Keywords
Postmenopausal, Hyperandrogenism, Virilization, Hyperthecosis, Ovarian tumor, Sertoli-Leydig cell tumor, Imaging
This article is included in the Oncology gateway.
This article is included in the The Multifaceted Aspects of Menopause collection.
During the postmenopausal period, hyperandrogenic manifestations include hirsutism and variable degrees of virilization. Metabolic syndrome is often associated. True hirsutism and acne should not be considered normal as they may reveal tumorous sources of excessive androgen secretion originating from either the adrenals or the ovaries. Once adrenal causes are excluded, virilizing ovarian tumors must be considered, with Sertoli-Leydig cell tumor being the most common histological subtype.
We report the observations of three female patients who presented with post-menopausal hyperandrogenism of ovarian origin during the year 2023, with details on clinical presentation, lab results, and aspects found both on ultrasound and magnetic resonance imaging. They all underwent surgery and two of them were diagnosed with Sertoli-Leydig cell tumors.
Postmenopausal hyperandrogenism, though rare, requires thorough assessment to avoid misdiagnosis of underlying androgen-secreting tumors, with Sertoli-Leydig cell tumors being the predominant subtype. Surgical intervention is the primary treatment modality, often resulting in complete postoperative recovery.
Postmenopausal, Hyperandrogenism, Virilization, Hyperthecosis, Ovarian tumor, Sertoli-Leydig cell tumor, Imaging
During the postmenopausal period, hyperandrogenic manifestations include hirsutism and variable degrees of virilization. Metabolic syndrome is often associated. The literature on virilizing ovarian tumors remains mostly limited to case reports. Sertoli- Leydig cell tumor is the most common histological subtype.1 Treatment is surgery and complete post-operative recovery is often observed. We report the observations of three female patients who presented with post-menopausal hyperandrogenism of ovarian origin during the year 2023.
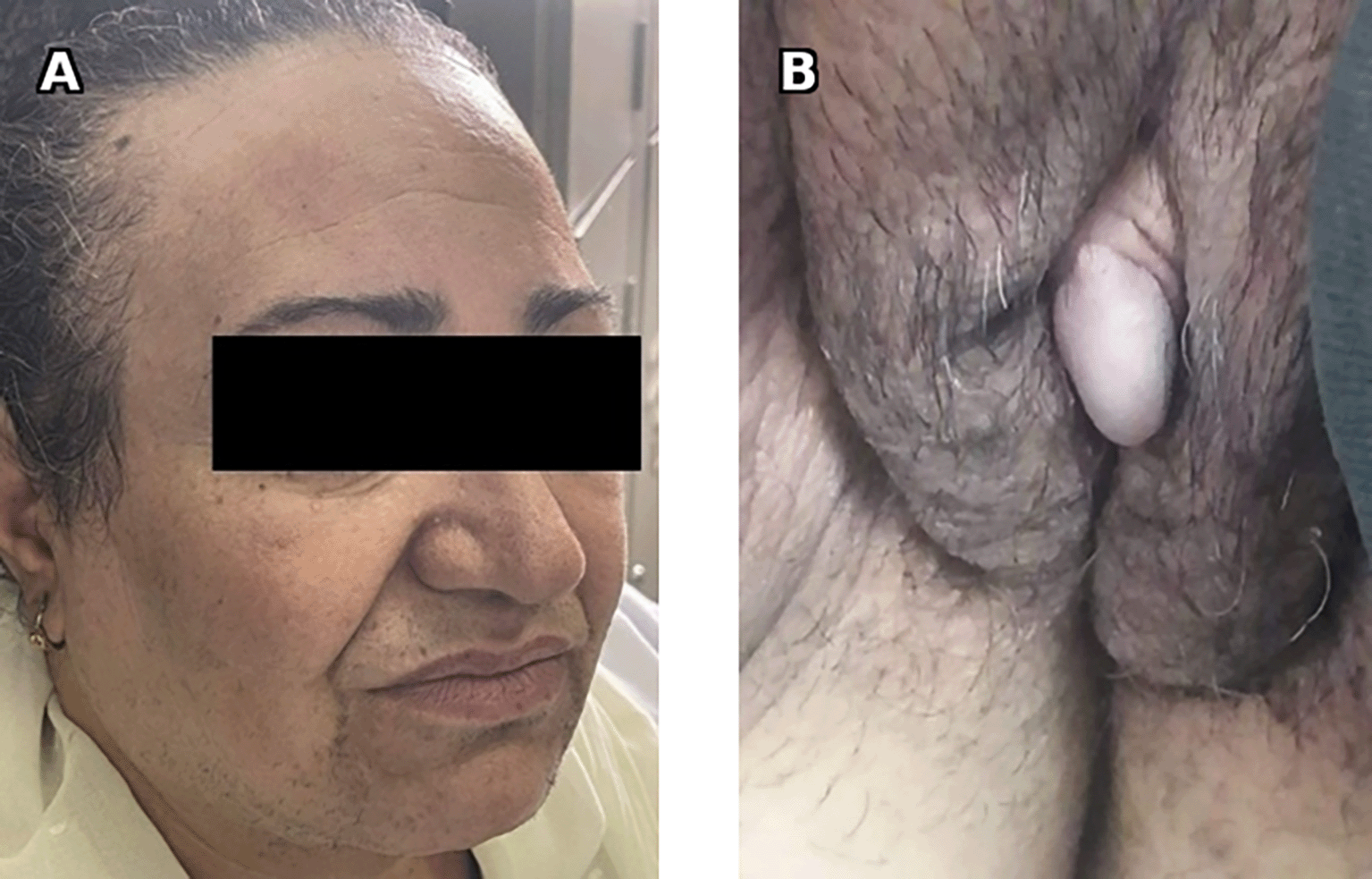
A 62-year-old female patient, Gravida 1 Para 1, menopausal at the age of 55, with a personal history of type 2 diabetes and hypertension, presented with signs of virilization discovered 3 years prior. On clinical examination, the patient was overweight with a body mass index calculated at 28 kg per m2 and an android-type fat distribution. Fronto-temporal alopecia and clitoral hypertrophy were noted with a Ferriman and Gallwey Score estimated at 23 ( Figure 1).
Laboratory studies revealed very high testosterone levels at 4.7 nmol/l, rechecked several times (N:0.2-3.5 nmol/l), normal levels of CA 125, DHEA-S and normal corticotropic balance with good dexamethasone control. A pelvic ultrasound was performed showing bilateral well defined hypoechoic round solid ovarian masses ( Figure 2).
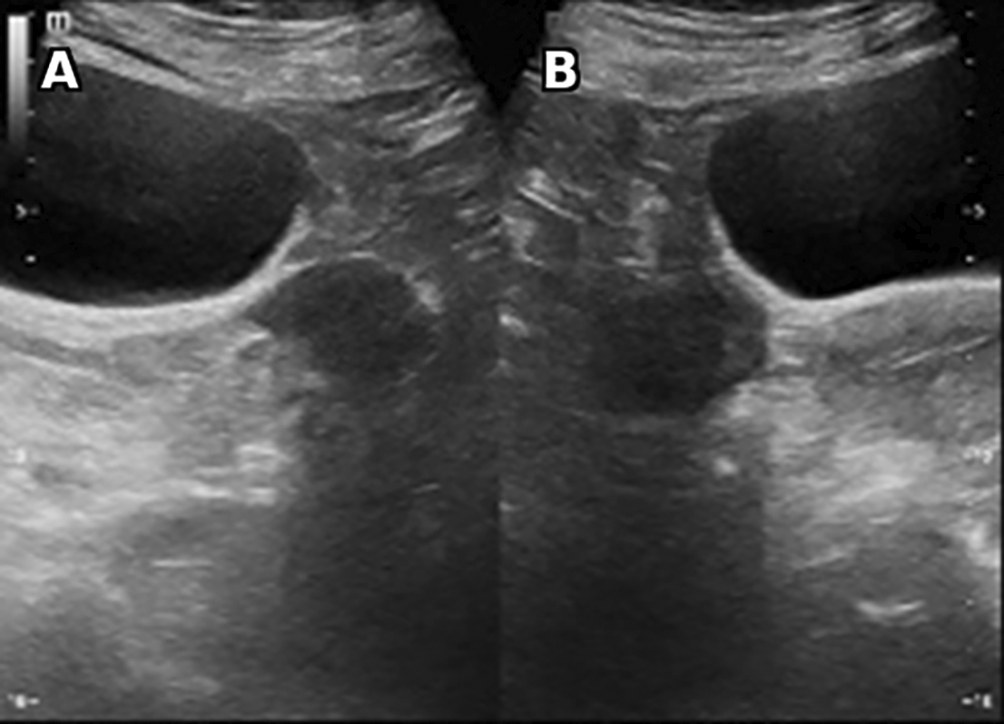
Vascularity is noted (not shown). No calcification, ascites or retroperitoneal lymphadenopathy were found.
On MRI, bilateral, round solid ovarian masses with homogenous low signal intensity on T2 were found. These masses were classified as ORADS category 2. No adrenal mass was noted ( Figure 3).
The patient underwent a total hysterectomy and bilateral adnexectomy with clitoral reduction ( Figure 4).
Pathologic report showed a morphological aspect that suggests bilateral ovarian fibroids or ovarian stromal hyperplasia ( Figure 5). No findings suggestive of hyperthecosis were reported. Clitoris histological examination found no tumor proliferation.
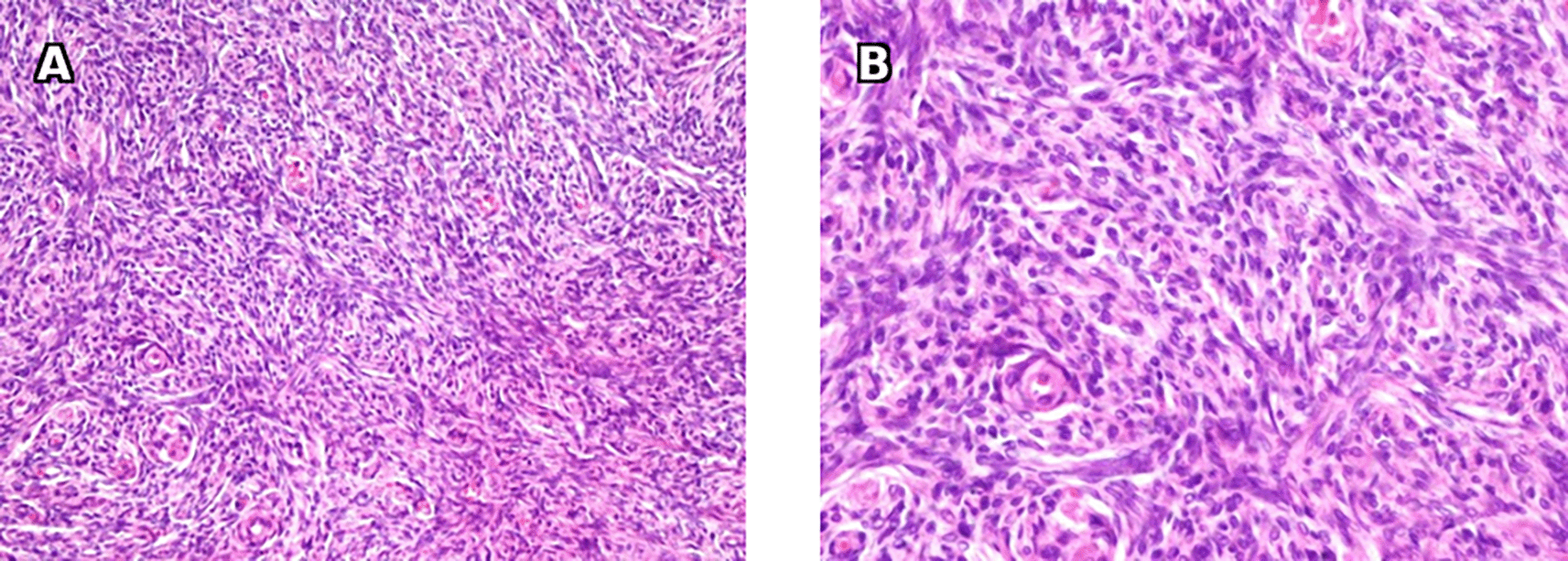
A: Microscopic examination with 200x magnification shows proliferation of spindle cells arranged in intersecting bundles. B: Microscopic examination with 400x magnification shows cells with spindle to ovoid nuclei with scant cytoplasm and no mitotic activity.
On follow up, complete recovery was noted with a decrease in levels of testosterone <0.7 nmol/l.
A 70-year-old female patient, Gravida 6 Para 5, menopausal at the age of 53, with a personal history of hypertension and android-type obesity, presented with severe hirsutism, alopecia, and clitoral hypertrophy (Figure 6) with a Ferriman and Gallwey Score estimated at 28. Testosterone level was high at 1.5 μg/L along with D4A levels (2.3 μg/L), AFP levels at 2.42 (<7 ng/ml) and CA 19-9 levels at 29.84 IU/l.
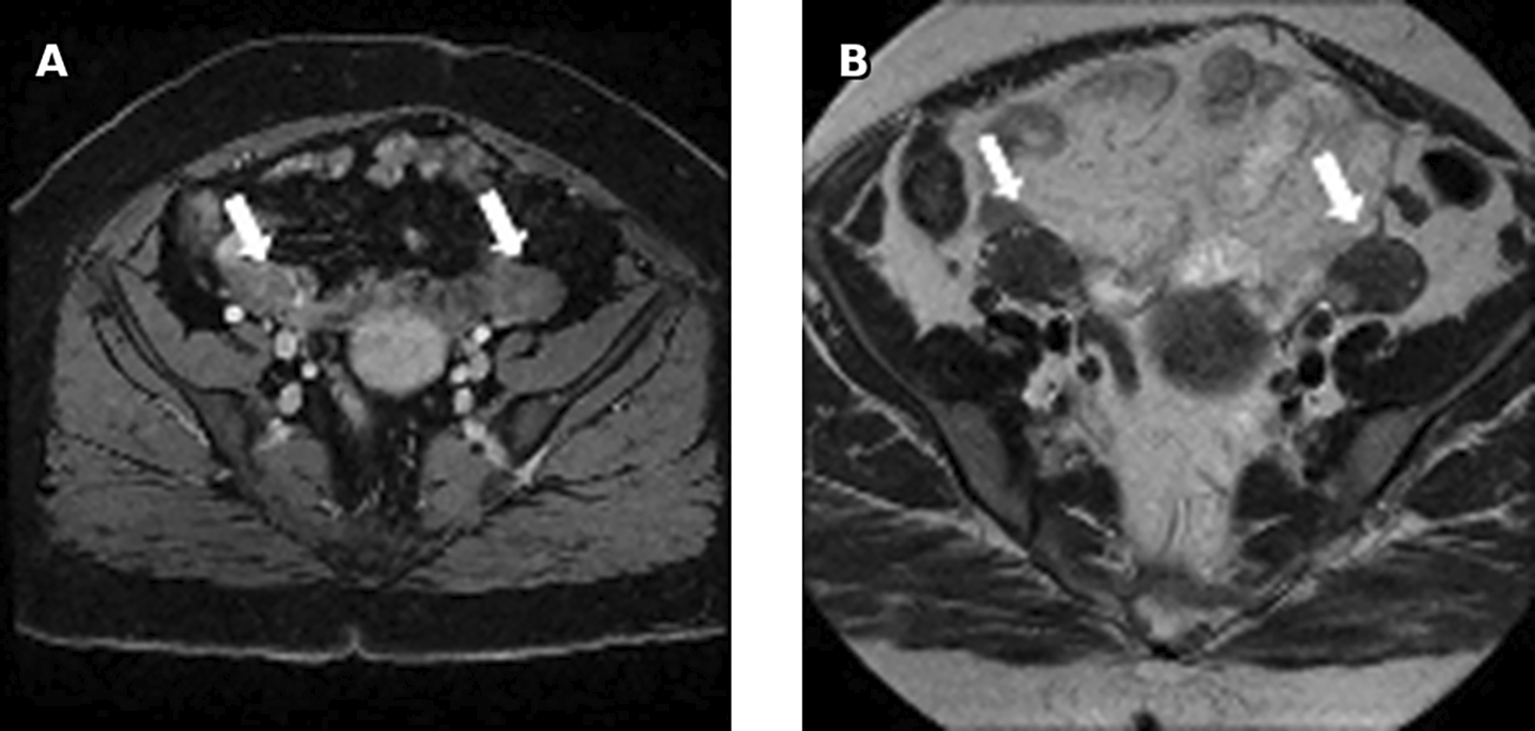
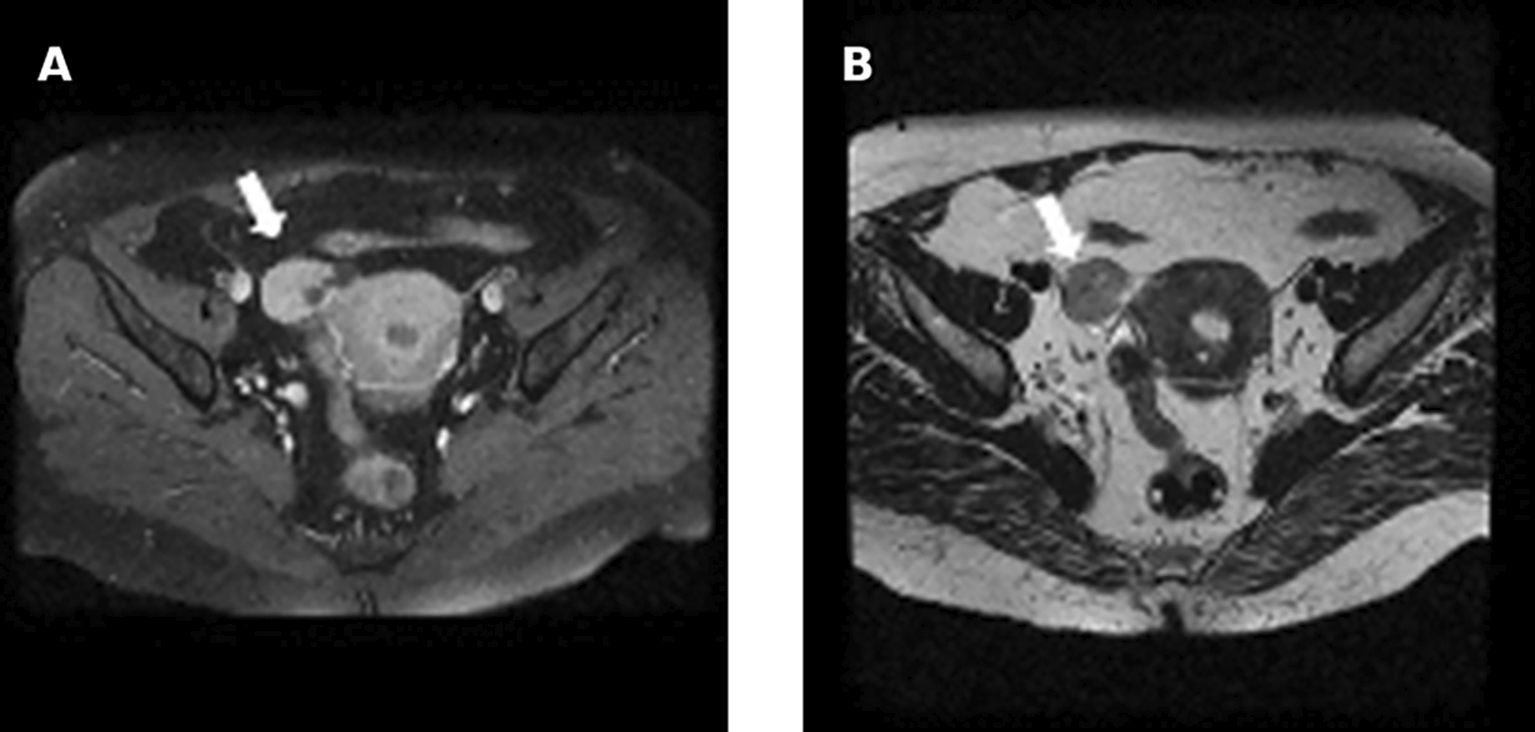
Pelvic ultrasound showed an enlarged uterus and right ovary (measured at 60*30 mm). MRI revealed an enlarged right ovary, with several follicles as well as a solid mass with intermediate signal on T2 and avid enhancement after gadolinium administration. The left ovary was of normal size 26*24 mm. No mass was found ( Figure 7).
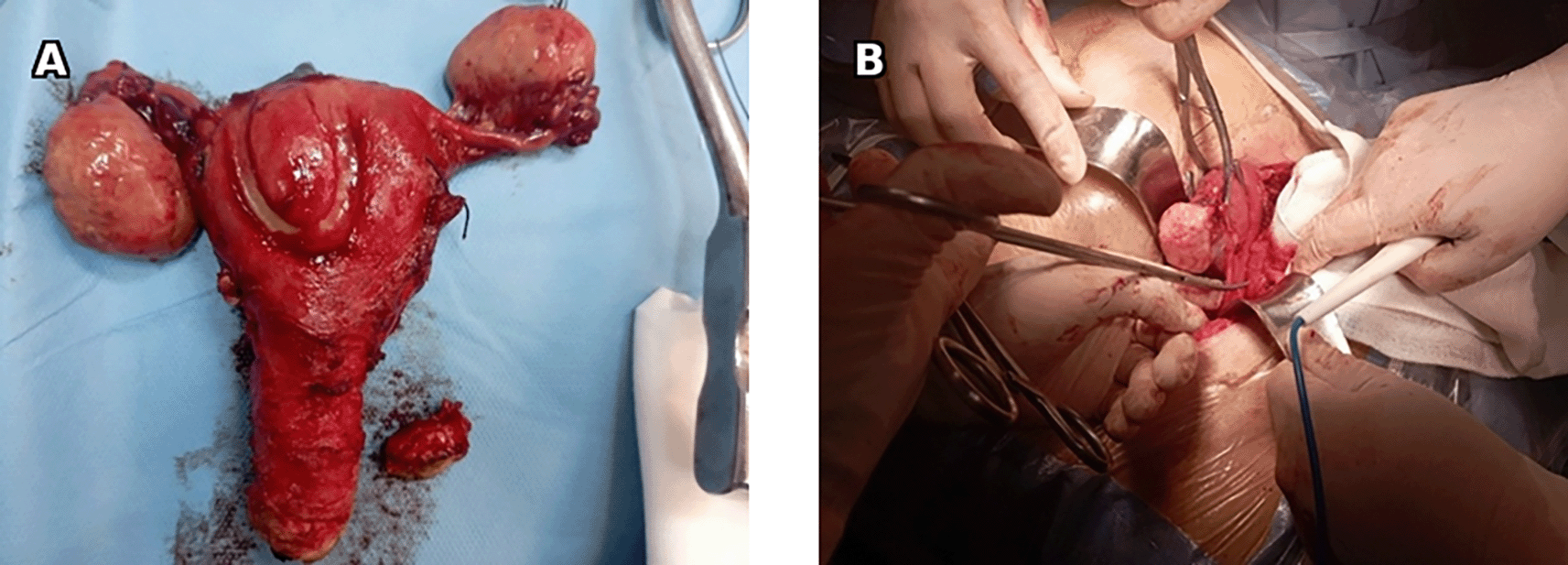
The patient underwent surgery with hysterectomy, bilateral adnexectomy, peritoneal cytology and peritoneal biopsies (Figure 8).
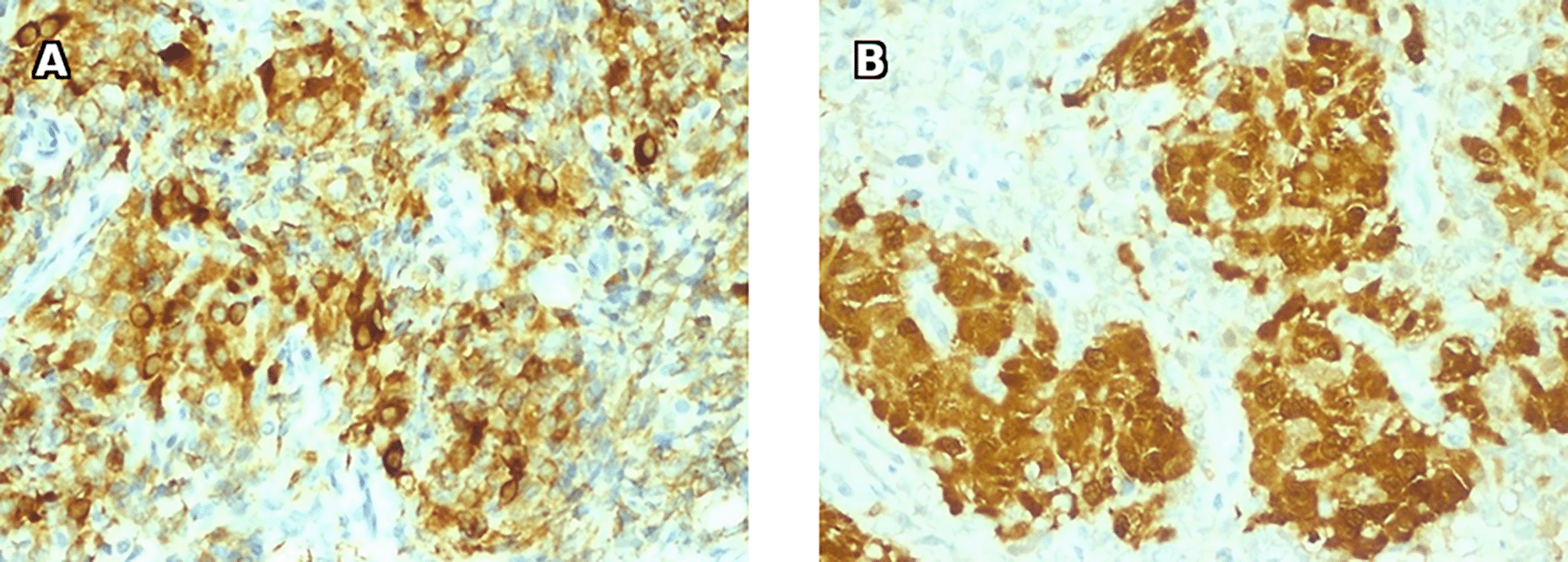
Histology results were compatible with a right ovarian fibroma with associated hyperplasia of Leydig cells, given the clinical signs of hyperandrogenism, the morphological aspects and the immunohistochemical profile ( Figures 9 and 10).
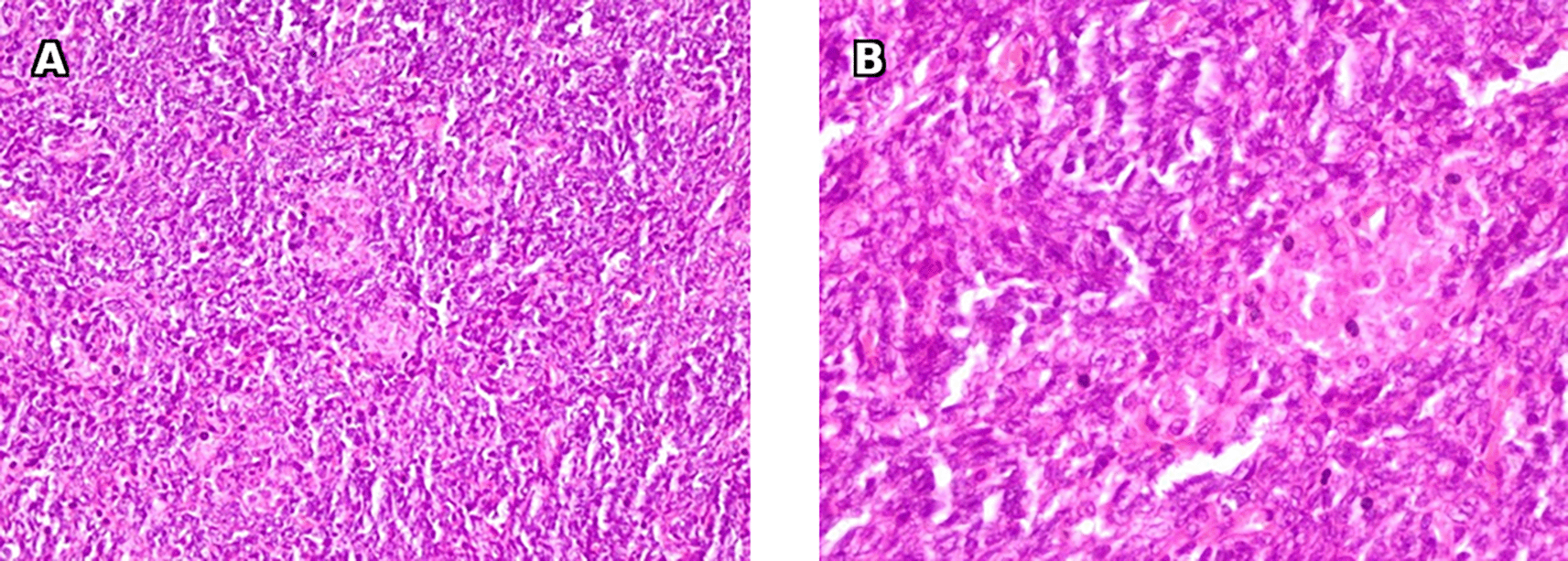
A: Microscopic examination with 200x magnification shows spindle cells proliferation with clusters of Leydig cells. B: Microscopic examination with 400x magnification shows large polygonal cells with eosinophilic non granular cytoplasm and large round nuclei and prominent nucleoli consistent with Leydig cells.
Upon follow-up, there was observed a complete remission, with testosterone levels decreasing to below 0.7 nmol/l.
A 60-year-old female patient, nulligest, menopausal at the age of 54, with a personal history of hypertension, type IIb dyslipidaemia and long-standing insulin treated type 2 diabetes with associated degenerative complications, presents with hirsutism ( Figure 11), androgenic alopecia, excessive sweating, and increased libido. Ferriman and Gallwey score was estimated at 26 with signs of virilization (muscle hypertrophy and male-pattern baldness).
Testosteronemia was at 9.22 nmol/l with normal levels of SDHEA (873 ng/ml). Lab tests revealed a normal basic cortisol level with no findings suggestive of cushing’s syndrome. A Thoraco-abdominopelvic CT was performed showing a 30mm right adrenal mass with spontaneous density at 7 HU and an absolute wash out at 35% suggestive of an adenoma. Pelvic MRI revealed a solid left ovarian mass with heterogeneous signal on T2 with an intermediate risk time intensity curve (B) on dynamic contrast enhancement, classified as ORADS category 4 ( Figure 12).
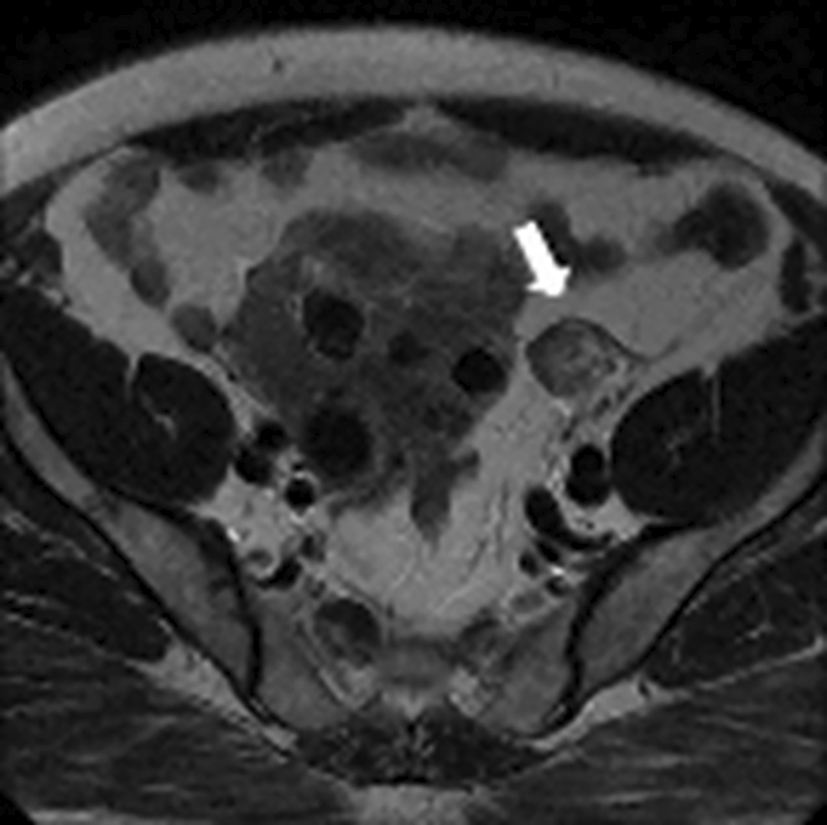
The patient underwent left adnexectomy ( Figure 13).
Histology found a fleshy, brownish, and friable nodular formation, with a well-circumscribed tumor proliferation made of large polyhedral or rounded epithelioid cells with abundant eosinophilic cytoplasm sometimes including dense, elongated rod-shaped eosinophilic crystals (Reinke crystals). The nuclei were monomorphic, rounded and nucleolated. They were frequently grouped into clusters leaving free eosinophilic cytoplasmic intervals. No tumor necrosis or capsular rupture was found. Therefore, it concluded to a benign Leydig cell tumor of the ovary ( Figure 14).
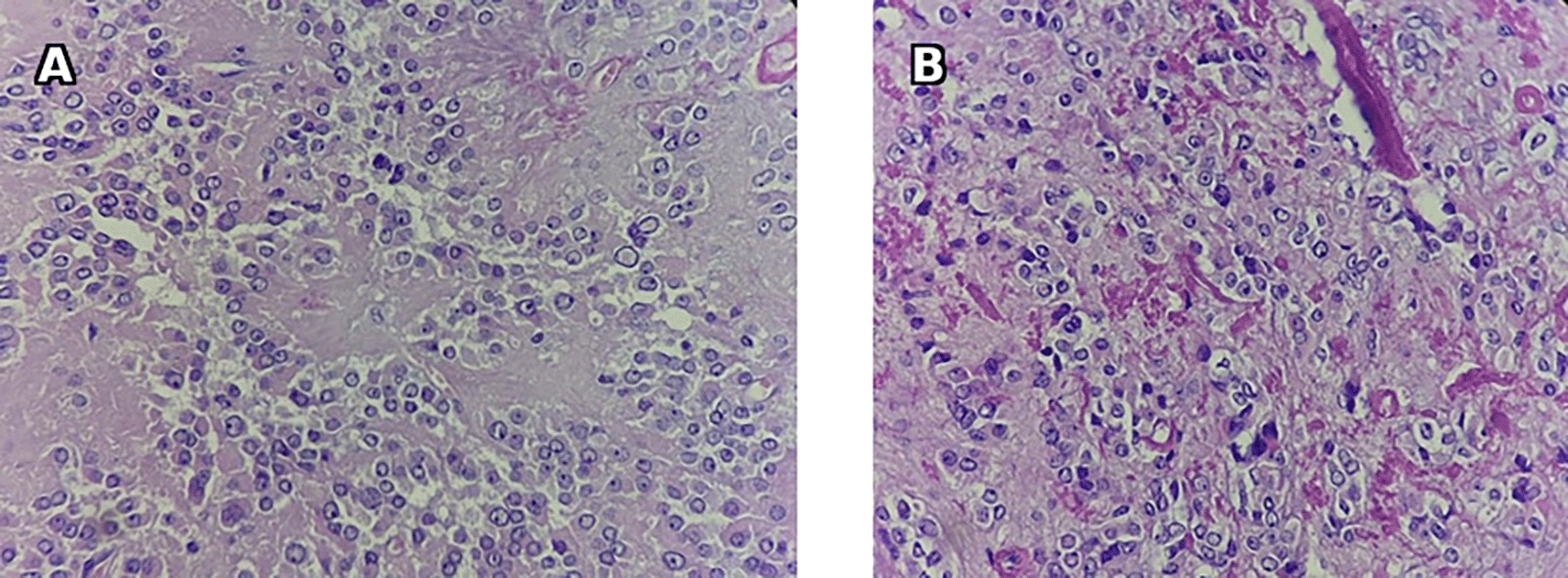
During follow-up, there was observed full resolution accompanied by a reduction in testosterone levels to <0.7 nmol/l.
The diagnosis of a tumorous origin of hyperandrogenism after menopause is mainly based on the rapid onset, progression, and severity of clinical symptoms.1 Thus, recent, and severe signs of virilization such as baldness, clitoral hypertrophy and deepening of the voice are in favor of a tumorous source of excessive androgen secretion. In our patients, the absence of hyperandrogenism in pre-menopause, the rapid progression over the years, as well as the presence of signs of virilization are clinical arguments in favor of a tumor etiology. Based on the consensus of the French Society of Endocrinology, when testosterone levels are high, blood testing of SDHEA (dehydroepiandrosterone sulfate) must be performed and increased levels may point to an adrenal cause.2 In the study by Elhassan YSla et al, normal SDHEA levels did not exclude the diagnosis of adrenocorticaloma. Furthermore, high levels of SDHEA were found in cases of hyperandrogenism of ovarian origin.3 In pre- and post-menopausal women, the two sources of androgen secretion are the ovaries and the adrenal glands. Imaging and more specifically adrenal CT and pelvic MRI are the most appropriate tools that may allow the identification of the etiology and therefore help establish a final diagnosis.4 Sertoli-Leydig cell tumors constitute less than 1% of overall ovarian tumors with 10% of them being well-differentiated and 90% moderately and poorly differentiated tumors.5,6 Sertoli-Leydig cell tumors usually occur in young women but can also occur in postmenopausal women. In studies, the mean age at diagnosis was 24 years.7 Women with well-differentiated Sertoli-Leydig cell tumors are, on average, 40 years old, 10 to 15 years older than those with moderately or poorly differentiated tumors.8,9 In our series, all women were postmenopausal. Two of them were diagnosed Sertoli-Leydig cell tumors.
Approximately 50% of Sertoli-Leydig cell tumors produce steroid hormones, and 40% of patients are virilized. Patients present with different symptoms of virilization which are evaluated using the Ferriman and Gallewey score.10 Sertoli-Leydig cell tumors tend to be unilateral and confined to the ovary (stage IA) at diagnosis. Unilateral salpingo-oophorectomy is the main treatment for most patients.11,12 Hysterectomy and bilateral salpingo-oophorectomy may be suggested for older patients. Lymph node metastases are exceptional. Regression of symptoms usually follows tumor removal.13 Well-differentiated Sertoli-Leydig cell tumor is a clinically benign tumor that does not recur after complete excision. The prognosis is usually good for patients with moderately and poorly differentiated Sertoli-Leydig cell tumors.14 In our series, patients underwent total hysterectomy with bilateral adnexectomy with complete recovery on follow up.
Hyperandrogenism after menopause is a rare condition that needs careful evaluation in order not to misdiagnose an underlying androgen-secreting tumors. Sertoli-Leydig cell tumors is the most common subtype of virilizing ovarian tumors. Treatment is surgery and complete post-operative recovery is often observed.
Written informed consent for publication of the clinical details and/or clinical images was obtained from the patients or their legal guardians. All identifiable information has been anonymized to protect patient confidentiality.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)