and MSS subtypes have distinct immunogenic microenvironments besides variable genomic instability. It has been established that the MSI tumors had more immune infiltrating cells than the MSS tumors, which typically have a higher abundance of immune infiltrating cells than MSS tumors.63 Among these immune cells, cytotoxic CD8+ T-lymphocytes (CTL) are considered the primary defense mechanism against tumor cells. The abundance of T cells is a significant and important prognostic indicator for the effectiveness of immunotherapy and chemotherapy.64 Regulatory T-cells (Tregs) are another crucial form of T-cells closely associated with colorectal tumors.
High infiltration of immune cells, including CD8+, CD4+, neutrophils and NK cells, was correlated with KLF3 expression in our analysis. In contrast, KLF8 expression was significantly negatively correlated with CD8+ and NK cells, whereas it was significantly positively correlated with Tregs and M2-type macrophages. These results suggest that KLF3 and KLF8 can potentially serve as prognostic indicators for immunotherapy efficacy. The differential associations of these transcription factors with specific immune cell populations highlight their potential role in shaping the tumor microenvironment and influencing the response to immunotherapy in CRC.
Autophagy has a major influence on the tumor-specific CD8+ T cells65 and is found to be crucial for the activity of effector and memory T-cells.66 However, the role of autophagy in tumor immunity is complex and can have both promoting and inhibitory effects on tumor progression. Autophagy has been shown to contribute to the degradation of cytolytic granules secreted by cytotoxic CD8+ T cells and natural killer (NK) cells, thereby dampening their antitumor activity.67,68 Additionally, autophagy plays a role in the immunosuppressive function of regulatory T (Treg) cells, which rely on autophagy for their suppressive effect.69 In the tumor microenvironment, tumor-associated macrophages (TAMs) of the M2 phenotype have been shown to promote tumor growth, angiogenesis, and metastasis.70
Conversely, M1 macrophages have been implicated in inhibiting tumor progression.71 Autophagy has been found to participate in the production and polarization of macrophages, with Toll-like receptor-2 (TLR2) deficiency resulting in impaired autophagy and the generation of M2-type macrophages that support tumor progression.72 Furthermore, autophagy induction in TAMs has been shown to promote apoptotic cell death, restrain proliferation, and enhance radiosensitivity in colorectal cancer.73 These findings highlight the complex role of autophagy in modulating the tumor microenvironment and its impact on cancer progression. It is important to note that the role of autophagy in cancer is dynamic and can vary depending on the specific context and stage of tumor development. While our bioinformatics analysis using the TIMER web tool explored the correlation between immune infiltrating cells and autophagy genes in a comprehensive CRC patient dataset, further studies are needed to investigate the specific subtypes and stages of CRC to gain a deeper understanding of the relationship between autophagy and the immune microenvironment in different contexts.
In colorectal cancer (CRC), the differential expression of autophagy genes is likely regulated through epigenetic mechanisms rather than gene mutations. In the presence of microsatellite instability (MSI), alterations in the crucial autophagy gene UVRAG can lead to truncated mutations, resulting in dysregulated UVRAG expression and subsequent tumor growth.74 Similarly, KLF8 has been found to have an amplification mutation and higher mRNA levels in CRC compared to KLF3. To further investigate the regulation of gene expression, we evaluated the promoter methylation levels of KLF3 and KLF8. Promoter methylation is known to affect mRNA expression by influencing the accessibility of DNA to transcriptional machinery. Previous studies in prostate cancer cells have shown a negative association between DNA methylation at the promoter region of various KLF genes, including KLF3 and KLF8, and their mRNA expression levels.75 In our analysis, we observed a significant decrease in KLF3 promoter methylation levels in late CRC stages. In contrast, KLF8 promoter methylation levels were significantly decreased in stage 4 CRC and late stages of lymph node metastasis.
Autophagy, in this context, promotes cell survival and progression by suppressing cell death.28,76 In various cancers, including colorectal cancer (CRC), a significant correlation has been observed between the expression of autophagy genes and the activation of the RAS-MAPK pathway. Specifically, ATG7 has been found to correlate with the activation of the RAS-MAPK pathway in CRC. Furthermore, autophagy has been implicated in activating epithelial-to-mesenchymal transition (EMT), a critical process in tumor progression and metastasis. While many autophagy genes are generally correlated with EMT activation in different cancer types, specific to CRC, UVRAG and ULK2 have shown a significant correlation with EMT activation. These findings highlight the intricate relationship between autophagy and key signaling pathways, such as RAS-MAPK and EMT, in promoting cancer development and progression. Targeting autophagy, particularly in the context of these specific pathways, may hold promise for future therapeutic interventions in CRC and other cancers.
One of the most potent inducers of EMT is transforming growth factor-beta (TGF-β), which activates both Smad and non-Smad signaling pathways. Notably, KLF8 has been identified as one of the downstream targets of TGF-β signaling.77 Inhibition of extracellular signal-regulated kinase (ERK) signaling has been shown to reduce both KLF8 expression and cellular motility.78
The primary limitation of our study lies in its reliance on a single cell line, HCT 116 to perform CRISPR/Cas9 gene editing technique to knockout ATG5/7. However, we used several cell lines in our experiments. Generalizing our findings to broader biological scenarios should be approached with caution, recognizing the need for further investigation across multiple cell lines to enhance the robustness and applicability of our results.
The current study highlights the potential therapeutic significance of targeting both KLF8 and autophagy in treating metastatic colorectal cancer (CRC), in particular, MMR deficient subtypes. A synergistic effect may be achieved by simultaneously inhibiting KLF8, which is implicated in cellular motility, proliferation, and transcriptional regulation, and autophagy, which has been linked to tumor progression and resistance to chemotherapy. This combined approach could potentially enhance treatment efficacy by targeting multiple pathways involved in CRC metastasis and chemoresistance. Further studies are warranted to explore the feasibility and effectiveness of this therapeutic strategy in preclinical and clinical settings.
Eglal Mahgoub: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jalal Taneera: visualization and revision. Samrein Ahmed: for CRISPR-Cas9 gene editing experiments and data analysis. Shirin Hafezi: Experimental work (CRISPR-Cas9) and data analysis. Thenmozhi Venkatachalam: Transcriptomics analysis, Methodology, writing. Mahmood Hachim: Bioinformatics analysis. Nabil Sulaiman: Reviewing. Rifat Hamoudi: Bioinformatics Analysis and revision. Maha Saber-Ayad: Conceptualization, Writing – review & editing, Supervision, Resources, Methodology, Project administration, Funding acquisition.
Figshare: Unraveling the interplay of autophagy genes and KLF3/KLF8 in Colorectal cancer metastasis: A bioinformatics and cellular exploration, https://doi.org/10.6084/m9.figshare.25655457.v2. 41
This project contains the following underlying data:
• Data file: The data is whole transcriptomics analysis (RNAseq) of 7 samples of isogenic colorectal cancer cell line HCT116 (parental wild HCT116, HCT116_negatic control, HCT116_Knocked out for ATG5, HCT116_Knocked out for ATG7). The cells were knocked out using Caspase Cas9 (ribonucleoprotein). RNA-seq data were analyzed using the Ion Torrent Software Suite version and the alignment was carried out using the Torrent Mapping Alignment Program (TMAP).
• Data file: Full western blot gel for protein expression of ATG5 and ATG7 in HCT 116, HT29, LoVo and SK-CO-1).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Colorectal cancer genomics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Reggiori F, Klionsky DJ: Autophagic processes in yeast: mechanism, machinery and regulation.Genetics. 2013; 194 (2): 341-61 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Cell signalling; cancer
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Jul 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)