Keywords
heat polymerized acrylic resin, avocado seed extract, mono-species biofilm, polymicrobial biofilm, denture plaque, MBIC
This article is included in the Antimicrobial Resistance collection.
Heat polymerized acrylic (HPA) resins are known to have high porosity that contributes to increased surface roughness and microcrack formation in stress areas. This facilitates the attachment and growth of polymicrobial biofilms contributing to increased antimicrobial resistance. Many research had been carried out on avocado seeds, but no research that studies the effect of avocado seeds on denture-plaque microorganism biofilm on HPA resin has been found.
This study used 144 samples (n=144), namely HPA resin discs covered with mono-species and polymicrobial biofilms consisting of Candida albicans, Candida glabrata, Actinomyces odontolyticus, Streptococcus gordonii, and Staphylococcus aureus. The discs were soaked for 8 hours in the 5%, 10%, 15%, 20% avocado seed extract, positive control (alkaline peroxide), and negative control (aquadest). Each disc was shaken with a vortex mixer for 1 minute, and 100 μL was added into 96-well microplates with three times repetition and incubated for 24 hours. The inhibition values were determined from the percentage inhibition value formula which required absorption values from a microplate reader (595 nm).
In this research, it was found that the MBIC50 of avocado seed extract against the mono-species of C. albicans (5%), C. glabrata (5%), A. odontolyticus (15%), S. gordonii (15%), S. aureus (10%), while against the biofilm was 20%. There was a significant effect of soaking HPA resin in avocado seed extract of 5%, 10%, 15%, 20% on the inhibition of mono-species and polymicrobial biofilms of denture-plaque microorganisms with a value of p<0.001 (p<0.05).
The MBIC50 of avocado seed extract in polymicrobial biofilm group was higher than that in the mono-species biofilm groups. Although alkaline peroxide showed higher inhibition value than that of the MBIC50 in polymicrobial biofilm group, 20% avocado seed extract was effective in inhibiting polymicrobial biofilm because it was able to inhibit more than 50% polymicrobial biofilm.
heat polymerized acrylic resin, avocado seed extract, mono-species biofilm, polymicrobial biofilm, denture plaque, MBIC
The denture base is a part of the denture which rests on the supporting tissue and serves as a place for the arrangement of tooth elements.1 Denture base materials vary greatly, but the most commonly used and popular material is polymethyl methacrylate acrylic resin (PMMA) with more than 95% of fabricated denture bases are made from acrylic resin.2,3 Acrylic resin itself has various types, one of which is heat polymerized acrylic resin.4 Heat polymerized acrylic (HPA) resin has better strength properties and a higher degree of polymerization, less residual monomer, and more stable color.5,6 However, it still has limitations, some of which have porous properties and high surface roughness which can increase the attachment of fungal and bacterial biofilms.4
Colonization in a biofilm requires strong attachment of oral microorganisms by integrating into the salivary pellicle to form plaque on the denture material. Surface roughness and surface free energy are two factors that can promote plaque development.7 Surface roughness of acrylic resin can be reduced by adequate polishing. However, this cannot prevent the build-up of plaque on the denture due to the presence of microporosity in the acrylic resin which cannot be completely avoided.4 This area of porosity becomes an environment which can protect microorganisms in the biofilm.7 In addition, the abiotic surface of the denture causes less exposure of the denture biofilm to the host immune system so that microorganisms can grow without hindrance and have sufficient time to develop into plaque with varying compositions.8
O’Donnell et al. (2015) stated that the composition and diversity of dental plaque was different from denture plaque. Denture plaque in the oral cavity was found to be colonized by Candida spp. against the denture surface which co-aggregated with bacteria in the oral cavity.8 As many as 60% to 100% of denture wearers were found to carry Candida in the oral cavity in higher quantities compared to those who did not wear dentures.8,9 The commonly found Candida species in denture plaque is Candida albicans. Another Candida species that is found in denture plaque and increases with age is Candida glabrata. Together with C. albicans, these two fungal species can form more pathogenic and invasive biofilms and increase the severity of denture stomatitis.8 Several studies have found that denture plaque compared to dental plaque has a higher proportion of obligate anaerobic Actinomyces spp., a low proportion of Gram-negative rods, and the common presence of Staphylococcus aureus a.10 Shi et al. (2016) found that the genus of bacteria which was most commonly found on both surfaces of denture teeth and remaining natural teeth was the genus Actinomyces, followed by Streptococcus, Veillonella, Capnocytophaga, Neisseria, Prevotella, and Corynebacterium.11 Based on the genus mentioned, the bacterial species which will be used in this study were Actinomyces odontolyticus, Staphylococcus aureus, and Streptococcus gordonii.
The presence of these three bacteria in dentures can increase the virulence of C. albicans thereby increasing damage and invasion of mucosal tissue which increases the risk of denture stomatitis. Morse et al. (2018) found a significant increase in tissue damage from mixed Candida and bacterial biofilms where the composition of the biofilm was broadly the composition of denture plaque.8,12 The difference between biofilms and planktonic bacteria or fungi is that biofilms are a community of microbial cells enveloped in a matrix, while planktonic bacteria or fungi do not have this matrix layer. The presence of matrix can cause failure of treatment with antimicrobial agents, relapse of infection, and increased mortality.13 Penetration of antimicrobial agents can be complicated due to the formation of extracellular polysaccharides (EPS) which reduce the permeability of the biofilm thereby protecting microorganisms in the deepest layers of the biofilm from antimicrobial agents, minor mechanical stress, and host immune response.14,15 To determine the inhibitory effect of an antimicrobial agent on biofilm formation, it can be done using the Minimum Inhibitory Biofilm Concentration (MBIC), which is almost the same as the MIC. The difference between the two is that MBIC is defined as the lowest concentration of an antimicrobial agent at which there is no time-dependent increase in the average number of cells capable of surviving in the biofilm. Meanwhile, MIC is defined as the lowest concentration of an antimicrobial agent against planktonic microorganisms.13
To prevent the accumulation of denture plaque, adequate and routine denture cleaning needs to be done. Denture cleaning can be done chemically using alkaline peroxide type denture cleaning agent. However, alkaline peroxide was found not to show stable biofilm cleaning efficacy with previous studies showing that alkaline peroxide was not effective in cleaning biofilm and was only effective in cleaning new plaque.16,17 Therefore, it is necessary to develop a denture cleanser product in solution preparation with natural ingredients that have antimicrobial effects which can effectively clean denture plaque. One example of natural ingredient that can be used as an antimicrobial and antibiofilm agent is avocado seeds.
Avocados (Persea americana Mill.) are one of the most popular types of fruit among Indonesian and are widely used as food ingredients (salads, sandwiches, cakes) and drinks (juice, ice cream), cosmetic ingredients, medicines and ornamental plants.18 However, avocado seeds have no practical use and have not been utilized optimally so they tend to be an organic waste.19 Avocado seed can actually be used as an antimicrobial agent because of the higher amounts of phytochemical components contained in avocado seed, namely flavonoids, tannins, saponins, and alkaloids, than in avocado skin and pulp, which are 64% in seed, 23% in skin, and 13% in pulp.20,21 The inhibitory effects of avocado seed extract has been studied. Anggraini et al. (2017) studied the inhibition zone of avocado seed extract at concentrations of 10%, 20%, 40%, 80%, 100% on the growth of C. albicans, and found that the 10% concentration was the most effective concentration in inhibiting C. albicans.22 Another study by Talib et al. (2018) tested the effectiveness of avocado seed extract in inhibiting Streptococcus mutans at concentrations of 2%, 4%, 6%, 8%, 10% and found that the most effective concentration was 10%.23
However, most studies using avocado seed extract were carried out on planktonic bacteria or fungi, which is different from denture plaque in the patient’s oral cavity, which is a polymicrobial biofilm that tends to be more resistant to antimicrobial agents. This can be seen in a study by Hamzah et al. (2019) who found an increase in the minimum inhibitory concentration of tannin in polymicrobial biofilms (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans) when compared to the concentration in mono-species biofilms. The minimum inhibitory concentration of tannin in mid-phase polymicrobial biofilms (24 hours) is 1%, while the minimum inhibitory concentration of tannins in mono-species biofilms (24 hours) varies widely, namely E. coli (0.125%), S. aureus (0.5%), P. aeruginosa (0.25%), C. albicans (0.5%).24 Hence, this study aimed to determine the effect of avocado seed extract (Persea americana Mill.) with concentrations of 5%, 10%, 15%, 20% on denture-plaque microorganisms, which were Candida albicans, Candida glabrata, Actinomyces odontolyticus, Streptococcus gordonii, and Staphylococcus aureus, in the form of mono-species and polymicrobial biofilms on HPA resin.
Avocados were obtained from Brastagi, Karo Regency, North Sumatra Province, Indonesia. The avocado fruit used in this research has been determined by the Medanense Herbarium Plant Systematics Laboratory (MEDA) at the University of Sumatera Utara with letter number 1835/MEDA/2023.
This research used in vitro experimental methods with post-test only control group design. The sample used in this research was HPA resin in the shape of a disc with a diameter of 10 mm and a thickness of 2 mm (Figure 1). The number of samples used in this study was determined using Federer formula, hence the number of samples for each group was 4 samples. There were 6 treatment groups in this study which were avocado seed extract groups of 5%, 10%, 15%, 20%, as well as the positive control group (alkaline peroxide) and the negative control group (aquadest). As this research was conducted on mono-species biofilms: Candida albicans, Candida glabrata, Streptococcus gordonii, Actinomyces odontolyticus, Staphylococcus aureus, and on polymicrobial biofilm which is a combination of the five microorganisms, the final total amount sample that would be used in this research was 144 samples (n=144).
A disc-shaped brass metal master models with a diameter of 10 mm and a thickness of 2 mm were made to be used as a research sample mould. The dental cuvette, which had been smeared with Vaseline, was poured with a type II blue dental stone gypsum mixture made with a ratio of 300 g of gypsum: 90 mL of water to fill the bottom cuvette while being shaken with a vibrator so that no bubbles were trapped in the mixture. The master models, which had been smeared with vaseline, were then placed in the dough in a cuvette, avoiding the surface of the master models being flat with the gypsum surface. The gypsum was left to harden for ± 30-45 minutes and then smeared with vaseline. The upper cuvette was attached to the lower cuvette and filled with the same gypsum mixture as described previously. After the plaster had hardened, the cuvette was opened and the master models were taken out to obtain a mould.
The surface of the mould was smeared thinly with cold mould seal and left for 10 minutes. The HPA resin mixture was prepared with a weight ratio of 2.5:1. When it reached the dough stage, the acrylic resin mixture was put into the mould, then covered with plastic cellophane along with the top cuvette. The cuvette was pressed slowly with a hydraulic press until the pressure reached 1000 psi. Excess dough was cleaned with dental lecron, then the cuvette was closed and pressed again with a pressure of 2200 psi. The cuvette was reopened and cleaned of excess acrylic resin mixture. The cuvette was closed again and locked with the cuvette bolts, then left for 30 minutes. The cuvette was inserted into a water bath filled with aquadest, then the temperature and time were set at 70°C for 90 minutes, then at 100°C for 30 minutes. After 30 minutes, the cuvette was left in the water bath until the water reached room temperature for the cuvette cooling process. The samples were removed from the cuvette, then the sharp parts and plaster residue were trimmed with a fraser bur and sand paper.
Avocado seeds were extracted using a maceration technique. The avocado seeds would be cut into slices which would be dried in a drying cabinet at a temperature of ±40°C for about 24 hours, then coarsely grounded and blended until they became a fine powder. The avocado seed powder was put into a vessel and poured with 70% ethanol solvent with a ratio of 1:10 (10 g: 100 mL), then stirred until evenly mixed and left for 1×24 hours protected from light while stirring periodically every 6 hours so that the solution was evenly mixed. The solution was filtered until macerate I was obtained and the remaining filtered dregs were subjected to a second maceration process. The results of macerate I and II would be mixed and transferred into a closed vessel, then left in a cool place protected from light for 2×24 hours. The extract was concentrated using a rotary evaporator at a temperature of ±50°C to evaporate the solvent until a thick extract was obtained. The thick extract was then made into a concentration of 5%, 10%, 15%, 20%.
The thick extract of avocado seeds was sent to the Pharmaceutical Biology Laboratory, University of Sumatera Utara, Medan for phytochemical examination and quantitative testing of phytochemical compounds.
The microorganisms used in this study were cultured and maintained under the following conditions. Candida albicans ATCC® 24433TM and Candida glabrata ATCC® 90030TM were each cultured on Sabouraud Dextrose Agar (SDA) with yeast nitrogen base supplemented with 100 mmol L−1 glucose and cultured at 37°C under aerobic conditions for 24 hours. Actinomyces odontolyticus ATCC® 10558TM was cultured on fastidious anaerobe agar with 5% (v/v) defibrinated bovine blood at 37°C under anaerobic conditions for 24 hours. Streptococcus gordonii ATCC® 10558TM was cultured on blood agar with 5% (v/v) defibrinated bovine blood at 37°C under aerobic conditions for 24 hours. Staphylococcus aureus ATCC® 25923TM was cultured on blood agar with 5% (v/v) defibrinated bovine blood and incubated at 37°C under aerobic conditions for 24 hours.
Sterile HPA resin discs were preconditioned for 24 hours by immersion in artificial saliva. The density of the microorganism cultures must be adjusted using a densitometer following the 0.5 McFarland standard, namely 1.5 × 108 CFU/mL for bacterial suspensions (A. odontolyticus, S. gordonii, S. aureus) and 1.0 McFarland standard, namely 3.0 × 108 CFU/mL for fungal suspensions (C. albicans and C. glabrata). The preconditioned discs were then placed aseptically into 24-microplates, and 100 μL of standardized microorganisms were added to each surface of the disc. Biofilm preparations carried out were mono-species biofilms for each microorganism studied (C. albicans, C. glabrata, A. odontolyticus, S. gordonii, S. aureus) and polymicrobial biofilms which were a combination of the five microorganisms studied. Sterile Dulbecco’s Modified Eagle Medium (DMEM) (supplemented with 50 mmol L-1 L-glutamine per liter) was added to a final volume of 2 mL in each plate. Culture media discs in 24-wells microplates were shaken on an orbital shaker for 30 minutes to homogenize the media and culture solutions, then incubated at 37°C for 24 hours.
HPA resin discs which had been grown by mono-species and polymicrobial biofilms would be treated with immersion in avocado seed extract of 5%, 10%, 15%, 20%, as well as positive control (alkaline peroxide) and negative control (aquadest) for 8 hours at room temperature. The discs were then cleaned with distilled water, then put into a test tube together with 5 mL of Mueller Hinton broth and each shaken with a vortex mixer for 1 minute. A total of 100 μL of test solution was taken from the dilution and added into 96-wells microplates with a repetition of three times. Microplates were incubated at 37°C for 24 hours. After incubation, the microplates were cleaned with distilled water and patted vigorously on a lab mat to remove as much distilled water as possible. As much as 125 μL of 1% crystal violet solution was added to each microplate to colour the formed biofilm and left for 15 minutes. The crystal violet solution was discarded, then cleaned with distilled water and patted hard on a lab mat. The stained biofilm plates were allowed to dry until the remaining water in the microplates evaporated, then 150 μL of 95% ethanol was added to each plate and left for 10 minutes. The absorption value (OD) reading was carried out with a microplate reader at a wavelength of 595 nm and the results were calculated using the percentage inhibition value formula of which Control OD was defined as negative control absorption value and Sample OD was defined as test sample absorption value.24
The treated sample which had an inhibition value of at least 50% of biofilm formation could be considered as the Minimum Biofilm Inhibitory Concentration (MBIC50).24
Univariate analysis was carried out to determine the average (mean) and standard deviation of the inhibition values for immersion of heat polymerized acrylic resin discs in each group. The conversion of absorption value to inhibition value in percentage is counted using the percentage inhibition value formula that had been coded in Excel 2021 software. The normality test was carried out using the Shapiro-Wilk test (p>0.05) and the homogeneity test was carried out using the Levene test (p>0.05). Data analysis was carried out using one-way ANOVA, which could be accompanied by Welch ANOVA on non-homogeneous data, to determine the effect of treatment in each group. Data were analyzed with IBS SPSS Statistics (RRID: SCR_016479) v.22.0 software and presented in tabulation and graphic form as mean and standard deviation. Significant differences were defined at p<0.05.
The SEM procedure was carried out at the USU Integrated Research Laboratory, Medan. HPA resin disc samples that had been preconditioned with artificial saliva were then grown with polymicrobial biofilm according to the previous biofilm formation procedure and given a soaking treatment in avocado seed extract. HPA resin disc samples that had biofilm grown on were cleaned with distilled water three times and fixed with 2.5% (w/v) glutaraldehyde in cacodylate buffer for about 6 hours. The wet sample was then coated with a thin layer of gold to make the sample conductive. Sample reading using SEM was carried out with a voltage of 5 kV.
In this study, there were 6 treatment groups consisting of samples of HPA resin discs soaked in avocado seed extract 5%, 10%, 15%, 20%, as well as a positive control (alkaline peroxide) and a negative control (aquadest). The HPA resin disc samples were grown with mono-species biofilms of C. albicans, C. glabrata, A. odontolyticus, S. gordonii, S. aureus and polymicrobial biofilms so that the number of samples in this study was 144 samples (n=144).
The following are the results of avocado identification by the Medanense Herbarium, University of Sumatera Utara.
Kingdom: Plantae
Division: Spermatophyta
Class: Dicotyledoneae
Order: Laurales
Family: Lauraceae
Genus: Persea
Species: Persea americana Mill.
Local Name: Avocado Seed
The phytochemical test on the ethanol extract of avocado seeds was done using specific reagents to determine the presence of secondary metabolite compounds which were alkaloids, flavonoids, glycosides, saponin, tannin, triterpenoids/steroids (Table 1). The test showed positive results of all the tested secondary metabolite compounds and none negative results.
The secondary metabolite compounds existing in the avocado seed ethanol extract could be further assessed by doing a quantitative analysis to determine the amount of the secondary metabolites in the sample extract which were flavonoids, phenol, saponin, and alkaloids (Table 2). The analysis showed a total amount of phenol (66,8157 mgGAE/g extract), total amount of flavonoids (4,0888 mgQE/g extract), total percentage of saponin (1,59%), and total percentage of alkaloids (1,22%).
Each sample in each group was repeated three times to obtain three absorption values (OD) which then using univariate analysis, the mean and standard deviation were obtained. The obtained absorption value was calculated using the percentage inhibition value formula with an inhibition value of 50% as a parameter for determining MBIC50 (Figure 2). Based on calculations, MBIC50 of avocado seed extract in mono-species C. albicans biofilm was 5% avocado seed extract. MBIC50 avocado seed extract in mono-species C. glabrata biofilm was 5% avocado seed extract. MBIC50 avocado seed extract in mono-species A. odontolyticus biofilm was 15% avocado seed extract. MBIC50 avocado seed extract in mono-species S. gordonii biofilm was 15% avocado seed extract. MBIC50 avocado seed extract in mono-species S. aureus biofilms was 10% avocado seed extract.
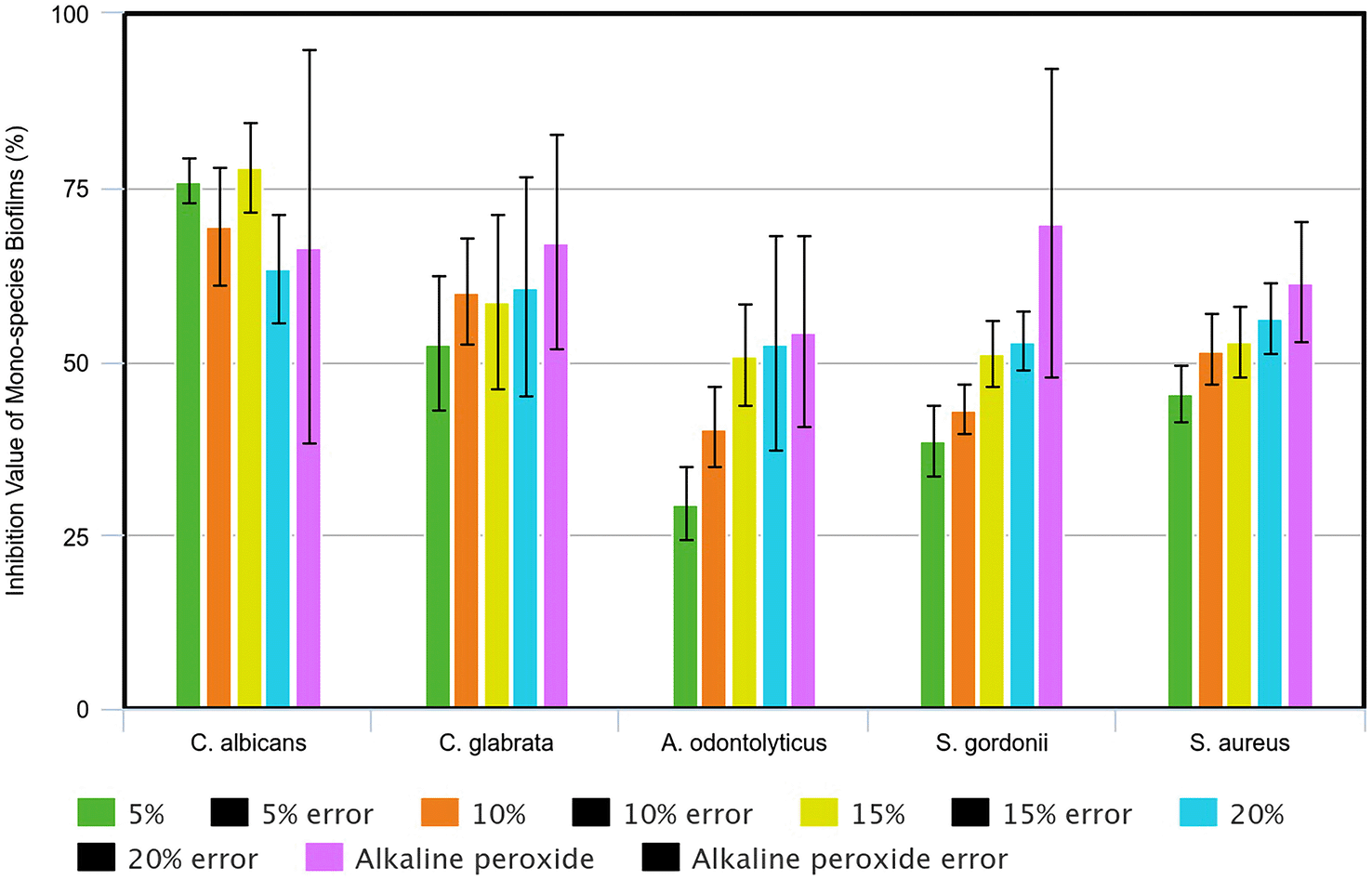
The sample was tested for normality and a value of p>0.05 was obtained, hence the data were normally distributed. Then, a homogeneity test was carried out by which the mono-species C. albicans biofilm sample obtained a value of p<0.001 (p<0.05), the mono-species A. odontolyticus biofilm sample obtained a value of p=0.002 (p<0.05), and the mono-species S. gordonii biofilm sample obtained a value of p≤0.001 (p<0.05) so the data were not homogeneous, while the mono-species C. glabrata biofilm sample obtained a value of p=0.054 (p>0.05) and the mono-species S. aureus biofilm sample obtained a value of p=0.116 (p>0.05) so the data were homogeneous. Data which was normally distributed and homogeneous was tested using one-way ANOVA and data which was normally distributed but not homogeneous was analysed using Welch ANOVA. This study found that mono-species biofilm samples of C. albicans, C. glabrata, A. odontolyticus, S. gordonii, S. aureus obtained a value of p≤0.001 (p<0.05) which indicated a significant effect of 5%, 10%, 15%, 20% avocado seed extract in inhibiting mono-species biofilms of C. albicans, C. glabrata, A. odontolyticus, S. gordonii, S. aureus (Table 3).
| Treatment groups | Absorption values | p |
|---|---|---|
| Mono-species C. albicans Biofilm | ||
| Avocado Seed Extract of 5% | 0.2949 ± 0.0397 | <0.001* |
| Avocado Seed Extract of 10% | 0.3782 ± 0.1057 | |
| Avocado Seed Extract of 15% | 0.2738 ± 0.0812 | |
| Avocado Seed Extract of 20% | 0.4527 ± 0.0968 | |
| Positive control (+) Alkaline Peroxide | 0.4140 ± 0.3489 | |
| Negative control (-) Aquadest | 1.2332 ± 0.1059 | |
| Mono-species C. glabrata Biofilm | ||
| Avocado Seed Extract of 5% | 1.0400 ± 0.2107 | <0.001* |
| Avocado Seed Extract of 10% | 0.8743 ± 0.1651 | |
| Avocado Seed Extract of 15% | 0.9057 ± 0.2735 | |
| Avocado Seed Extract of 20% | 0.8576 ± 0.3452 | |
| Positive control (+) Alkaline Peroxide | 0.7160 ± 0.3368 | |
| Negative control (-) Aquadest | 2.0627 ± 0.2138 | |
| Mono-species A. odontolyticus Biofilm | ||
| Avocado Seed Extract of 5% | 1.4803 ± 0.1093 | <0.001* |
| Avocado Seed Extract of 10% | 1.2479 ± 0.1185 | |
| Avocado Seed Extract of 15% | 1.0302 ± 0.1534 | |
| Avocado Seed Extract of 20% | 0.9946 ± 0.3225 | |
| Positive control (+) Alkaline Peroxide | 0.9595 ± 0.2909 | |
| Negative control (-) Aquadest | 2.0972 ± 0.1524 | |
| Mono-species S. gordonii Biofilm | ||
| Avocado Seed Extract of 5% | 1.0829 ± 0.0887 | <0.001* |
| Avocado Seed Extract of 10% | 1.0048 ± 0.0635 | |
| Avocado Seed Extract of 15% | 0.8602 ± 0.0834 | |
| Avocado Seed Extract of 20% | 0.8316 ± 0.0751 | |
| Positive control (+) Alkaline Peroxide | 0.5322 ± 0.3910 | |
| Negative control (-) Aquadest | 1.7614 ± 0.0895 | |
| Mono-species S. aureus Biofilm | ||
| Avocado Seed Extract of 5% | 0.9012 ± 0.0666 | <0.001* |
| Avocado Seed Extract of 10% | 0.7968 ± 0.0832 | |
| Avocado Seed Extract of 15% | 0.7779 ± 0.0832 | |
| Avocado Seed Extract of 20% | 0.7225 ± 0.0820 | |
| Positive control (+) Alkaline Peroxide | 0.6362 ± 0.1407 | |
| Negative control (-) Aquadest | 1.6475 ± 0.0884 | |
In this study, three absorption values (OD) of polymicrobial biofilm samples were obtained from which the mean and standard deviation were obtained using univariate analysis. Using the percentage inhibition value formula, the inhibition values were obtained for each group of avocado seed extract of 5%, 10%, 15%, 20%, and the positive control (alkaline peroxide) where the 50% inhibition value was set as a parameter for determining MBIC50 (Figure 3). Hence, the MBIC50 avocado seed extract in polymicrobial biofilm was 20% avocado seed extract.
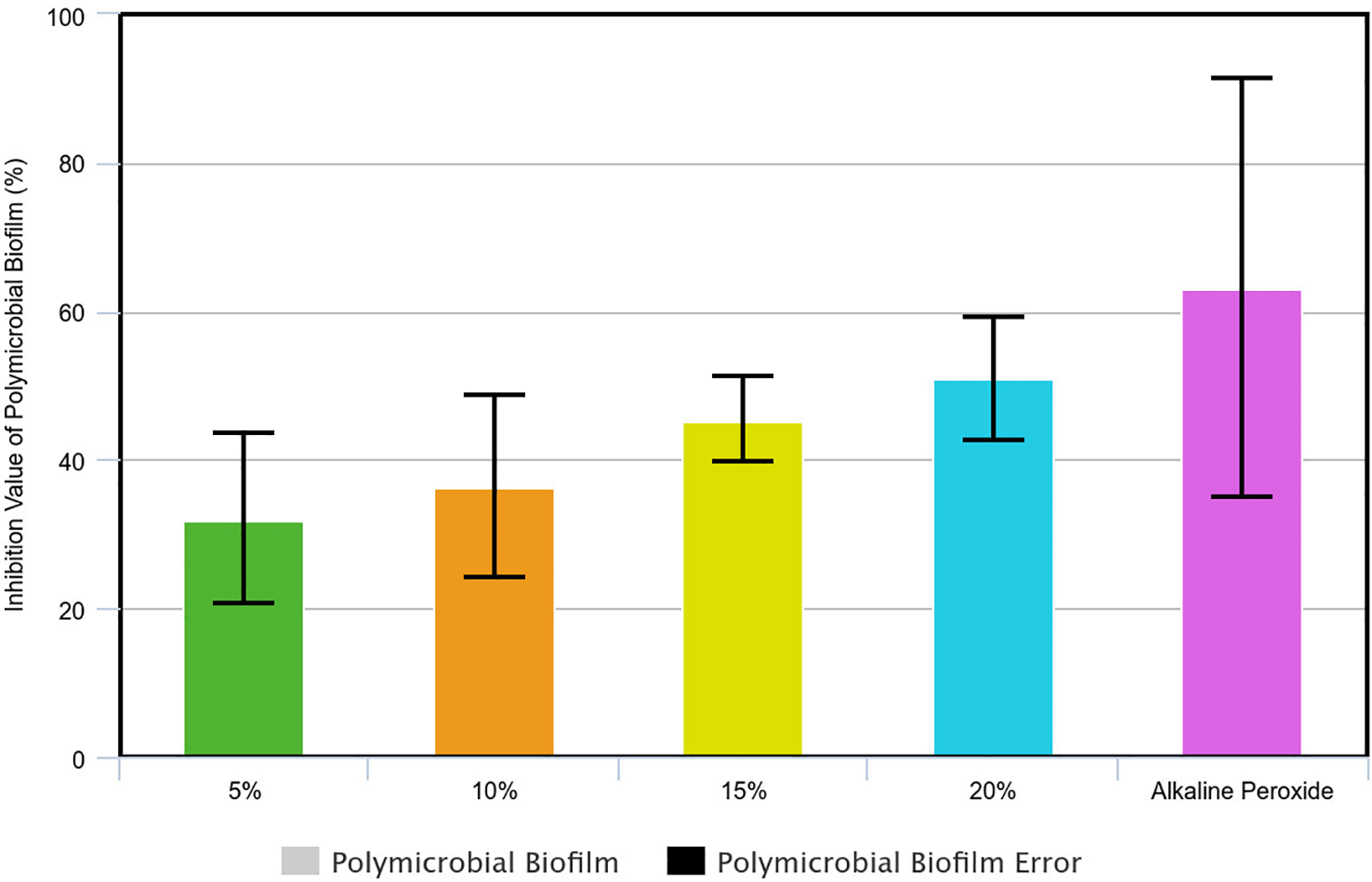
The sample was tested for normality and a value of p >0.05 was obtained, hence the data was normally distributed. Then, a homogeneity test was carried out by which the polymicrobial biofilm samples obtained a value of p=0.006 (p<0.05) so that the data was not homogeneous. Data that were normally distributed but not homogeneous were analysed using the Welch ANOVA. This study found that the polymicrobial samples obtained a value of p≤0.001 (p<0.05) which indicated a significant effect of soaking in 5%, 10%, 15%, 20% avocado seed extract in inhibiting polymicrobial biofilm (Table 4).
| Kelompok | Absorption Values of Polymicrobial Biofilm | p |
|---|---|---|
| Avocado Seed Extract of 5% | 1.3573 ± 0.2300 | <0.001* |
| Avocado Seed Extract of 10% | 1.2699 ± 0.2442 | |
| Avocado Seed Extract of 15% | 1.0897 ± 0.1137 | |
| Avocado Seed Extract of 20% | 0.9810 ± 0.1658 | |
| Positive control (+) Alkaline Peroxide | 0.7359 ± 0.5637 | |
| Negative control (-) Aquadest | 1.9942 ± 0.6417 |
Based on the Integrated Laboratory Test Results Report of the University of Sumatera Utara with the number 113/UN5.4.6.K/KPM/2024, the results of SEM tests carried out on HPA resin discs with polymicrobial biofilm which had been soaked with avocado seed extract could be detected and clearly seen in Figure 4 below. SEM results showed that there were microorganisms growing on the HPA resin disc. The soaking in 5% avocado seed extract showed a denser formation of biofilm compared to soaking in 15% avocado seed extract.
Denture plaque is not the same as dental plaque, although the microbial composition of denture plaque is influenced to a certain extent by dental plaque because the microbiota on the denture surface and the tooth surface originates from the same oral cavity. However, Fujinami et al. (2021) and O’Donnell et al. (2015) found lower diversity in denture plaque compared to dental plaque which may be caused by differences in the surface on which it grows.8,25 Low diversity in denture plaque, and the denture environment in the underlying tissue which is low in oxygen levels and saliva flow, as well as denture surface characteristics in the form of porosity and non-specific factors such as hydrophobicity, van der Waals forces, Brownian motion forces, and electrostatic interactions support the adhesion and growth of Candida spp. to colonize denture surfaces and form co-aggregates with bacteria to form complex microbial communities.8,26,27
Candida albicans has the pathogenic ability in the form of morphogenesis, which is the ability to transition C. albicans from a unicellular yeast form to a pathogenic filament form (pseudohyphae or hyphae) reversibly.28,29 C. albicans yeast cells will adhere to the denture surface via A1s1-8p adhesin, then proliferate to form microcolonies which become the basal layer of the biofilm and produce extracellular matrix (ECM). As the biofilm matures, there is an increase in biomass with the presence of yeast cells, hyphae and pseudohyphae encapsulated in the extracellular matrix. These hyphae are fundamental and important components in supporting the structural integrity of the biofilm and provide a means of attachment for additional yeast cells, pseudohyphae, other hyphae, and bacteria due to their ability to express specific adhesins such as Hwp1p and Hyr1p.15 These hyphae are also capable of damaging epithelial cells and destabilizing membranes through induced calcium ion influx and release of lactate dehydrogenase.9 In this study, the MBIC50 of avocado seed extract in mono-species C. albicans biofilm was 5% avocado seed extract with an inhibition value of 76.09 ± 3.21%. This is in accordance with previous research by Wulandari et al. (2023) who tested the effect of avocado seed extract on C. albicans biofilms. Using the inhibition percentage formula, the lowest concentration of avocado seed extract tested, which is a concentration of 3.13%, was able to inhibit C. albicans biofilms incubated for 24 hours by 75.37%.30
Candida glabrata is the second most frequently isolated cause of candidiasis and is often found together with C. albicans in the form of co-isolation in which C. glabrata budding yeast is found attached to C. albicans hyphae.31 There was still little to none research done on the effect of avocado seed extract on C. glabrata. This study found that the MBIC50 of avocado seed extract in mono-species C. glabrata biofilm was 5% avocado seed extract with an inhibition value of 52.41 ± 9.64%. When compared with the C. albicans inhibition value, there was a decrease in the biofilm inhibition activity of avocado seed extract against C. glabrata. This is due to the higher antifungal resistance in C. glabrata than in C. albicans, and the rapid ability of C. glabrata to develop resistance to currently used antifungal agents.32 Farahyar et al. (2016) found that C. glabrata had Candida drug-resistant (CgCDR) genes CgCDR1 and CgCDR2, and Fatty Acid Activator 1 (FAA1) which was positively regulated twice as much in resistant strains.33 Yu et al. (2018) found that another factor that played an important role in the antifungal tolerance and cell wall integrity of C. glabrata is ADA2 which was mediated by the ERG6 gene.34
Bacteria are thought to play an important role in the formation of denture plaque considering that denture plaque can contain 1011 microbes per milligram.8 Actinomyces is a genus commonly found in denture plaque with a large proportion which can be caused by the ability of C. albicans biofilms to provide a positive anaerobic environment to some anaerobic bacteria so that Actinomyces which is an anaerobic bacteria can easily grow in oxygen-rich areas.10,29 However, the clinical significance of Actinomyces spp. still needs to be proven and the available data regarding the antimicrobial susceptibility of Actinomyces is still limited with the susceptibility method which has not been standardized. In this study, the MBIC50 of avocado seed extract against the mono-species Actinomyces odontolyticus biofilm was high, namely 15% avocado seed extract with an inhibition value of 50.88 ± 7.31%. This can be explained by several studies which had found the existence of antimicrobial agent resistance or antibiotic resistance in A. odontolyticus. Wolff et al. (2022) found an A. odontolyticus isolate that showed multi-drug resistance (MDR) to benzylpenicillin, meropenem, moxifloxacin, and daptomycin.35 Steininger et al. (2016) tested the susceptibility of Actinomyces spp. taken from 387 patients over a 7-year period and found that Actinomyces spp. was susceptible to β-lactam antimicrobial agents with and without β-lactamase inhibitors and there was an A. odontolyticus isolate that was resistant to tetracycline.36
Shi et al. (2016) found that S. gordonii colonized denture teeth in healthy denture users at a significantly higher rate.11 In this study, the MBIC50 of avocado seed extract in mono-species S. gordonii biofilm was 15% avocado seed extract with an inhibition value of 51.16 ± 4.74%. There was still no research done on the effect of avocado seed extract on S. gordonii, but most researches on S. mutans had been carried out, where these researches focused on treating caries and dental plaque rather than denture plaque. Calosa et al. (2023) found that the minimum inhibitory level of avocado seed extract against S. mutans as seen from the sample absorbance was 12.5%.37 S. gordonii usually competes with S. mutans where S. gordonii metabolically produces hydrogen peroxide which is able to inhibit the growth of S. mutans, and produces alkaline ammonia which is able to mitigate acidity on the tooth surface. The presence of S. mutans in plaque is strongly and positively associated with caries while S. gordonii is negatively associated with caries.38 Considering the antagonistic relationship between S. mutans and S. gordonii, the presence of S. gordonii in denture plaque minimizes the presence of S. mutans.37 Further research into the effects of avocado seeds on S. gordonii needs to be carried out.
S. aureus is often associated with higher amount in the elderly, seriously ill patients, individuals with low salivary secretion, and denture wearers.39 S. aureus is also commonly found in patients with oral infections associated with Candida albicans, such as denture stomatitis and angular cheilitis, due to the nature of S. aureus which tends to attach more easily to the hyphal phase of C albicans compared to abiotic surfaces.40 In this study, the MBIC50 of avocado seed extract in mono-species S. aureus biofilm was 10% avocado seed extract with a value of inhibition was 51.63 ± 5.05%. Research on the effect of avocado seed extract on S. aureus that has been carried out has found the Minimum Inhibitory Concentration (MIC) of avocado seed extract, but not the MBIC. Santosa et al. (2019) using the zone of inhibition test concluded that avocado seed extract was effective in inhibiting multi-resistant S. aureus at a concentration of 6.25%.41
This study found a significant effect of soaking in avocado seed extract (Persea americana Mill.) concentrations of 5%, 10%, 15%, 20%, as well as the positive control of alkaline peroxide in inhibiting the growth of denture plaque microorganisms on HPA resin discs in the form of C. albicans, C. glabrata, A. odontolyticus, S. gordonii, and S. aureus mono-species biofilms, each with a value of p≤0.001 (p<0.05). If the inhibition value of avocado seed extract was compared with the inhibition value of the positive control alkaline peroxide, only the MBIC50 inhibition value of avocado seed extract on mono-species C. albicans biofilm (76.09 ± 3.21%) was found to be higher than the inhibition value of the positive control (66, 43 ± 28.29%). In other mono-species biofilms, such as C. glabrata, A. odontolyticus, S. gordonii, S. aureus, the inhibition value of avocado seed extract was lower than the inhibition value of the positive control. Morelli et al. (2023) stated that effervescent tablets showed good antimicrobial activity against C. glabrata, S. mutans, and S. aureus on a cobalt-chromium surface. However, none of these peroxide-based solutions showed a reduction in C. albicans biofilms or substantially eliminated aggregated biofilms.42
From the research results, the MBIC50 of avocado seed extract against polymicrobial biofilm in this study was 20% avocado seed extract with an inhibition value of 50.81 ± 8.32%. When compared with MBIC50 in mono-species biofilms, it was found that polymicrobial biofilm required a higher concentration of avocado seed extract. This is in accordance with research results which state that polymicrobial biofilms have higher resistance to antimicrobial agents compared to mono-species biofilms. O’Brien et al. (2022) who tested three clinically relevant antimicrobial agents namely colistin, fusidic acid, and fluconazole against polymicrobial populations containing P. aeruginosa, S. aureus, and C. albicans found a higher antimicrobial agent resistance in polymicrobial biofilm compared to mono-species biofilms. These researchers found that there was a decrease in antimicrobial activity against target microorganisms in polymicrobial cultures compared to mono-species cultures.43 However, Kart et al. (2014) stated that polymicrobial biofilms did not always have higher resistance compared to mono-species biofilms as its susceptibility to antimicrobial agents depends on the nature of the microbial species present and the disinfectant used.44
In polymicrobial biofilms, the interactions between microbes are very complex, some of which include cooperative and antagonistic interactions. Synergism between species in polymicrobial biofilms can produce effects on growth enhancement, antimicrobial resistance, virulence, and greater exopolysaccharide production compared to individual species alone.45 C. albicans and C. glabrata are often found together in the form of co-isolates that cause increased pathogenicity of both species.46 This is due to the ability of C. albicans to damage host tissue which can be exploited by C. glabrata to reach deeper tissues. C. glabrata itself has very high antifungal resistance capabilities, and is able to modify the maturation of macrophage phagosomes so that they can hide inside macrophages from the host immune system so C. glabrata can produce infections that are much more severe and require quite complicated treatment.47 Other microorganisms that were found to have a very synergistic interaction were S. gordonii and C. albicans. S. gordonii was found to be capable of promoting filamentation and increasing fungal biofilm formation. Higher biomass was also found in polymicrobial biofilms formed by C. albicans and S. gordonii.48 Diaz et al. (2014) showed an increase in the ability of oral streptococci to form biofilms on abiotic surfaces in the presence of C. albicans.49 This is caused by C. albicans adhesins which facilitate the interaction of bacterial species, such as Als1p, Als2p, Als3p, Hwp1p.28 On the other hand, these bacteria is able to influence the local environment of C. albicans by altering nutrient supply and carbon dioxide levels thereby favouring C. albicans’ hyphal transition and virulence.49 The interaction of the two species causes increased resistance to antimicrobial agents.48 The relationship between S. aureus and C. albicans has also been studied extensively where C. albicans can increase S. aureus resistance to vancomycin by 100-fold due to the production of the cell wall component β-1,3-glucan. These compounds were identified as matrix constituents that provide bacteria with increased drug tolerance. In addition, the production of farnesol and prostaglandin E2 by C. albicans can increase S. aureus biofilm formation.50
Antagonistic interactions are a type of competitive interaction where one species will inhibit the growth of another species by producing a variety of secondary metabolites that can inhibit or kill competing species so that the biofilm architecture can be disrupted.45 Guo et al. (2015) found an inhibitory effect of A. odontolyticus on proliferation, adhesion, metabolic enzyme activity, hypha formation, and biofilm development of C. albicans. Actinomyces was found to produce many metabolites with antifungal activity, including lincomycin and geldanamycin.51 However, another study by Morse et al. (2019) showed opposite results and found that polymicrobial biofilms of S. sanguinis, S. gordonii, A. odontolyticus, and A. viscosus were able to increase the number of C. albicans hyphae.52
In this study, there was a significant effect of soaking in avocado seed extract (Persea americana Mill.) concentrations of 5%, 10%, 15%, 20%, as well as the positive control of alkaline peroxide in inhibiting the growth of polymicrobial biofilm on HPA resin discs with a value of p≤0.001 (p<0.05). The results of this analysis were also supported by SEM results which showed a much sparse biofilm on HPA resin discs soaked in 15% avocado seed compared to those soaked in 5% avocado seed extract. This showed that avocado seed extract had the ability to damage the mucus layer of polymicrobial biofilms. Polymicrobial biofilms are highly structured associations of microorganisms encased in an extracellular matrix (ECM) which attached to biotic or abiotic surfaces. One of the advantages of biofilms to the microorganisms within them is the presence of collective recalcitrant which is defined as the ability of pathogenic biofilms to survive in the presence of high concentrations of antibiotics. Cells in biofilms were found to be 10-1000 times more resistant to various antimicrobial agents than their planktonic forms.53 Polymicrobial biofilms were found to have tolerance to antimicrobial agents and increased virulence due to an extracellular matrix (ECM) containing abundant extracellular polymeric substances (EPS) to protect all microbial cells from various dangers.54 The presence of extracellular matrix (ECM) can influence pH, oxygen concentration, and nutrient availability in the deepest layers of the biofilm. In addition, ECM can limit the penetration of antimicrobial agents and cause the accumulation of antibiotic-degrading enzymes.44 Therefore, increasing the permeability of polymicrobial biofilms is one of the targets of antimicrobial agents to inhibit the microorganisms within them.
In the phytochemical tests that have been carried out, flavonoid, tannin, alkaloid, saponin, triterpenoid and polyphenol class compounds were found present in avocado seed extract. Followed by quantitative tests of phytochemical compounds, it was found that the total flavonoid content in avocado seed extract was 4.0888 mgQE/g, the total phenol content was 66.8157 mgGAE/g, the total alkaloid content was 1.22%, and the total saponin content was 1. 59%. Vinha et al. (2013) found higher levels of flavonoids and total phenolics in avocado seeds compared to avocado flesh and skin.55 The extraction technique used in the research was the maceration technique, which is a method that is very suitable for secondary metabolite compounds that are sensitive to heat, such as polyphenolic compounds, especially flavonoids, causing the discovery of high levels of flavonoids and polyphenols.26 Flavonoids and tannins are a family of polyphenolic components that are widely distributed in Kingdom Plantae.56 Flavonoids were found to have an antibiofilm effect by penetrating the biofilm layer and inhibiting bacterial growth and attachment. surface. The presence of hydrophilic parts of the chemical structure of flavonoids, including glycoside and hydroxy groups, could increase penetration of the biofilm structure and increase antibiofilm activity.27 Matilla-Cuenca et al. (2020) found that the antibiofilm activity of flavonoids which could inhibit S. aureus biofilm formation was specifically mediated by Bap.57 Tannins were also found to influence the gene expression of virulent factors such as biofilm, enzymes, adhesins, motility and toxins, and act as quorum sensing inhibitors.58 Villanueva et al. (2023) found that all unmodified natural tannins had broad spectrum activity due to their ability to exhibit very significant anti-biofilm activity against Gram-positive and Gram-negative bacteria at least at a concentration of 150 mg/L.59
Alkaloids have been found to damage bacterial cell membranes, inhibit efflux pumps, inhibit ATP synthesis which affects the metabolic processes of microorganisms, damage DNA/RNA molecules or inhibit DNA thereby preventing the expression of virulent genes, and inhibit FtsZ protein synthesis by participating in the diaphragm formation and forming a ring structure in division sites to control the division process and growth of bacterial cells.60 Saponin can reduce the surface tension of bacterial cell walls and damage cell permeability so that saponin can diffuse into the cell and bind to the cytoplasmic membrane which can lead to cell lysis.58 This activity can facilitate the influx of antimicrobial agents to the deeper layers of the polymicrobial biofilm. Brahim et al. (2015) found that the combination of saponin extract with fluconazole showed good synergism against C. albicans, C. parapsolosis, C. krusei, and C. glabrata.61 Monte et al. (2014) showed the potential of saponins in controlling the shape of plankton and biofilms of E. coli and S. aureus.62 Triterpenoids with more polar groups such as hydroxyl, carboxyl and carbonyl have anti-biofilm activity due to their hydrophilic nature so they are able to penetrate the exopolysaccharide polymeric matrix in bacterial biofilms and has an effect on bacterial cells in the biofilm, and shows anti-quorum sensing activity.63
The inhibition value of the positive control alkaline peroxide against polymicrobial biofilm was found to be higher (63.10 ± 28.26%) than the MBIC50 inhibition value of 20% avocado seed extract (50.81 ± 8.32%). This shows that alkaline peroxide has a good anti-biofilm effect. Kaypetch et al. (2023) found that acrylic resin soaked in alkaline peroxide for more than 3 hours could efficiently penetrate and inhibit multispecies denture biofilm with an effect comparable to immersion in 0.5% NaClO for 10 minutes.64 Research by Lucena-Ferreira et al. (2013) found that daily use of alkaline peroxide could improve denture cleanliness by reducing total microorganisms and total Streptococcus, but had no effect on the Candida spp. population.65 This is contrary to research by Li et al. (2010) who examined the effect of alkaline peroxide on C. albicans biofilms mixed with microorganisms taken from human saliva samples that were conditioned in cases of denture stomatitis and found that alkaline peroxide was able to reduce the viability of Candida growing on the surface of acrylic resin by 3-4 times.66 However, MBIC50 avocado seed extract has been declared effective in inhibiting polymicrobial biofilm with an inhibition value exceeding 50% so that 20% avocado seed extract has the potential to be applied clinically as a natural denture cleanser.
Several limitations have been found in this study. First, the diversity and composition of microorganisms in the polymicrobial biofilm in this study is a broad generalization of the diversity and composition of denture plaque in denture wearers. Second, the research was carried out in vitro, which means that all research variables were under the control of the researcher, which cannot be used to represent the condition of the oral cavity in patients using dentures that can be influenced by factors such as age, gender, habits, and so on. Third, this research can only tell how much of the biofilm biomass that can be inhibited with avocado seed extract, but cannot know what microorganisms are inhibited in the polymicrobial biofilm.
This study did not include any human participants or animal. The denture base subjects’ research was approved on 27th February 2024 and performed according to the ethical standards by the Health Research Ethics Committee of the University of Sumatera Utara, Indonesia as stated in letter number 166/KEPK/USU/2024.
Figshare: Avocado Seed Extract on Inhibiting Mono-species and Polymicrobial Biofilm. https://doi.org/10.6084/m9.figshare.25996006. 67
This project contains the following underlying data:
• Ethical Clearance No. 166KEPKUSU2024. pdf
• Determination of Avocado Fruit Plants. pdf
• Phytochemical Test Results of Avocado Seed Ethanol Extract. pdf
• Quantitative Analysis for Phytochemical Compounds. pdf
• Research Data of Mono-species C. albicans Biofilm. docx
• Research Data of Mono-species C. glabrata Biofilm. docx
• Research Data of Mono-species A. odontolyticus Biofilm. docx
• Research Data of Mono-species S. gordonii Biofilm. docx
• Research Data of Mono-species S.aureus Biofilm. docx
• Research Data of Polymcrobial Biofilm. docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Prosthodontics, Dental materials, Implants, Maxillofacial prosthodontics, Systematic reviews, Fixed prosthodontics, Removable prosthodontics.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Anggraini V, Masfufatun M: EFEKTIVITAS KOMBINASI EKSTRAK DAUN SIRIH MERAH (Piper Crocatum) DAN EKSTRAK BIJI ALPUKAT (Persea americana) DALAM MENGHAMBAT PERTUMBUHAN Candida albicans. Jurnal Kimia Riset. 2017; 2 (2). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: prosthodontics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Prosthetic dentistry, dental materials, Microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 25 Mar 25 |
|||||
|
Version 2 (revision) 29 Oct 24 |
read | read | read | ||
|
Version 1 20 Aug 24 |
read | read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)