Keywords
Mature Teratoma, Intracranial Germ Cell Tumor, Case Report, Intraoperative Hemorrhage, Intraventricular tumor
This article is included in the Oncology gateway.
Intracranial teratomas are rare central nervous system germ cell tumors, and their occurrence within the third ventricle of pediatric patients presents significant surgical challenges owing to the proximity of critical neurovascular structures. We report the case of an 8-year-old boy who presented with hydrocephalus and seizures caused by a large multiloculated cystic and solid mass in the third ventricle. An initial attempt at gross total resection via a transcortical transventricular approach was complicated by a massive, life-threatening hemorrhage upon dissection of the tumor from the ventricular floor, which was suspected to be an injury to the internal cerebral vein. The procedure was aborted, and bleeding was controlled with hemostatic packing. After a 10-day period of stabilization in the pediatric intensive care unit, the patient underwent a second craniotomy to remove the packing and resect the tumor. Histopathological analysis confirmed the diagnosis of mature teratoma. Postoperatively, the patient required a new ventriculoperitoneal shunt to treat persistent hydrocephalus and residual left-sided hemiparesis. This case highlights the profound risk of vascular injury during resection of large intraventricular teratomas. It also demonstrates that a staged surgical approach can be a crucial life-saving strategy for managing severe intraoperative complications, allowing patient stabilization before definitive tumor removal.
Mature Teratoma, Intracranial Germ Cell Tumor, Case Report, Intraoperative Hemorrhage, Intraventricular tumor
Intracranial teratomas are rare germ cell tumor subtypes of the central nervous system, comprising only approximately 0.5% of all intracranial tumors and less than 5% of the pediatric population. Intracranial teratomas, like other teratomas, commonly affect midline structures, such as the pineal gland, quadrigeminal plate, wall of the third ventricle, suprasellar region, or cerebellar vermis.1 Intraventricular teratomas are large at presentation with hydrocephalus and lobulated cystic masses on imaging modalities.2 We presented a case of intraventricular lobulated cystic mature teratoma in an 8-year-old male with massive intraoperative bleeding.
An 8-year-old boy presented to our center with a history of decreased consciousness, generalized seizures, progressive headache, and vomiting one week prior to admission to our hospital. The patient had previously undergone a VP shunt for communicating hydrocephalus because of tuberculous meningitis 1 year prior and clinically improved. He underwent a head CT scan due to this latest clinical deterioration, which showed hydrocephalus with a lobulated cystic and solid mass in the 3rd ventricle. Subsequent contrast-enhanced CT imaging indicated the presence of vascular structures surrounding the tumor. The internal cerebral vein was visible, crossing beneath the tumor (Figure 1). The patient also underwent contrast head MRI, and the result showed a well-defined multiloculated mass with cystic and solid components filling the 3rd Ventricle which was enhanced inhomogenously with gadolinium contrast administration and enlarged lateral ventricles (Figure 2). An endoscopic tumor biopsy was performed, and the histopathological results suggested that the tumor was an arachnoid cyst.
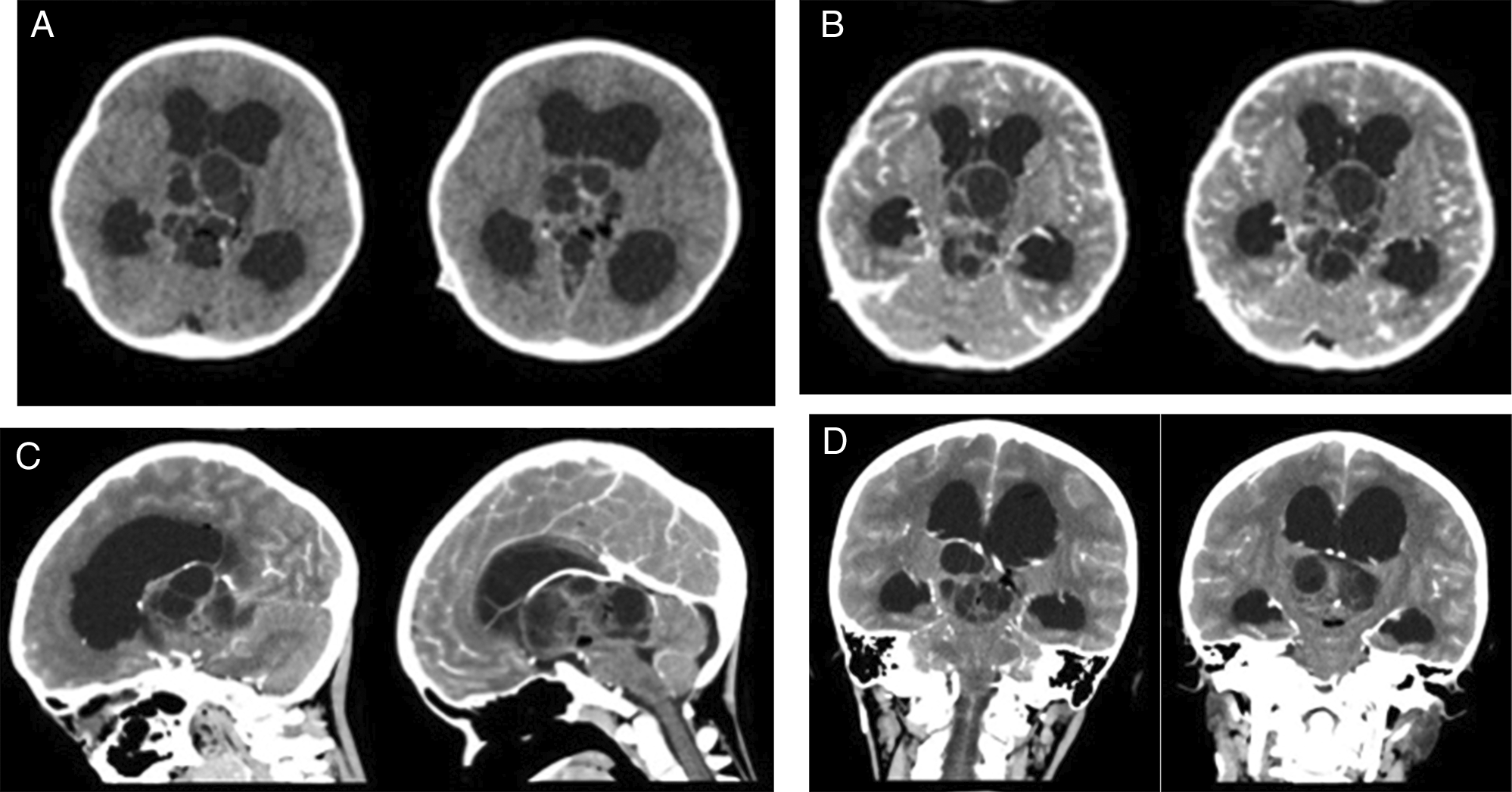
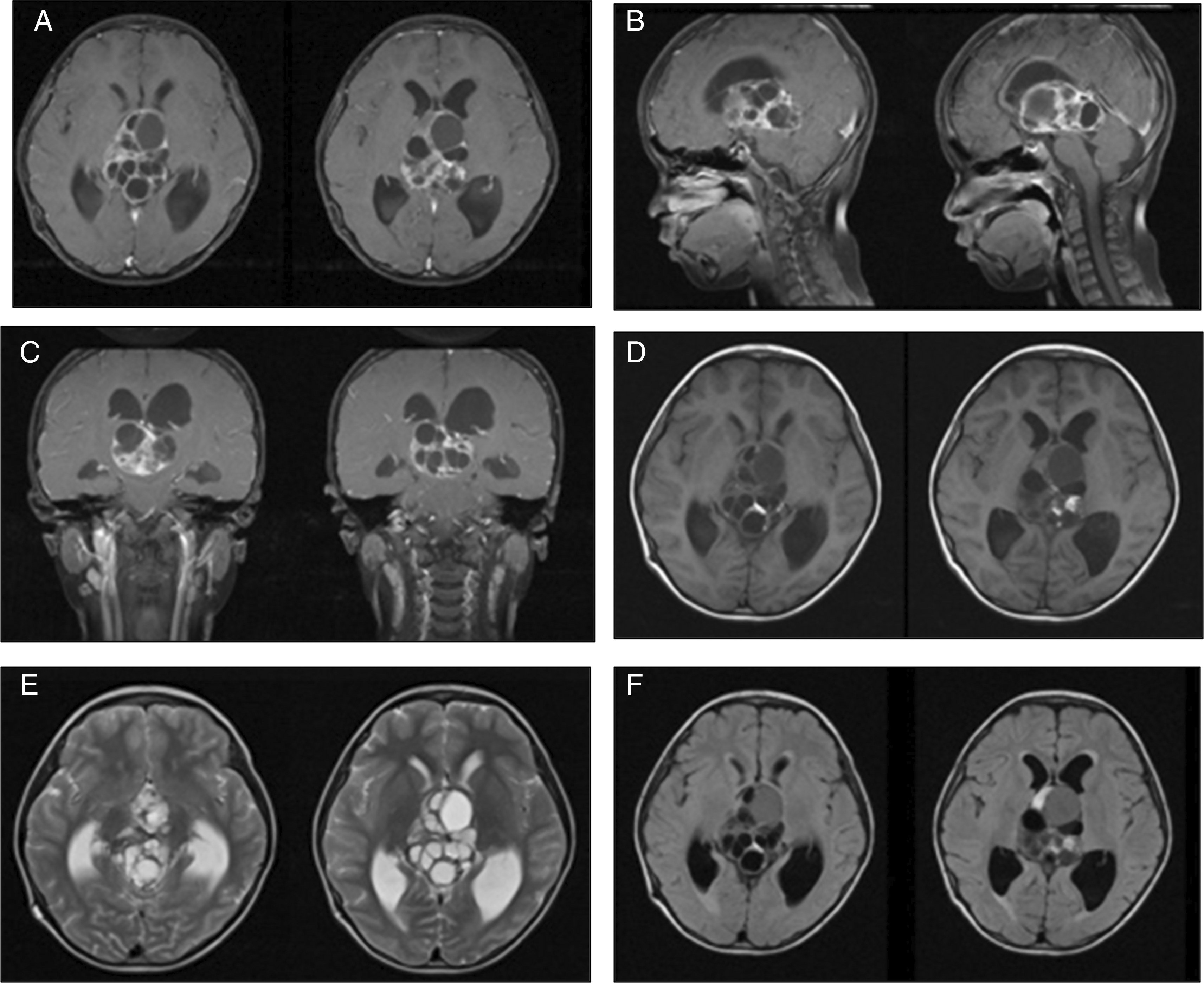
These images showed heterogenous intraventricular multilobulated thick-walled mass with solid and cystic component in T1WI and contrast T1WI imaging (A-D). T2W1 and Flair showed cystic component of the mass with minimal peritumoral edema (E, F).
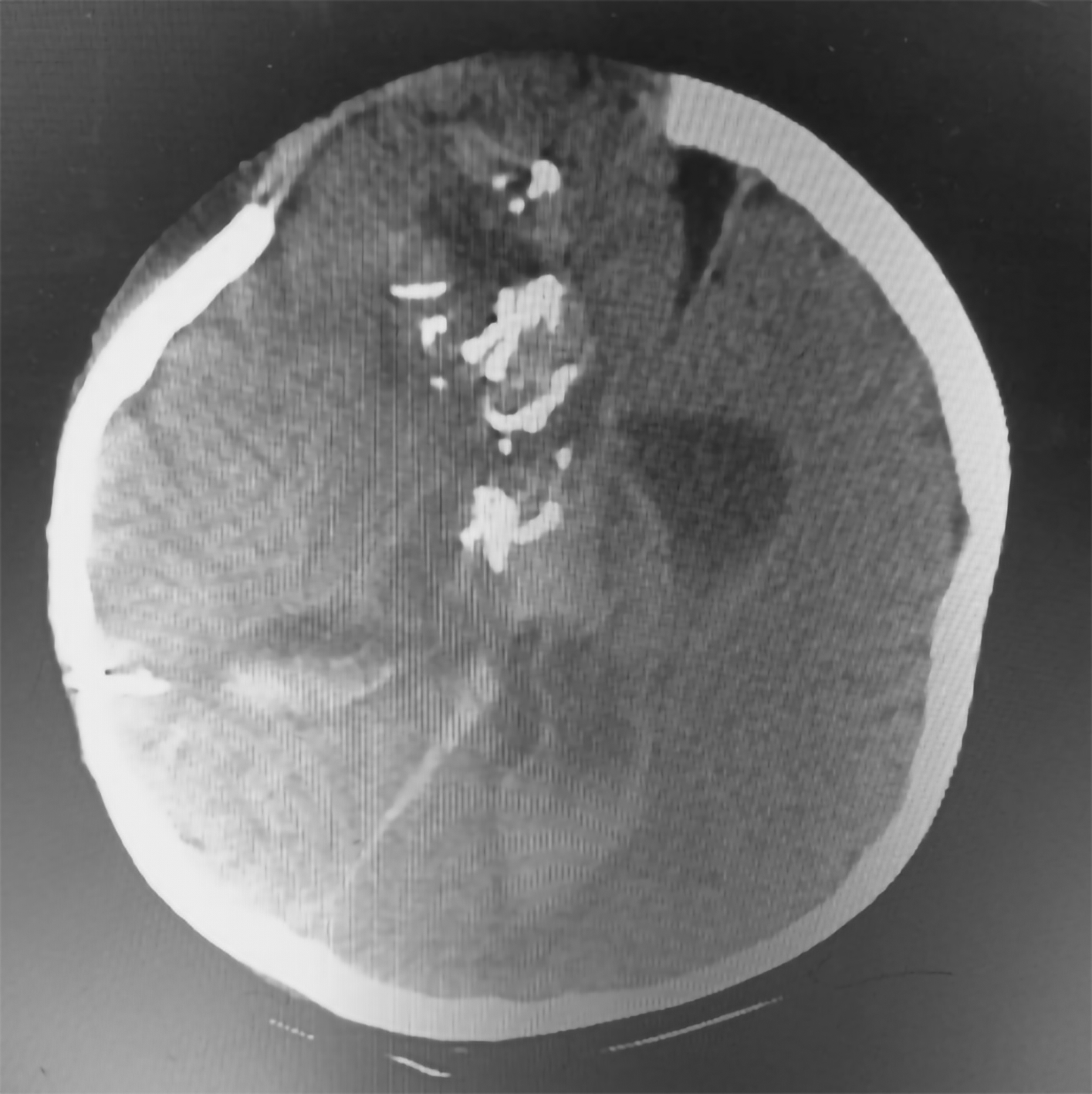
Tumor removal was performed through a transfrontal transcortical transventricular approach towards the 3rd ventricle. We aimed for gross total tumor removal and found a multiloculated solid cystic mass with thick glistening greyish content attached to the septum pellucidum and bilateral fornix. Massive bleeding occurred upon removal of the tumor from the bed, and we suspected that the internal cerebral vein was injured. The patient lose almost 1.5 L of blood and suffered from hemorrhagic shock during fluid resuscitation and blood transfusion were being given. We packed the bleeding with cotton patties, and the surgery was stopped for salvage. The patient was sent to the pediatric ICU for stabilization, knocked down for three days, and regained consciousness. Another CT scan revealed intraventricular hemorrhage (Figure 3). We performed another craniotomy to remove the hemostasis pack 10 days later. Histopathological examination revealed a mature teratoma consisting of flat epithelial cells, fat cells, cartilage, chondrocytes, and ciliated pseudostratified epithelial cells (Figure 4). The patient was discharged one week after the 2nd craniotomy. The postoperative CT scan revealed a small remaining intraventricular tumor and hydrocephalus; therefore, another VP shunt was placed on the contralateral side (Figure 5). After surgery, the patient had a GCS score of 15, but the developed left hemiparesis. The clinical timeline is shown in Figure 6.
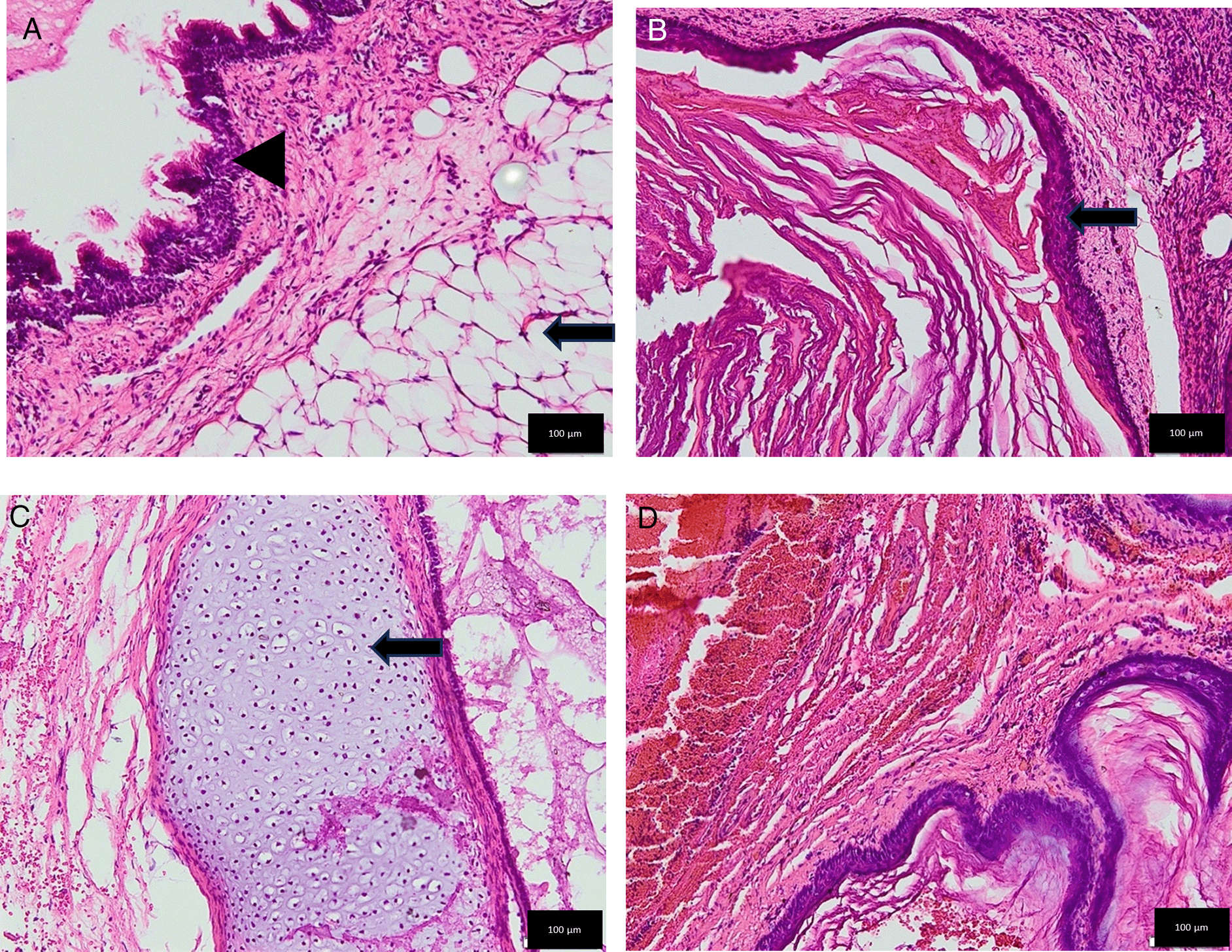
(A) The mesoderm component appeared as mature fat cells with nuclei at the edges within normal limits (black arrow) and the endoderm component in the form of ciliated pseudostratified epithelial tissue with nuclei within normal limits (black arrowhead). (B) The ectoderm component showed layered squamous epithelium. (Black arrow). (C) The mesoderm component revealed as cartilage tissue with chondrocytes (Black arrow). (D) No neuroectodermal component was found.
Intracranial Germ cell tumors (GCTs) are a rare entity that typically arise from primordial germ cells that grow ectopically in the Central Nervous System.3 While they can occur in individuals of all ages, they are most frequently diagnosed in patients under 20 years of age, predominantly male. GCTs account for approximately 3% of all pediatric intracranial tumors.4 According to the 2021 WHO Classification of Tumors of the Central Nervous System, GCTs include teratomas, germinomas, embryonal carcinomas, yolk sac tumors, choriocarcinomas, and mixed germ cell tumors.5 Teratoma accounts for approximately 0.5% of all primary intracranial tumors and 20% of all intracranial GCTs.6
Pathogenesis of teratoma starts from embryogenesis. During primitive streak formation, embryonic cells at various developmental stages may be displaced within the bilaminar embryonic disc. They could become part of the lateral mesoderm stream and be carried to the neural plate area, where they may be incorrectly incorporated into the brain during neural tube formation. Tumors composed of cells resembling those seen in the earlier stages of embryonic development (ontogenesis) tend to be more malignant than those composed of cells resembling cells from later stages of embryonic development. Teratomas appeared to arise from misinvolved, misenfolded cells during the embryonic period during the 4th week post-conception.7
On histological examination, teratomas were classified into three forms based on the maturation rate of the germ cells: 1) mature teratomas composed of fully differentiated cells, such as bone, fat, muscle, cartilage, epithelium, and brain. 2) Immature teratoma consist of primitive cell forms that have a higher potential for malignant transformation than mature forms. 3) Teratoma with somatic type malignancy: teratomatous neoplasm with additional malignant somatic tissue.8,9 In our case, histopathological results showed fully differentiated cells from all layers of germ cells, without immature or malignant components, indicating that it was a mature teratoma. Macroscopically, the teratoma appeared as a solid cystic lobulated mass with a greyish red color and sometimes consisted of hair, fat, or bones.10 However, we did not find grossly mature structures, but observed them microscopically.
Patients with intracranial germ cell tumors, including teratomas, usually present with a wide range of clinical pictures, but mostly due to increased intracranial pressure, including headache, nausea and vomiting, polyuria and/or polydipsia, double vision, changes in visual acuity or visual field defects, fatigue, and hormonal disturbances. Nearly half of patients had hydrocephalus at diagnosis. This correlates strongly with the location of tumors; most intracranial GCTs arise at the midline in or around the suprasellar, third ventricle, or pineal region. Pineal tumors manifest as symptoms of hydrocephalus, whereas suprasellar tumors cause symptoms related to endocrinopathies.11,12
In terms of imaging modalities, intracranial teratomas often present as single or multiloculated ovoid or lobulated masses. Intracranial teratomas are frequently observed to have fatty components, cystic areas, and calcifications on CT images.13 In MRI, teratomas usually appear as well-defined masses with mixed signals on T1- and T2-weighted images. Fatty and highly proteinaceous material typically appear brighter on T1WI, while calcification and blood are usually darker on both T1WI and T2WI. The cystic, solid, and hemorrhagic regions showed varied signals on T2WI. Gadolinium contrast enhancement is generally heterogeneous.2 Liu et al. (2013) showed that malignant teratomas exhibit significant irregular enhancement on contrast-enhanced T1-weighted images, indicating vascular proliferation in the solid region of the tumor. This marked enhancement is key to differentiating between mature and malignant teratomas.10
The objective of treatment is to completely remove tumors when feasible, with a specific approach depending on their location. A CSF diversion procedure may be needed in addition to tumor removal if associated hydrocephalus is present. Adjuvant therapies, such as radiotherapy and chemotherapy, should be used only for immature or malignant tumors. Prognosis is generally unfavorable for immature teratomas and teratomas with malignant transformation, whereas mature teratomas tend to have a good prognosis.1,6,14 Mature teratomas are often firmly attached to neighboring tissues, especially after repeated surgeries. Gamma Knife radiosurgery has been successfully used as an alternative treatment for high-risk patients with residual or recurrent intracranial teratomas.15 We performed craniotomy through a transfrontal transcortical transventricular transforaminal approach towards the 3rd ventricle with the target of total tumor removal.
However, in our case, the internal cerebral vein was inadvertently injured during the tumor resection. Venous injury during neurosurgery is common; however, there is little concern in the literature is rather scarce. The occurrence of unpredicted postoperative complications in the field of neurosurgery is often attributed to the lack of preventive measures or failure to identify venous issues, particularly damage to critical venous structures such as the major dural sinuses, deep cerebral veins, and prominent superficial veins.16 During surgical procedures, electrocoagulation or ligation of the venous sinuses may be used to treat injured venous vessels. Venous injuries can result in conditions, such as cerebral venous infarction, brain edema, venous thrombosis, necrosis, and hemorrhage, each with variable outcomes and prognoses. While minor venous injuries may be asymptomatic, extensive damage can lead to mild edema, infarction, and minor bleeding, without significant complications. However, injuries to the central vein and vein of Labbé are associated with high morbidity and mortality rates.17 Various strategies can be employed to ensure proper venous protection during a craniotomy. These include meticulous preoperative planning to determine the most effective approach, optimal patient positioning, sacrificing adequate bone for skull base craniotomy to increase the operative field, and cisternostomy to maximize brain relaxation, while minimizing neurovascular complications. In cases where venous sacrifice occurs accidentally, venous reconstruction and ligation can be performed to manage complications.18 We tried to coagulate and pack the venous injury with cotton patties; however, when we unpacked the bleeding persisted. Therefore, we decided to leave them and perform re-craniotomy to remove the hemostatic plug several days later because the hemodynamic status of the patient was unstable during the bleeding, although massive fluid resuscitation and blood transfusion were administered to the patient. Furthermore, it was difficult to perform vascular reconstruction through a small transcortical opening towards the base of the ventricle where the tumor bed lay. Following subtotal resection of the intraventricular tumor, permanent shunting was sometimes needed for hydrocephalus treatment, as shown in our case.19
Intraventricular teratomas are rare tumors in the pediatric population. It can be detected through imaging modalities; however, histopathological results are mandatory for confirming the diagnosis. Complete surgical resection is the treatment of choice for mature intracranial teratoma. Meticulous care should be taken to preserve the surrounding neurovascular structures to limit morbidity and improve outcomes.
Written informed consent was obtained from both patient’s parents for the publication of this case report and any accompanying images.
Repository: CARE checklist for ‘Third Ventricle Multiloculated Cystic Mature Teratoma with Intraoperative Massive Bleeding: A Case Report’, https://doi.org/10.6084/m9.figshare.29978263.20
Data are available under the terms of the Creative Commons Zero “No rights reserved’ data waiver (CC0 1.0 Public domain dedication).
The authors would like to express their gratitude to TP Setiabudiawan for helping with the outline of this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Saloum A, Al-Nuaimy Y, Baloi D, Karsy M, et al.: Advances in the Multimodal Management of Pediatric Arteriovenous Malformations: A 10-Year Review. Life. 2025; 15 (10). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: SAH
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical Pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 29 Oct 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)