Keywords
Meta-analysis, Radioactive iodine, Second primary malignancy, Thyroid cancer.
This article is included in the Oncology gateway.
While the prognosis of thyroid cancer (TC) was generally excellent, there was a long-term concern regarding the risk of subsequent second primary malignancies (SPM) following radioactive iodine (RAI). This study aimed to comprehensively estimate the pooled risk of SPM occurrence following RAI therapy. The study searched the literature across three databases (PubMed, Scopus, and ScienceDirect), and was followed by citation searching. Original observational studies assessing the risk of SPM following RAI in TC were included. The Newcastle Ottawa scale was used for methodological assessment. STATA 17.0 was used for statistical analysis. A total of 18 studies encompassing 1,189,992 patients were included (638,275 in the RAI group and 551,717 in the non-RAI group). Pooled meta-analysis demonstrated no statistically significant increase in the risk of SPMs in the RAI-treated group, with a pooled RR of 1.07 (95% CI: 0.96 –1.21; p = 0.23). This result was deemed robust based on leave-one-out analysis. Subgroup analysis across publication year, follow-up duration (>10 years vs ≤10 years), population size, and SPM site (including breast, genitourinary, gastrointestinal-hepatobiliary, respiratory, skin, hematology, central nervous system, head and neck) consistently showed comparable results. There was no evidence of a significantly increased risk of SPM among TC survivors treated with RAI.
Meta-analysis, Radioactive iodine, Second primary malignancy, Thyroid cancer.
Thyroid cancer (TC) has been increasing globally over recent decades, mainly driven by greater detection of small papillary lesions due to improved imaging techniques and more extensive screening.1 GLOBOCAN 2022 has established TC as the seventh most common malignancy worldwide, with an estimated 821,214 new cases and 47,507 deaths reported in that year.2 While the prognosis of TC is generally excellent, with 10-year disease-specific survival rates often exceeding 90%, long-term survivors remain at risk of subsequent second primary malignancies (SPM).3,4
SPM is defined as a new, histologically confirmed malignancy arising in a different anatomical site (non-synchronous) from the original thyroid carcinoma, and not a metastasis or recurrence.5 Reported incidence of SPM in TC survivors varies widely, ranging from about 2.4% to over 19%, depending on patient demographics, length of follow-up, and whether or not radioactive iodine (RAI) therapy was used.6,7 Given the long-expected survival after TC, even modest elevations in SPM risk may translate into meaningful excess morbidity and mortality over time.
To date, only a limited number of meta-analyses have investigated the risk of SPM following RAI. For instance, Nappi et al. (2022) evaluated breast cancer as a second risk of cancer after RAI treatment and found no significant risk elevation.8 However, that study was limited to a single tumor site and did not assess overall SPM risk. No comprehensive meta-analysis has yet synthesized evidence across all non-thyroidal SPMs (both solid and hematologic) while accounting for these potential sources of variation. Herein, the present study aims to comprehensively estimate the pooled risk of SPM occurrence following RAI therapy.
The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.9 The protocol was registered in advance on PROSPERO (registration number CRD420251150102), and all methodological decisions reported here follow the outlined protocols.
PubMed, Scopus, and ScienceDirect databases were used as a comprehensive literature search database, from inception to March 2025, to identify studies that evaluate the risk of SPMs following RAI therapy in patients with TC. A search strategy was employed using a mix of keywords and MeSH (Medical Subject Headings) terms pertinent to thyroid cancer, radioactive iodine, and secondary primary malignancies. The specific search terms included: (“thyroid cancer” OR “thyroid carcinoma” OR “differentiated thyroid carcinoma”) AND (“radioactive iodine” OR “radioiodine” OR “I-131”) AND (“second primary malignancy” OR “second cancer” OR “second primary neoplasm”). Additionally, the reference lists of eligible articles and relevant reviews were manually screened to identify further studies.
Studies were considered eligible in this study if they fulfilled inclusion criteria: (1) original observational articles; (2) enrolled patients with histologically confirmed TC; (3) compared patients who received RAI with those who did not; (4) reported the number of SPM events and total patients in each group or provided effect estimates (risk ratio [RR], hazard ratio [HR], or odds ratio [OR]) with 95% confidence intervals (CI); and (5) reported a minimum follow-up duration of at least 2 years after thyroid cancer diagnosis. Studies were excluded under the following criteria: (1) they reported only synchronous malignancies, recurrences, or metastases; (2) lacked a non-RAI comparison group; and (3) were case reports, editorials, reviews, or conference abstracts.
Two independent reviewers were responsible for screening the titles, abstracts, and full texts of the literature to determine eligibility and for extracting relevant data from the included studies. A third party resolved any discrepancies. The extracted data comprised the following elements: the first author, year of publication, country of study, study design, population size, number of patients who received RAI, number of patients who did not receive RAI, the number of SPM events in each group, and the mean or median duration of follow-up.
The primary objective of the study was to evaluate the likelihood of patients diagnosed with TC developing non-thyroidal SPMs following treatment with RAI. This was compared to the risk faced by patients who did not receive RAI as part of their treatment regimen. The effect measure was the natural logarithm of the RR (logRR), which was pooled across studies and then back-transformed to RR for interpretability. Secondary outcomes included pooled RR estimates stratified by tumor site and the predefined subgroup variables.
The methodological quality of the studies included in the analysis was assessed using the Newcastle-Ottawa Scale (NOS), which evaluates three key domains: selection, comparability, and assessment of outcome/exposure. Studies that received a score of 7 points or higher were categorized as high-quality, while those scoring between 5 and 6 points were classified as moderate-quality. Studies with scores below 5 points were regarded as low quality. Two authors independently evaluated the risk of bias, and a third-party adjudicator resolved any discrepancies that emerged.
All statistical analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX, USA). LogRRs and SEs were calculated for each study, and pooled using a random-effects model (DerSimonian–Laird method) to account for between-study heterogeneity. Results were presented as pooled RRs with 95% CIs. Statistical heterogeneity was evaluated using Cochran’s Q test (p < 0.10 indicating significant heterogeneity) and quantified with the I2 statistic, with values of 25%, 50%, and 75% considered low, moderate, and high heterogeneity, respectively.
Leave-one-out sensitivity analyses were conducted to evaluate the influence of individual studies on the overall pooled effect. Subgroup analyses were performed in Stata to explore differences in effect estimates across predefined study-level variables, including publication year, follow-up duration, and population size. A two-sided p-value of <0.05 was considered statistically significant.
A total of 2,361 records were identified from electronic databases. After removing duplicates and ineligible records, followed by screening and full-text assessment, 18 articles were included in this study.10–27 Subsequent citation searching yielded two articles that were included in the current analysis. The complete screening process is outlined in the PRISMA flowchart ( Figure 1).
A total of 1,189,992 patients diagnosed with thyroid cancer were included in this analysis, drawn from eighteen studies. Among these patients, 638,275 received treatment with RAI, whereas 551,717 did not undergo this treatment. The studies, published between 2003 and 2023, primarily utilized retrospective or population-based cohort designs and were conducted in various countries, including South Korea, the United States, several European nations, China, Taiwan, Israel, Saudi Arabia, and Brazil. Follow-up durations ranged from 2 to 16.2 years, with the majority of studies focusing on papillary or follicular thyroid carcinoma. Comprehensive baseline characteristics are presented in Table 1.
| Author, year | Study design | Country | RAI | SPM RAI | No RAI | SPM No RAI | Type of TC | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Rubino et al., 200310 | Retrospective cohort | Swedish, Italian, and French | 37,702 | 751 | 40,253 | 652 | DTC (PTC or FTC) | 13 (2–55) years |
| Bhattacharyya and Chien, 200611 | Cohort | SEER | 10,349 | 501 | 18,882 | 1,271 | DTC (PTC and FTC) | 61.84 months (mean) |
| Cappagli et al., 202012 | Retrospective cohort | Italy | 1050 | 78 | 46 | 3 | DTC (PTC and FTC) | 13.9 ± 9.5 years |
| Hong et al., 202313 | Retrospective cohort | South Korea | 147,747 | 8,322 | 215,481 | 11,063 | TC | 8.11 ± 3.48 years |
| Kim et al., 202314 | Retrospective cohort | South Korea | 100,448 | 6,148 | 117,329 | 5,772 | TC | 7.7 (5.5-10.3) years |
| Lang et al., 201215 | Cohort | China | 643 | 56 | 252 | 8 | DTC (PTC or FTC) | 93.5 months (range, 23.4–570.8) |
| Lin et al., 201516 | Cohort | China | 7,069 | 91 | 3,292 | 38 | TC | 6.58 years (IQR 4.40–9.12) |
| Mei et al., 202117 | Cohort | SEER | 51,212 | 2,289 | 52,814 | 2,339 | DTC (PTC or FTC) | 91 (57–133) months |
| Molenaar et al., 201818 | Cohort | SEER | 68,374 | 366 | 79,033 | 417 | DTC (PTC or FTC) | 6.6 (3.1-11.4) years |
| Pasqual et al., 2022 (solid tumor)19 | Cohort | SEER | 14,877 | 764 | 12,173 | 760 | DTC (PTC or FTC) | 12.7 (IQR 4.8; 24.5) years |
| Pasqual et al., 2022 (hematological malignancies)19 | Cohort | SEER | 14,477 | 65 | 17,694 | 81 | DTC (PTC or FTC) | 12.7 (IQR 4.8; 24.5) years |
| Souza et al., 201520 | Retrospective cohort | Brazil | 252 | 13 | 161 | 4 | DTC (PTC or FTC) | 11.0 ± 7.5 years |
| Wu et al., 202321 | Retrospective Cohort | SEER | 61,210 | 4,154 | 69,692 | 4,450 | DTC (PTC or FTC) | 85 (41-141) months |
| Hakala et al., 201522 | Retrospective cohort | Finland | 840 | 83 | 170 | 26 | DTC (PTC or FTC) | 16.2 ± 6.9 years |
| Hirsch et al., 201623 | Cohort | Israel | 1,335 | 132 | 296 | 29 | DTC (PTC or FTC) | 9.3 ± 9.6 years |
| Ko et al., 201524 | Cohort | Taiwan | 1,834 | 78 | 1,834 | 61 | DTC (PTC or FTC) | 5.55 ± 3.27 years |
| Seo et al., 202125 | Retrospective cohort | South Korea | 9,548 | 81 | 9,069 | 43 | TC | 79 months (IQR = 46–108 months) |
| Silva-Viera et al., 201726 | Retrospective cohort | Portugal | 1,570 | 108 | 461 | 22 | DTC (PTC or FTC) | 8.8 years (range 5.0–17.0 years) |
| Al-Qahtani et al., 201527 | Retrospective cohort | Saudi | 732 | 41 | 91 | 6 | DTC (PTC or FTC) | 8.05 years (range: 1.62-11.4) |
The evaluation of the study quality revealed that 11 studies were rated as of good quality, with scores ranging from 7 to 9; six were considered of fair quality, receiving scores of 5 to 6; and one was identified as of poor quality, earning a score of 3 ( Table 2). Most population-based studies (e.g., Rubino et al. 2003; Bhattacharyya & Chien, 2006; Kim et al., 2023; Pasqual et al., 2022) demonstrated clear cohort selection, strong outcome ascertainment through cancer registries, and adequate follow-up durations. In contrast, several institutional studies faced challenges due to limited sample sizes and insufficient adjustment for confounding variables.
The pooled analysis revealed no statistically significant elevation in the risk of SPMs among the RAI-treated cohort. The pooled RR was calculated to be 1.07 (95% confidence interval: 0.96–1.21; p = 0.23), with substantial heterogeneity noted (I2 = 95.96%; see Figure 2). Leave-one-out sensitivity analysis yielded a robust result, as the sequential omission of each study resulted in a pooled logRR that remained consistent with all comparable p-values ( Figure 3).
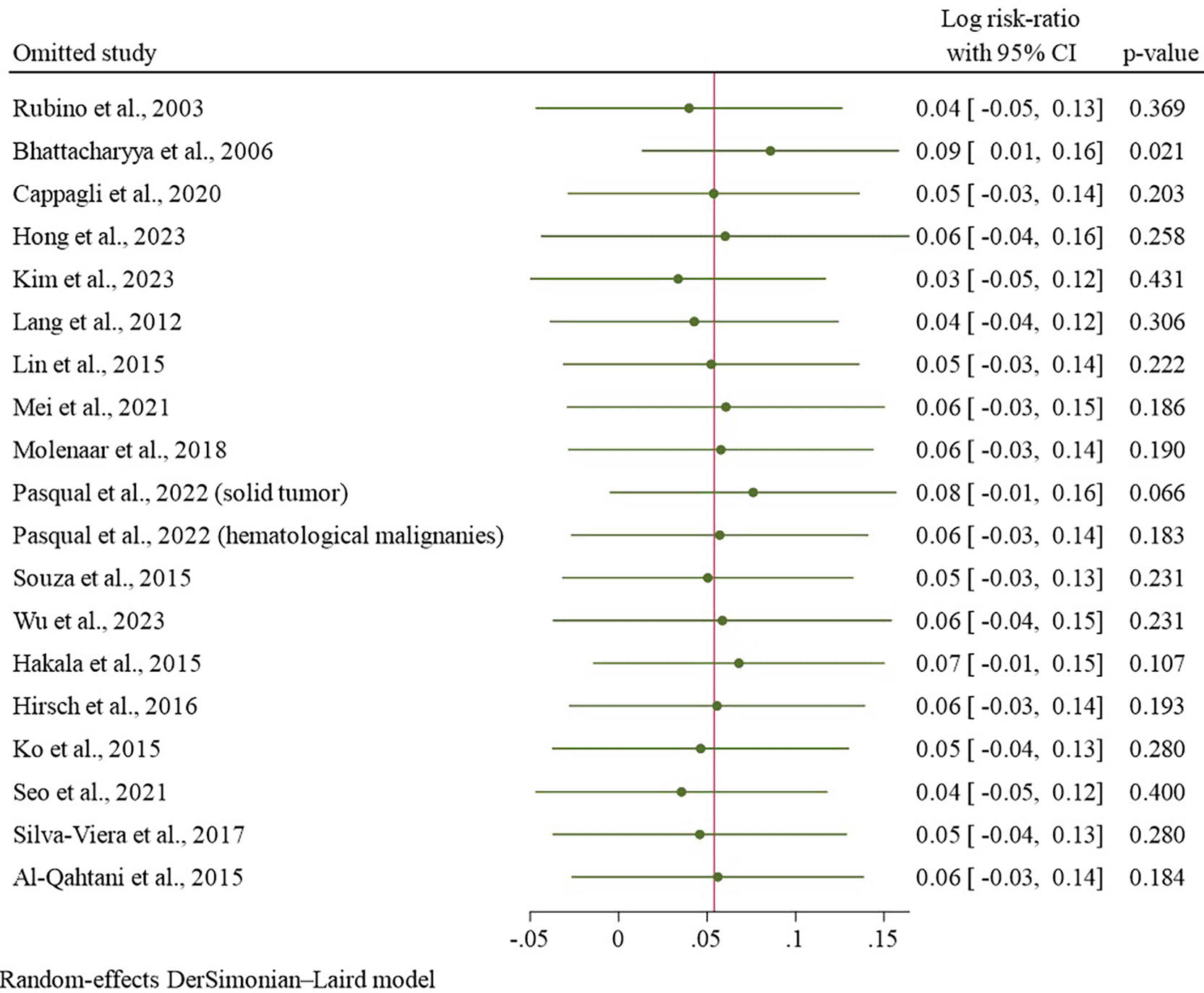
Subgroup analyses were performed to investigate potential factors contributing to the heterogeneity in the relationship between RAI therapy and the incidence of SPMs among TC survivors, as detailed in Table 3. No significant differences were observed by publication year (≤2015: logRR = 0.08; >2015: 0.07), follow-up duration (≤10 years: 0.08; >10 years: –0.03), or population size (≤10,000: 0.17; >10,000: 0.04). Similarly, site-specific analyses showed no significant increase in risk for any tumor group, including breast (–0.09), genitourinary (0.36), gastrointestinal/hepatobiliary (–0.15), respiratory (–0.22), skin (–0.02), hematologic (–0.16), central nervous system (0.03), and head and neck (0.15). Overall, none of the predefined subgroups significantly modified the association between RAI therapy and SPM risk.
The evaluation of publication bias was conducted by creating a funnel plot and performing Egger’s regression test. The funnel plot indicated an approximately symmetrical distribution of effect sizes around the pooled estimate, with only minor deviations among studies that had larger standard errors, suggesting the absence of significant asymmetry (see Figure 4). Furthermore, Egger’s regression test did not indicate any evidence of small-study effects, with a bias coefficient of -0.53 (95% CI: -2.66 to 1.60; p = 0.609).
This systematic review and meta-analysis, encompassing 18 cohort studies and over 1.1 million TC survivors, found no statistically significant increase in the risk of SPM associated with RAI therapy. Our subgroup analyses, by publication year, follow-up duration, population size, and SPM tumor site, revealed a consistent effect across all potential confounders, highlighting the safety profile of RAI.
These findings align with prior large population-based studies, such as those by Rubino et al. (2003) and de Vathaire et al. (1997), which reported no significant overall increase in SPM risk after RAI despite modest elevations for certain organ-specific cancers.10,28 Conversely, some studies have suggested a dose-related increase in SPM risk, particularly for leukemia or salivary gland cancers, at high cumulative activities (>150 mCi). However, most of these studies involved relatively small cohorts and reported few SPM events, raising concerns about statistical power and the robustness of those associations.10,29,30 Our findings therefore provide more substantial evidence, derived from aggregated data, that the association between RAI and subsequent malignancies is likely weak or absent at the population level.
Several limitations of the included evidence warrant consideration. First, the predominance of retrospective and registry-based designs introduces potential for residual confounding, especially regarding cumulative RAI dose, radiation from surveillance imaging, and lifestyle factors such as smoking or obesity. Second, many studies lacked detailed dose stratification, which prevented the assessment of dose–response relationships. Third, follow-up durations varied widely (2–16 years), and some cohorts may not have had sufficient latency to capture late-onset SPMs. Finally, heterogeneity in outcome ascertainment (e.g., variable cancer registry linkages) could have contributed to the high I2 observed.
From a clinical perspective, our findings suggest that RAI therapy should not be withheld from appropriate DTC patients out of concern for inducing second malignancies, particularly at commonly used doses. The ongoing heterogeneity in the findings highlights the importance of exercising caution when generalizing these results. Future investigations should prioritize large-scale pooled analyses that employ standardized definitions of SPM, incorporate long-term follow-up periods surpassing 20 years, and utilize detailed dose data. This approach is essential to elucidate potential dose-response thresholds related to risk. Additionally, molecular epidemiological studies may help identify susceptible subgroups (e.g., younger patients, genetic predispositions) who may be at an increased risk of radiation-induced malignancies.
Our meta-analysis showed substantial evidence indicating there was no significant elevation in the risk of SPM among TC survivors who underwent RAI therapy. These results endorse the ongoing application of RAI in suitably selected TC patients. However, it also emphasizes the need for tailored risk-benefit evaluations and ongoing surveillance, particularly for individuals classified within higher-risk subgroups.
Zenodo: Risk of second primary malignancies following radioactive iodine therapy in thyroid cancer: A meta-analysis of 1,189,992 patients. Doi: https://doi.org/10.5281/zenodo.1735068531
This project contains the following underlying data:
• PRISMA_2020_checklist.pdf
• Breast.xlsx
• CNS.xlsx
• Follow-up less 10.xlsx
• Follow-up more 10.xlsx
• Gastrointestinal and Hepatobilier.xlsx
• Genitourinary.xlsx
• Head and neck.xlsx
• Hemato.xlsx
• Large population.xlsx
• Publication less 2015.xlsx
• Publication more 2015.xlsx
• Respiratory.xlsx
• Skin.xlsx
• Small population.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Zenodo: Risk of second primary malignancies following radioactive iodine therapy in thyroid cancer: A meta-analysis of 1,189,992 patients, Doi: https://doi.org/10.5281/zenodo.1736157332
The Project contains the following reporting guidelines:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Zenodo: Risk of second primary malignancies following radioactive iodine therapy in thyroid cancer: A meta-analysis of 1,189,992 patients, Doi: https://doi.org/10.5281/zenodo.1736131133
The Project contains the following reporting guidelines:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to express their sincere gratitude to the Institute for Research and Community Service (LPPM), Universitas Andalas, for supporting this work through the Research Program for Fiscal Year 2025 (Grant No. 423/UN16.19/PT.01.03/PAR/2025).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)