Keywords
cerebral venous thrombosis, puerperal eclampsia, seizures, barbiturate coma.
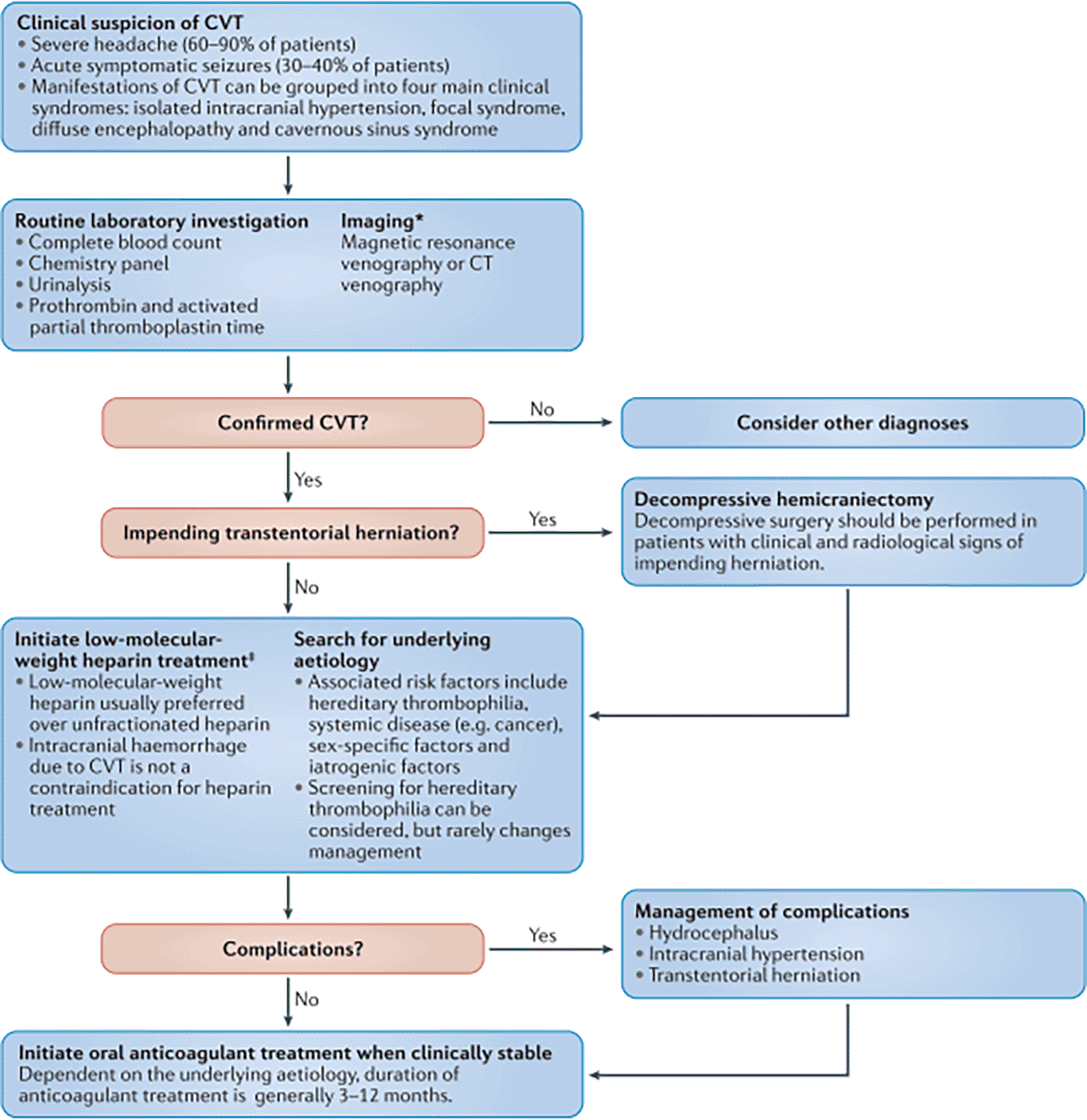
Cerebral Venous Thrombosis (CVT) is a rare and anatomically specific form of stroke that occurs in the cerebral veins, with pregnant and postpartum women at particular risk. Obstetric CVT carries high mortality due to its atypical presentation, often mimicking or being masked by other conditions such as preeclampsia. We report a case of a 37-year-old Sasak woman who, on the 32nd day postpartum following a cesarean section, experienced more than five seizures beginning with left arm weakness and heaviness. Upon presentation to the emergency department, the patient was conscious but complained of a severe headache and left-sided limb weakness. Her vital signs showed blood pressure of 152/87 mmHg, pulse of 84 beats/min, and a temperature of 37.1°C. During her stay in the ER, she experienced three additional seizures. Initially treated as eclampsia with magnesium sulfate by the obstetrics team, the patient underwent urgent brain MRI and MR venography, which confirmed a diagnosis of CVT in the superior sagittal sinus, complicated by intracranial hemorrhage, cerebral edema, and uncal herniation. The patient did not respond to anticoagulant therapy and subsequently underwent delayed decompressive craniectomy. To further manage elevated intracranial pressure, barbiturates were administered as a third-tier treatment. This case emphasizes the diagnostic challenges and high-risk nature of postpartum CVT, underlining the need for rapid neuroimaging and multidisciplinary intervention in postpartum women presenting with seizures and neurological deficits.
cerebral venous thrombosis, puerperal eclampsia, seizures, barbiturate coma.
Brain venous thrombosis (CVT) is a rare but dangerous illness that requires prompt medical attention due to its neurological consequences. CVT is a rare cause of stroke, affecting 0.5% to 1% of cases, mostly in younger people, but it can impact anyone. Unlike arterial stroke, cerebral venous thrombosis (CVT) affects younger people, notably women, and has different risk factors and treatment options. It has different clinical symptoms, a non-apoplectic onset, and a better prognosis. She had recurring focal seizures, decreased awareness, and hemiparesis shortly after delivery. These symptoms indicate superior sagittal sinus and dominant hemisphere cerebral venous thrombosis. A head CT scan showed a “hyperdense sinus sign” supporting the diagnosis, which was confirmed by MRI.1–4
Among women, the most common risk factors for CVT include oral contraceptive use, obesity, and pregnancy. Although the incidence of CVT in pregnant and postpartum women is reported to be less than 1%, the condition remains clinically significant—particularly in low- and middle-income countries with high fertility and childbirth rates.1–3 CVT is more frequently observed during the postpartum period (approximately 73%) compared to during pregnancy (about 29%), with the highest risk occurring in the third trimester through the sixth postpartum week (puerperium).5,6 The clinical presentation of CVT in this population is often atypical and overlaps with conditions such as preeclampsia and eclampsia, complicating early diagnosis.3,6,7
This case reflects a broader clinical challenge: the absence of clear surgical decision thresholds for postpartum CVT patients presenting with early herniation signs. In the context of limited surgical access and evolving neurological status, this report aims to fill the gap in guidance on when neurosurgical referral and decompression should be prioritized.
A women 27-year-old from the Sasak Lombok tribe, with one previous childbirth, presented to the emergency hospital following more than five seizures at home. Prior to the seizure, the patient reported a sensation of heaviness in the left hand, which was then followed by a seizure lasting approximately one minute, without any accompanying loss of consciousness post-seizure. Postictal symptoms were left-sided limb weakness and headache. The patient has a postpartum history of cesarean delivery 30 days prior, performed under spinal anesthesia due to a lengthy latent period, and has no prior or current history of hypertension during pregnancy. Upon admission at the emergency department, the patient exhibited stable vital signs: blood pressure 152/87 mmHg, pulse 84 beats per minute, and temperature 37.1°C. The patient was initially diagnosed with eclampsia, received magnesium sulfate therapy, and was then transferred to the intensive care unit while maintaining a clear state of awareness. Laboratory and radiological assessments were conducted (see Table 1).
The patient in the ICU remained conscious (E4V5M6) but experienced another seizure. Therapy was administered as a loading dose of mannitol at 1 g/kg body weight, followed by a maintenance dose of 0.5 mg/kg four times daily, continuous magnesium sulfate at 2-3 mg/kg/hour, citicoline at 500 mg three times daily, and sedation with dexmedetomidine at 0.5-0.7 mcg/kg/hour. The patient exhibited clinical and hemodynamic enhancement. On the subsequent day (ICU H-3), the patient reiterated complaints of intense headache and agitation (E3V5M6), which were succeeded by seizures and diminished consciousness. A neurological assessment revealed left hemiparesis, with right/left motor strength recorded at 5-5/4-4, and no nuchal rigidity was observed. A CT scan of the head revealed a “Dens sign” alongside intracranial hemorrhage (ICH) encircled by considerable edema in the superior sagittal sinus region, accompanied by subarachnoid hemorrhage. Nimodipine at a dosage of 6x60 mg and a hypertonic saline loading dose of 3 mg/kg, subsequently followed by 1 mg/kg body weight every 8 hours, were administered. The left side limbs exhibited a decline in strength to 5-5/2-2, accompanied by diminished motor function in the right side limbs (4-4/2-2). The CT scan of the head without contrast (NCCT, Figure 1) prompted a consultation with the neurology division, followed by a D-dimer test yielding a result of 4870 ng/ml. An MRI and MRV of the head revealed a hemorrhagic venous infarct in the right-left parietal lobe (dominant right, volume approximately 15.5 cm), accompanied by perifocal edema that displaced the right-left lateral ventricle inferiorly, resulting in subfalcine herniation to the left side due to thrombosis with anteromedial occlusion of the superior sagittal sinus ( Figures 2-3). Anticoagulant therapy commences with an initial dose of 5000 IU of low molecular weight heparin (LMWH), followed by a maintenance dose of 1.8 IU/kg body weight, and a consultation with the neurosurgery division for decompression craniectomy.
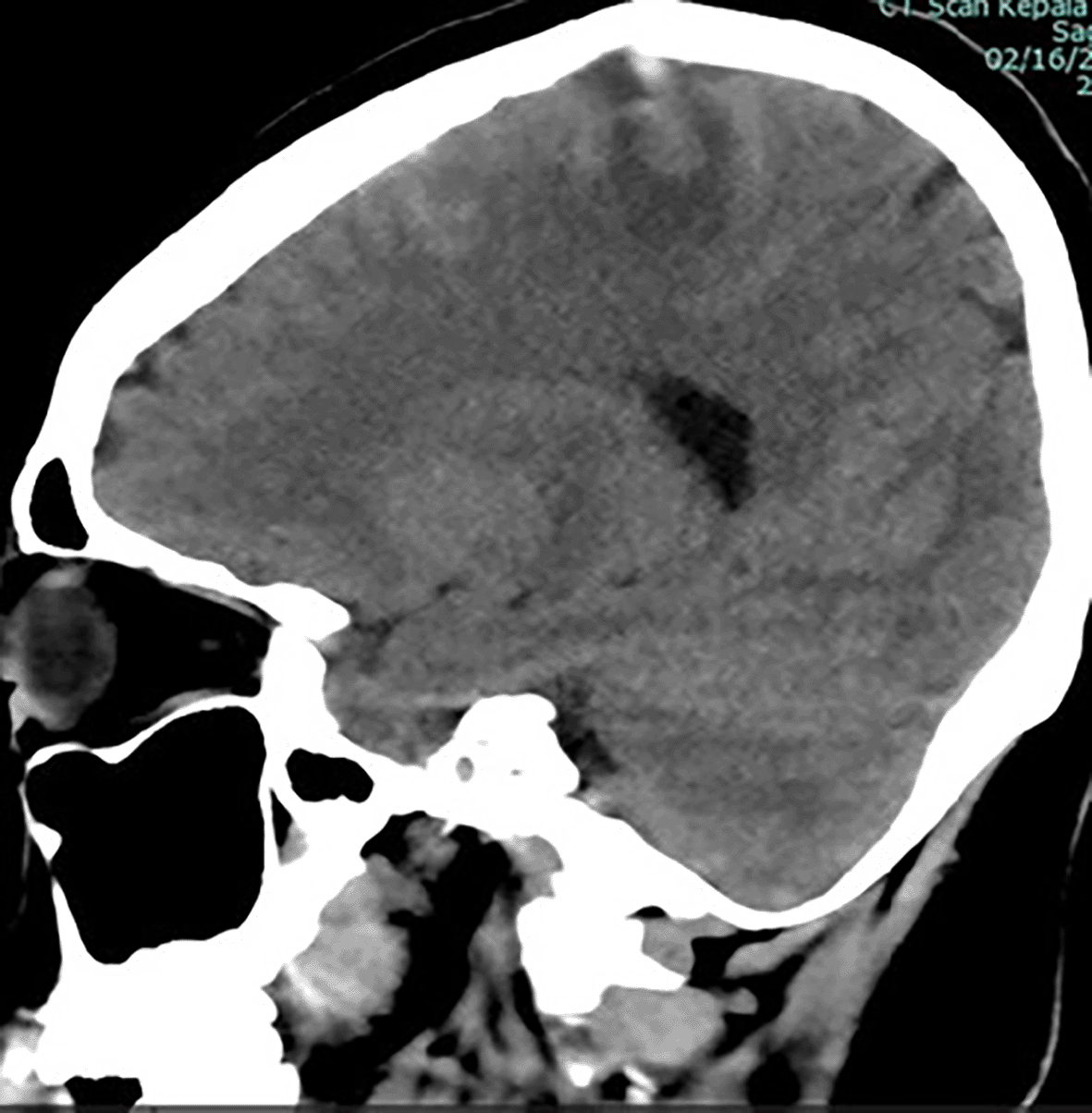
ICH is seen from rupture of the superior sagittal vein.
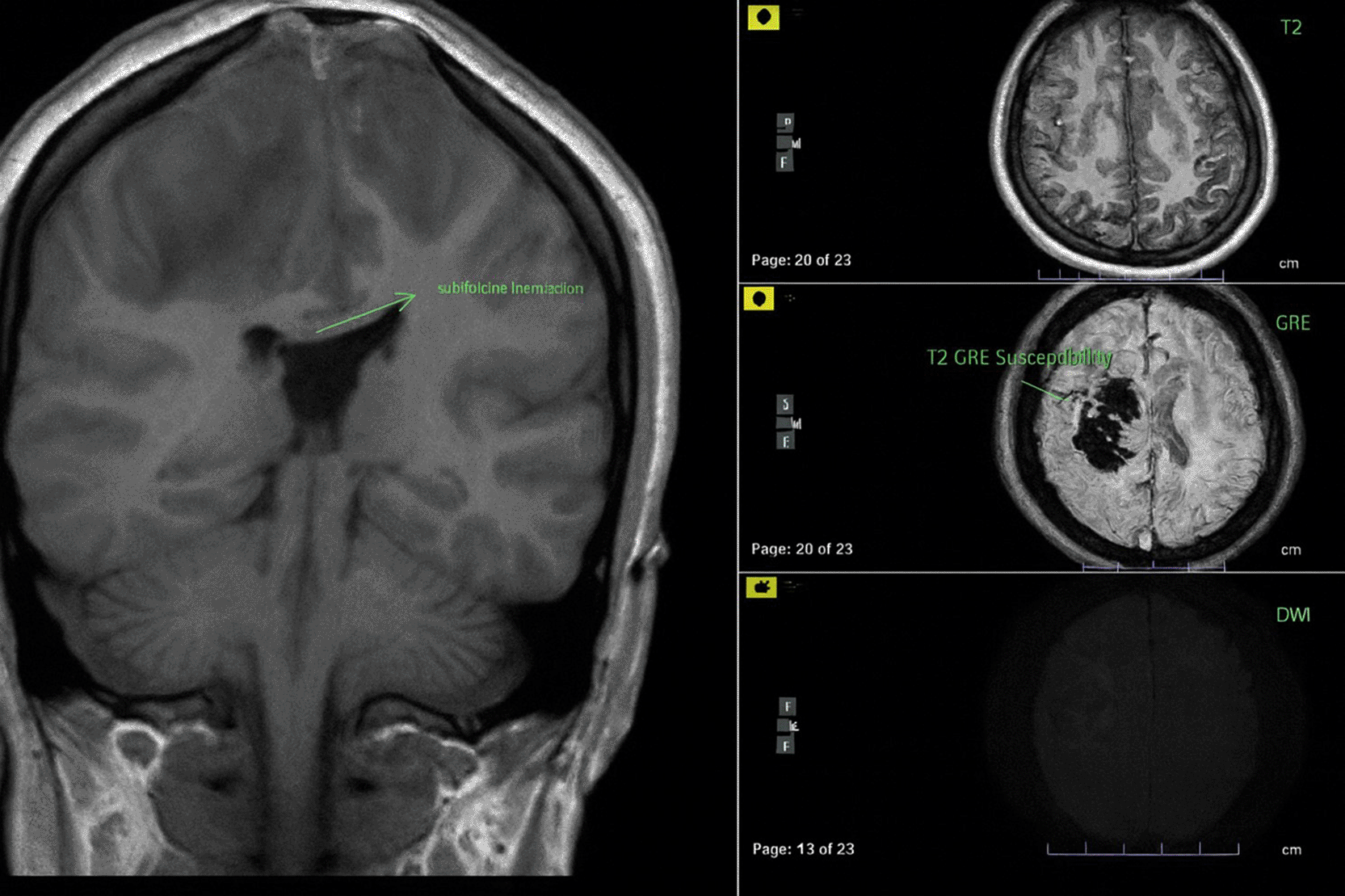
The lesion produced significant local mass effect with effacement of the surrounding sulcus and left subfalcine herniation (3.8 mm).
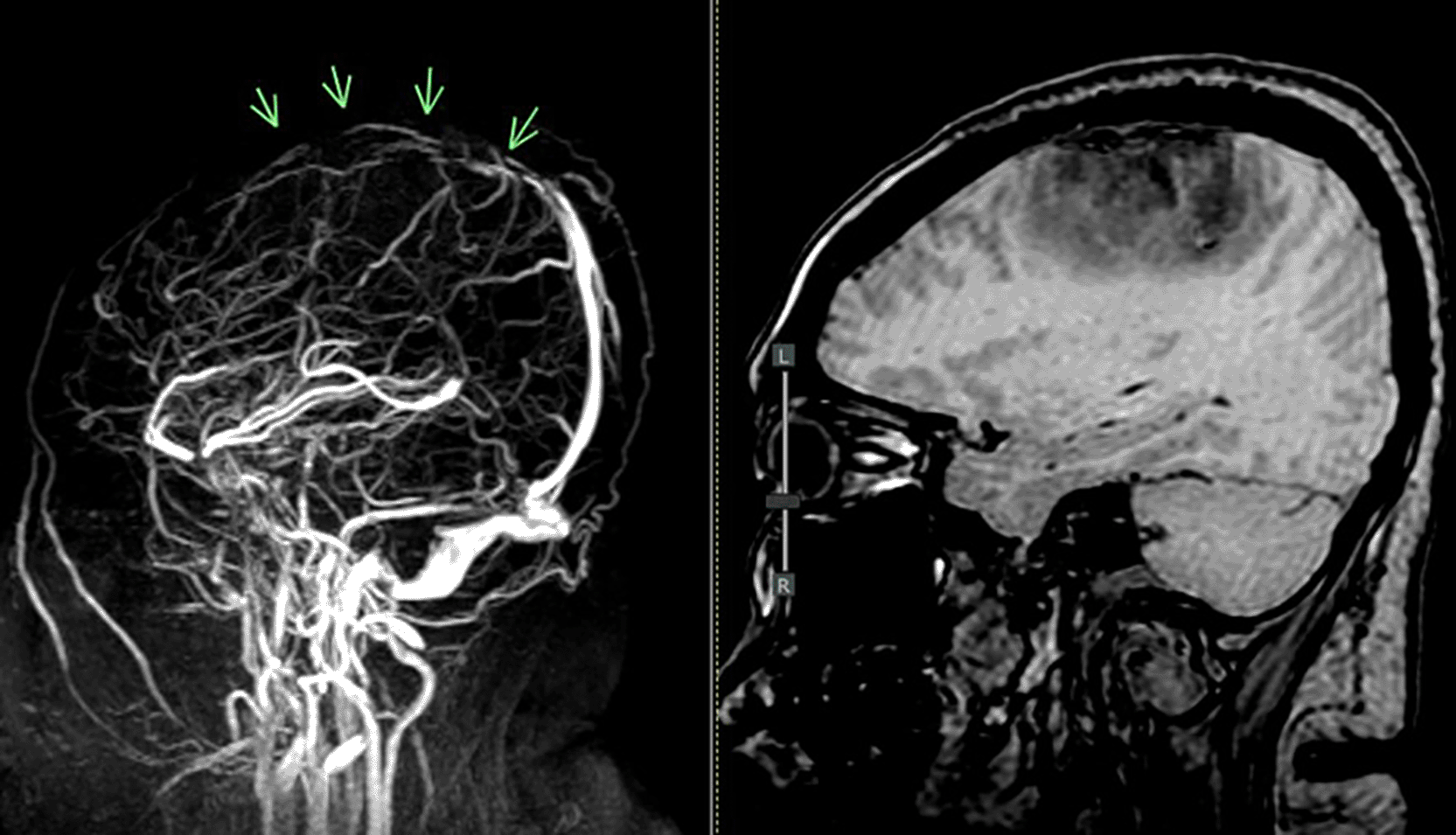
MRV = magnetic resonance venogram.
As a result of the tolerable GCS level of consciousness, the neurosurgery division postponed the surgery. Non-invasive hyperosmolar therapy was enhanced by administering mannitol every six hours, incorporating sedation with midazolam at a dosage of 0.1-0.5 mg/kg/hour, and providing Low Molecular Weight Heparin (LMWH) with an initial dose of 5000 IU, followed by 1 mg/kg every twelve hours, along with monitoring PT and APTT to achieve an INR target of 1.5 times the baseline APTT. Due to the absence of coagulability and the presence of persistent fever in the patient’s homeostatic function monitoring during anticoagulant medication, heparinization was terminated. We conducted sequential control CT scans and ultrasound assessments of the Optical Nerve Sheath Diameter (ONSD) to evaluate intracranial pressure (Figures 4-5). We administered third-tier therapy involving intubation, mechanical breathing, and barbiturate treatment, specifically continuous thiopental at a dosage of 1-5 mg/kg/hour ( Figure 6).
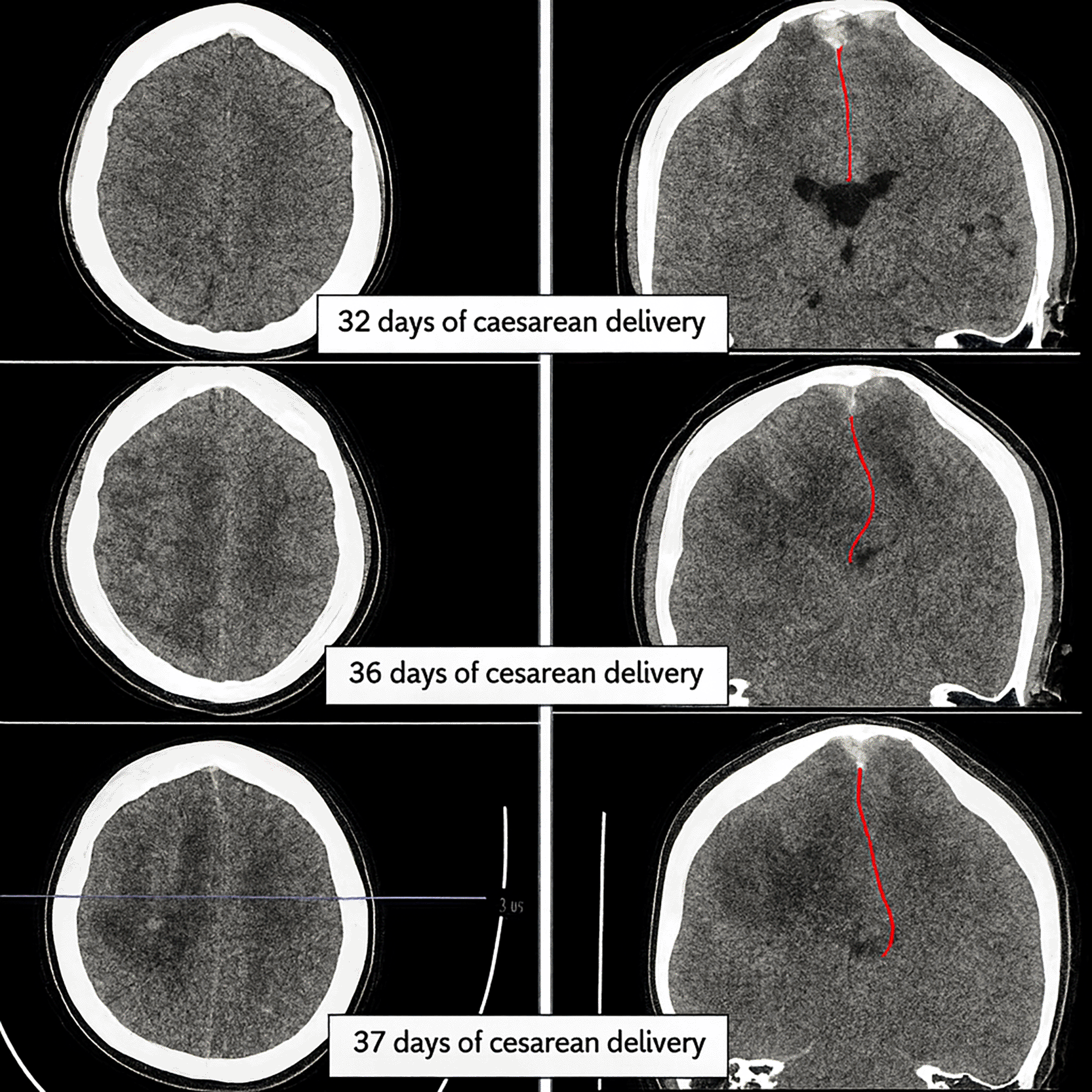
At each time point, hemorrhage volume (hyperdense cortical lesion) and mass effect (falcine herniation: red line) were estimated on reference slices to depict lesion progression. Initial examination on day 32 post-cesarean delivery showed venous hemorrhage with less infarction and no falcine herniation (top image). Follow-up examinations on days 36 and 37 showed that the infarction had become more extensive and the falcine herniation had increased relatively significantly (middle and bottom images).
Cerebral venous thrombosis during pregnancy and the puerperium (pCVT) is a rare but potentially fatal neurological condition. Its prevalence ranges from 0.018% to 0.2%, yet it represents a leading cause of stroke during this period, accounting for 6% to 64% of cases.4,7,8 Approximately 13.4% of pCVT cases result in poor outcomes, contributing to 10% of maternal deaths and lasting neurological impairments.3,7,9
The pathogenesis of cerebral venous thrombosis during this period is linked to a physiological hypercoagulable state characterized by elevated coagulation factors, notably procoagulants (factors I, II, VII, VIII, IX, and XII), alongside a reduction in the anticoagulant system (markedly diminished protein S activity and resistance to activated protein C), as well as decreased fibrinolytic activity attributed to heightened placental PAI-2 expression ( Table 2). Elevated estrogen levels during pregnancy stimulate venous dilation and congestion. Venous stasis resulting from coagulation abnormalities exacerbates the risk of venous thrombosis in pregnancy. These physiological changes, while protective against hemorrhage during delivery, simultaneously elevate the risk of thromboembolic complications.9–13
Pregnancy induces significant hematologic and hemodynamic changes, including a 60% increase in blood volume, elevated nitric oxide levels, enhanced venous capacitance, and increased arterial compliance—all of which contribute to venous stasis.4 Additional factors such as dehydration, hyperemesis gravidarum, prolonged bed rest, hypertensive disorders of pregnancy, delivery trauma, and postpartum hemorrhage further exacerbate hypercoagulability. Other risk factors include anemia (Hb <9.9 g/dL), young maternal age, infections, and cesarean delivery.4,6,7 The highest risk period for thrombotic events spans from the third trimester to the sixth postpartum week.3,6–8
Youth, postpartum status, and spinal anesthesia post-cesarean birth increase CVT risk. Insufficient hydration and mobility after delivery increase the risk of cerebral venous thrombosis in postpartum women. Few pregnant women have had cerebral venous thrombosis after spinal anesthesia3,14 and one after epidural analgesia.7
CVT is often challenging to diagnose due to its nonspecific and overlapping symptoms with other neurological disorders.15 Different thrombus sites cause different symptoms. Intracranial hypertension causes clinical symptoms and mass effect causes localized appearance. Intracranial hypertension is caused by sinus or cortical vein thrombus development, which raises venous pressure and reduces CSF absorption. It causes headaches, nausea, vomiting, and sudden symptomatic seizures due to high intracranial pressure. Thrombus-induced sinus occlusion increases blood flow into the venous system (venules and capillaries), raising local venous pressure. Reduced cerebral perfusion pressure (CPP) causes ischemia, cytotoxic edema, and blood-brain barrier breakdown, which may cause vasogenic edema. The structural variations between veins and arteries can increase capacitance and venous pressure, triggering venous wall rupture and intraparenchymal brain hemorrhage. Venous-capillary hypertension, cerebral edema, and brain parenchymal bleeding increase intracranial pressure (ICP) due to cerebral sinus thrombosis, which reduces cerebrospinal fluid absorption.4 Venous thrombosis is most prevalent in the superior sagittal sinus (SSS, 72%), followed by the lateral, deep, and cortical sinuses.3,16
In this case, recurrent focal seizures, decreased consciousness, and hemiparesis appeared rapidly during the puerperium. These are typical CVT symptoms, especially when the SSS and dominant hemisphere are affected. Symptoms may resemble preeclampsia or post-dural puncture headache (PDPH), presenting with headache, altered mental status, motor deficits, seizures, or visual disturbances.6,17 “Isolated cortical venous thrombosis” is characterized by headaches, convulsions, and focal neurological deficits.1 CVT-associated headaches are typically progressive and exacerbated by Valsalva maneuvers or changes in posture. In rare cases, acute onset may mimic subarachnoid hemorrhage with “thunderclap” headache and nuchal rigidity.2,4,18
Seizures are the second most frequent presenting symptom in CVT and a common reason for hospitalization. They occur more frequently than in arterial strokes and may be classified as acute symptomatic seizures (ASS, within 14 days of diagnosis; ~76%) or late-onset post-CVT epilepsy (>14 days; ~16%).4,19 Inflammation of cortical-subcortical parenchymal lesions or elevated ICP might cause seizures. Seizures are linked to superficial vein thrombosis (SSS), subcortical veins, and cortical veins, while deep venous thrombosis causes basal ganglia and thalamus edema, encephalopathy, mental impairment, lateralization, and coma.18 In isolated intracranial hypertension (IIH), headache, nausea, vomiting, papilledema, visual abnormalities, and tinnitus are common.2 Sudden symptomatic seizures and consciousness changes may result from supratentorial injuries like hemorrhages, motor or sensory impairments, and thrombosis in the central nervous system (SSS). Status epilepticus, which may be refractory, can result from several supratentorial lesions with or without hemorrhages. A clinical presentation with thrombosis in the superior sagittal sinus (SSS) and deep venous system (DVS), bilateral parenchymal lesions, significant cerebral edema, and/or herniation includes poor consciousness, encephalopathy, bilateral or multifocal symptoms, seizures, and status epilepticus.1,2,19 Early-onset seizures are associated with infarct hemorrhage, focal motor or sensory deficits, changed mental status, cerebral vein thrombosis, supratentorial edema/infarction, notably in the frontal lobe and superior sagittal sinus, and elevated D-dimer levels.20
Our patient presented with diffuse acute headache, positional worsening, bilateral motor deficits, and thrombosis in the SSS with bifrontal hemorrhagic infarction, edema, and subcortical damage—confirmed via CT and MRI-MRV ( Figure 4). Her symptoms, lasting over one month, reflect rare prolonged ICP elevation and venous congestion.
Diagnosing CVT is difficult due to nonspecific clinical and laboratory findings. D-dimer may support the diagnosis, showing ~94% sensitivity and ~90% specificity.6,18 In patients with a familial genetic background to thrombophilia, especially young ones, regardless of precipitating factors, it is recommended to test for congenital thrombophilia (including APC resistance, factor V Leiden mutation, factor III deficiency, protein S and C deficiencies, and prothrombin gene mutation) and systemic inflammatory markers associated with hypercoagulability.18 Head imaging is needed to rule out preeclampsia and eclampsia. For optimal outcomes during pregnancy and postpartum, diagnostic modalities must be used sequentially to avoid radiation and intravenous contrast. MRI and MRV are best for detecting venous and sinus thrombosis. MRI can detect intraluminal thrombi and intraparenchymal abnormalities, including infarction and hemorrhage, while MRV can pinpoint blocked veins or sinuses by measuring flow rate. When MRI/MRV is absent, CT venography (CTV) can detect intraparenchymal abnormalities and deep (cortical) venous thrombosis, but it is less sensitive.2,4,6 Digital subtraction angiography (DSA) can diagnose and remove thrombus clots.4
Supportive treatment, intracranial pressure reduction, seizure and infection control, and thrombosis prevention are the goals of interdisciplinary CVT management. Even with cerebral hemorrhage, anticoagulants are first-line treatment, unlike arterial stroke.21 Heparinization is the standard treatment for hyperacute CVT, although pregnant and postpartum women must consider fetal and labor risks. Enoxaparin Low molecular weight heparin (LMWH) reduces recurrent preeclampsia and eclampsia, is safe, has high absorption, a long plasma half-life, does not cross the placenta, and is not secreted in breast milk. Stop subcutaneous (SC) injection 24 hours before labor induction or cesarean section and resume 4-6 hours after normal birth or 6-12 hours after C-section. The recommended dose is 1 mg/kgBW/12 hours. Pregnant women should continue LMWH CVT for several weeks after birth. Activated partial thromboplastin time (APTT) determines the half-life and dose of unfractioned heparin (UFH), which can be antagonized with protamine sulfate but produce teratogenic consequences and fetal bleeding. Post-hyperacute oral vitamin K antagonists (DOACs) cross the placenta and harm fetal neural development and bleeding, making them unsafe during pregnancy. The best anticoagulant for pregnant, postpartum, or high-risk women with positive antiphospholipid test results for lupus, anticardiolipid, and anti-β2 glycoprotein antibodies is LMWH.4,6 LMWH was terminated due to coagulation difficulties and high fever, early infection symptoms. Sepsis and prothrombin time changes prevent anticoagulant medication, according to an Asian study.6
Treatment aim to reduces intracranial pressure. Intracranial hypertension causes fatal ischemia and herniation. The Brain Trauma Foundation (BTF) suggests triple therapy for Intracranial Hypertension (HI): hyperosmolar therapy (mannitol 0.25-1 g/kg iv), sedation, analgesia, and CSF draining. Second-tier therapy includes hyperventilation to achieve 30–35 mmHg PaCO2 and muscle relaxants, and third-tier therapy includes decompressive craniectomy, hypothermia, and high-dose barbiturates. Acetazolamide, diuretics, and CSF shunting are contraindicated in CVT.
In cases of CVT with intraparenchymal hemorrhage and herniation, DC significantly reduces mortality.4,21–23 Neurosurgery delayed decompressive craniectomy because the patient’s consciousness (GCS E3V5M6) was borderline, despite clinically progressive ICP elevation with extremity hemiparesis to hemiplegia, repeated seizures, decreased consciousness, and head CT results showing herniation. Only two cases (Gioti and Neelam) have reported successful DC during pregnancy/postpartum.24,25 These differ markedly from our case, as DC was planned after anticoagulant stabilization. DC may reduce ICP and prevent herniation, potentially improving prognosis—though long-term post-surgical complications remain a concern.26
The management of cerebral venous thrombosis (CVT) in postpartum patients remains a significant clinical challenge, particularly when patients develop signs of increased intracranial pressure (ICP) or transtentorial herniation. While decompressive craniectomy (DC) has been shown to improve survival in patients with malignant CVT accompanied by hemorrhagic infarction and herniation,27,28 the timing and criteria for surgical intervention in the postpartum context remain poorly defined. This uncertainty is compounded by the frequent clinical–radiological mismatch, where patients may exhibit borderline consciousness yet show radiological signs of impending herniation. Current recommendations such as those proposed by the European Stroke Organization (ESO) suggest considering DC in deteriorating CVT cases, but provide limited granularity regarding timing in obstetric populations.21 Moreover, neurosurgical hesitation to operate in borderline Glasgow Coma Scale (GCS) patients and and concerns about ongoing anticoagulation may delay timely intervention, leading to missed surgical windows.23,29
In our case, DC was deferred despite progressive neurological deterioration and imaging-confirmed herniation, highlighting a broader gap in decision-making frameworks for postpartum CVT. This issue is particularly acute in resource-limited settings, where delayed access to neurosurgical expertise and lack of standardized protocols may exacerbate poor outcomes. Our findings emphasize the urgent need for refined clinical criteria and prospective studies to define evidence-based thresholds for DC in high-risk postpartum patients with CVT.
As a final measure, the BTF’s third-tier intervention—thiopental coma—was initiated ( Figure 7). However, our center lacked burst suppression monitoring and hypothermia facilities. Ultimately, ischemia, infarction, and brain herniation led to brainstem death, confirmed by loss of reflexes and central temperature regulation ( Figure 4).
Cerebral venous thrombosis (CVT) in pregnant and postpartum women is rare yet associated with a significant incidence of disability and death. Diagnosis is difficult due to the numerous analogous clinical signs, especially those of obstetric preeclampsia and eclampsia, resulting in frequent misdiagnosis and inappropriate treatment. The predominant symptoms are headache and sudden (non-apopleptic) seizures; hence, it is advisable to contemplate a diagnosis of CVT in pregnant or postpartum patients exhibiting these symptoms.
The limits of our case encompass the postponed decompressive craniectomy, which is highly advised to mitigate the mortality risk in CVT exhibiting symptoms of herniation, evident in this patient from the beginning. Additionally, we conducted third-tier therapy without sufficient monitoring mechanisms.
Written Inform Consent to publish the case was obtained from the patient’s family. The authors confirm that this consent is held securely and is available for review by the editorial office upon request.
All data underlying the results are available as part of the article and the uploaded supplementary materials. The completed CARE checklist is openly available in Zenodo with the title A Missing Surgical Window in Postpartum Cerebral Venous Thrombosis: A Case Report and Clinical Reflection, accessible at: (https://doi.org/10.5281/zenodo.17066399).30
Data are available under the terms of the Creative Commons 1.0 Universal License (CC0 1.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)