Keywords
Asymptomatic Bacteriuria, Antibiotic Resistance, Escherichia coli, Public Health and Urinary Tract Infections
This article is included in the Pathogens gateway.
This article is included in the Antimicrobial Resistance collection.
Asymptomatic bacteriuria (ASB) is an under-recognized clinical condition, particularly in low-resource settings, with significant implications for antimicrobial resistance and public health. This study investigated the prevalence, microbial profile, and antibiotic susceptibility patterns of ASB among individuals residing in public housing in Obinze, Imo State, Nigeria.
A descriptive cross-sectional study was conducted with 150 randomly selected asymptomatic individuals. Structured questionnaires were used to collect sociodemographic data, assess hygiene practices, and evaluate ASB awareness. Midstream urine samples were collected and subjected to standard microbiological analyses, including culture, gram staining, and biochemical tests. The isolates were tested for antibiotic susceptibility using the Kirby-Bauer disc diffusion method. The data were analyzed using SPSS version 28.
A total of 75 bacterial isolates were recovered. Escherichia coli was the most prevalent organism (56%), followed by Staphylococcus aureus (28%), Enterococcus spp., Pseudomonas aeruginosa (6.7% each), and Klebsiella spp. (2.6%). Most participants exhibited poor awareness of ASB, with only 2% accurately identifying its diagnostic threshold. Hygiene practices varied, with a high proportion reporting inappropriate genital cleaning methods and urination in public spaces due to a lack of sanitation facilities. Antibiotic resistance was observed among the isolates, highlighting the potential threat of community-based reservoirs for resistant strains.
This study revealed a high burden of ASB and identified critical gaps in public awareness, hygiene practices, and sanitation infrastructure. These findings underscore the urgent need for targeted health education, routine ASB screening, improved sanitation, and antibiotic stewardship programs to mitigate public health risks in similar settings.
Asymptomatic Bacteriuria, Antibiotic Resistance, Escherichia coli, Public Health and Urinary Tract Infections
○ The study found a 100% prevalence of asymptomatic bacteriuria among participants, confirming that it is a widespread but silent condition in the study area.
○ A total of 75 bacterial isolates were recovered from 50 urine samples, indicating a high level of polymicrobial colonization within the population.
○ Escherichia coli was identified as the most prevalent uropathogen, accounting for 56% of the isolates, followed by Staphylococcus aureus at 28%, suggesting both enteric and environmental sources of infection.
○ Awareness of asymptomatic bacteriuria was critically low, with only 2% of participants correctly identifying the diagnostic threshold, reflecting a significant knowledge gap.
○ Hygiene and sanitation practices were generally poor, with widespread reports of public urination and improper genital cleansing methods among the participants.
○ Antibiotic susceptibility testing revealed notable levels of multidrug resistance, particularly among E. coli and S. aureus isolates, indicating a growing threat of antimicrobial resistance at the community level.
Urinary tract infections (UTIs) continue to represent one of the most common bacterial infections globally, affecting individuals across all age groups and sexes (Zhou et al., 2023). However, within the spectrum of UTIs lies a frequently overlooked condition asymptomatic bacteriuria (ASB) which remains largely undetected and underreported, particularly in low-resource settings (Chowdhury et al., 2024). Despite the absence of clinical symptoms, ASB can pose significant health risks, especially among vulnerable populations, such as pregnant women, individuals with diabetes mellitus, older adults, and immunocompromised patients. In these groups, persistence of bacterial colonization in the urinary tract without intervention has been associated with adverse outcomes. This includes pyelonephritis, preterm birth, low birth weight, and systemic infections, such as urosepsis (Schneeberger et al., 2014).
The neglect of asymptomatic bacteriuria (ASB) in public health policies and clinical guidelines is partly due to limited awareness and the absence of robust surveillance systems in many low- and middle-income countries (LMICs) (Alhassan & Wills, 2024). In regions such as sub-Saharan Africa, diagnostic services are often centralized, and routine urine culture screening is not commonly practiced owing to cost constraints, workforce shortages, and infrastructural limitations (UROKO & Morgan, 2024). These challenges are compounded by the widespread availability of over-the-counter antibiotics and high levels of self-medication, which promote the misuse of antimicrobials and contribute to the global threat of antimicrobial resistance (AMR) (Ofori et al., 2024).
The World Health Organization (WHO, 2019) has highlighted the urgent need for local epidemiological data to guide antimicrobial stewardship efforts and promote rational prescription practices. Similarly, the Centers for Disease Control and Prevention (CDC, 2022) emphasize the role of undiagnosed or inappropriately treated infections, such as ASB, as hidden drivers of antimicrobial resistance (Salam et al., 2023). Without accurate baseline data on the prevalence of ASB and resistance profiles of uropathogens, healthcare providers in LMICs are often forced to rely on empirical therapies that may be ineffective, thereby promoting resistance patterns and compromising patient outcomes (Schneeberger et al., 2014).
In Nigeria, most studies on ASB have focused narrowly on specific subpopulations, particularly antenatal women and HIV-positive individuals, and have been conducted predominantly in tertiary health facilities (Salihu et al., 2025). While these studies provide valuable insights, they do not reflect the broader population-level burden of ASB. This is especially true in semi-urban and peri-urban communities, where access to healthcare is variable and water sanitation is often inadequate. In such settings, environmental and behavioral risk factors, including poor personal hygiene, the use of untreated water, and limited healthcare-seeking behavior, can facilitate urinary tract colonization and bacterial persistence (Dickson-Gomez et al., 2023).
Imo State in southeastern Nigeria presents a relevant case for such an investigation. It comprises a mix of urban and semi-rural settlements, with varied levels of healthcare access and sanitation infrastructure (UROKO & Morgan, 2024). However, data on the prevalence and antimicrobial susceptibility of ASBs in the general population remain scarce. A comprehensive understanding of the local burden of asymptomatic bacteriuria is critical for designing evidence-based screening programs and for informing empirical treatment guidelines. This is important for developing context-specific strategies to combat AMR and improve patient outcomes (Ogbonna et al., 2024).
This study was undertaken to determine the prevalence of asymptomatic bacteriuria among individuals in Imo State and to analyze the antimicrobial susceptibility patterns of isolated uropathogens. The results will provide much-needed epidemiological evidence to support clinical decision-making and public health policy in Nigeria and in similar LMIC contexts.
This study adopted a descriptive cross-sectional design and was conducted between November 2024 and March 2025. This study aimed to determine the prevalence and antibiotic susceptibility patterns of asymptomatic bacteriuria (ASB) among individuals residing in public housing compounds in the Obinze, Owerri West Local Government Area of Imo State, Nigeria. A cross-sectional approach was chosen to provide a snapshot of the microbial and behavioral characteristics related to ASB within the study population at a single point in time. A total of 150 participants were selected using simple random sampling. Data were collected using structured questionnaires.
This study was conducted in Obinze, a semi-urban community located in the Owerri West Local Government Area of Imo State, southeastern Nigeria. Geographically, the community lies along the Owerri–Port Harcourt Expressway, approximately 3.5 kilometers from Avu, and is positioned at latitude 5°25′N and longitude 6°50′E, with an elevation of 61 meters above sea level. Obinze consists of several villages and shares borders with surrounding communities, such as Oforola, Avu, Ihiagwa, Eziobodo, Umuokanne, Nekede, and Mbirichi. The area hosts key institutions, including the 34 Artillery Brigade Nigerian Army Base and the Federal University of Technology Owerri (FUTO), which contribute to its growing urban influence. Residential patterns vary across communities, with some households relying on shared sanitation facilities and others occupying self-contained housing units. The predominant economic activities include subsistence farming and petty trading, which reflect the community’s semi-urban character and socioeconomic diversity. These environmental and demographic characteristics particularly inconsistent access to sanitation and varied living conditions make Obinze a suitable setting for studying the prevalence of asymptomatic bacteriuria and its associated risk factors within a population at potential risk of undiagnosed urinary tract colonization.
A comprehensive range of materials and equipment were employed during the experimental procedures to facilitate the accurate collection, handling, and microbiological analysis of urine samples. Urine specimens were obtained from individuals residing in public housing using sterile wide-mouth urine containers (Sterilin™, Thermo Fisher Scientific, Cat. No. 128A) to ensure aseptic sample collection. Each container was labeled using markers, masking tape, and adhesive stickers for proper sample identification and traceability. Standard laboratory equipment included conical flasks, beakers, syringes (5 ml, BD Plastipak™, Cat. No. 300185), test tubes (Borosil®, Cat. No. 9820U06), test tube racks, Petri dishes (90 mm, Greiner Bio-One, Cat. No. 633102), and inoculating wire loops. Disposable nitrile gloves were consistently used to maintain sterility and protect personnel. Microbiological analyses were conducted using cysteine lactose electrolyte-deficient (CLED) (Oxoid™, Thermo Fisher Scientific, Cat. No. CM301) agar to isolate and differentiate urinary tract pathogens. The core analytical instruments included a compound light microscope (Olympus CX23), for microbial observation, an incubator (Memmert IN30, 37°C), to support controlled bacterial growth, a centrifuge (Hettich Rotina 420R), for sediment separation, and an autoclave (All American 25X, 121°C, 15 psi) for sterilization of reusable materials and media. Distilled water (Milli-Q®, Merck Millipore) was used for the preparation of reagents and for cleaning purposes. All the materials and equipment were thoroughly cleaned, sterilized, and appropriately preserved to ensure the validity and reliability of the experimental outcomes.
The representative sample consisted of 150 urine specimens. These samples were carefully collected randomly from households that lived in public compounds in Obinze. The number of samples collected was determined using Cochran’s formula for prevalence, and experimental and epidemiological studies. The nearest estimated proportion used for this study was that used by (Elo-Ilo et al., 2013) in carrying out the prevalence of asymptomatic bacteriuria among preschool children in Nnewi in South-East Nigeria.
Each urine specimen (10–20 ml) was transferred into labeled test tubes and centrifuged at 2000 rpm for 15 min using a laboratory centrifuge. Following centrifugation, the supernatant was carefully decanted and discarded, and the sediment (pellet) was retained for microscopic examination and bacteriological analysis.
The cysteine lactose electrolyte-deficient medium (Oxoid™, CM301, 36 g/L) was prepared according to the manufacturer’s specifications and according to the method used by (Bale et al., 2023). The agar medium was autoclaved at 121oC°C for 15 min at 15 psi and then allowed to cool. About 15-20 ml of the prepared molten agar was dispensed into disposable petri dishes when the temperature was reduced to approximately 45oC°C. They were allowed to set and ready for inoculation.
A total of (150) urine samples were examined using standard methods. First, the urine samples were serially diluted. From a suitable dilution factor (10−5), a sterile wire loop was used to carefully collect about 0.01 ml of the pellets of the urine sample and was used to inoculate the cysteine-lactose electrolyte deficient (CLED) using streaking method (Bashir et al., 2016). The culture plates were incubated under aerobic conditions at 37°C for 24 h. Plates without visible growth were further incubated for an additional 24 h before they were discarded. The number and types of colonies grown in the media were recorded as insignificant when the samples had a colony count of less than 104 CFU/ml. Samples with a colony count equal to or greater than approximately 105 CFU ml of urine samples were considered to have significant bacteriuria. The bacterial isolates were identified based on a combination of cultural, morphological, and biochemical characteristics. The physiological characteristics of the colonies and their numbers on the culture plates were used as the basis for counting, taking into consideration their dilution number. Counts were made from plates containing 30-300 colonies which were according to the standard (Bashir et al., 2016). Isolated microorganisms were subjected to Gram staining and biochemical tests to confirm them.
Urinalysis with Combi 9 stripe: After processing the urine samples, the combination of 9 stripes (Roche Diagnostics, Cat. No. 11894489216) was inserted aseptically into the urine samples, which were then observed and compared to the standard to determine the normality or abnormality of the following parameters: glucose, ketones, protein, blood, nitrite, pH, ascorbic acid, urobilinogen, and bilirubin.
Gram-staining was used to differentiate bacterial isolates into gram-positive and gram-negative bacteria. For the Gram staining process, clean slides were taken. A well-sterilized wire loop was used to make a smear on the slides, which were air-dried and fixed using heat. Crystal violet was poured on the slide, incubated for 1 min, and washed thoroughly with tap water. Gram iodine was used to flood the smear, allowed to stand for 1 min, and rinsed with tap water before re-flooding with 95% alcohol. The slides were allowed to stand for 15–20 s and then washed with tap water. Then, the secondary dye, safranin, was applied to the smear, allowed to stand for 1 min, and then rinsed with water. The slides were allowed to stand in normal air and then examined under a microscope with oil immersion at ×100 magnification. Staining enabled the differentiation of the bacteria into gram-positive and gram-negative bacteria. The procedure was performed according to the method described by Chessbrough et al. (Carrillo et al., 2015).
Biochemical tests few biochemical tests were performed to confirm the microorganisms isolated from the urine samples.
a. Indole test: This was performed using Kovac’s reagent (Sigma-Aldrich, Cat. No. K2252) as adopted by (Carrillo et al., 2015). The test was conducted over a 24-hour period. Bacterial isolates were first cultured in peptone water, after which 0.5 ml of Kovac’s reagent was added. The mixture was gently shaken and observed after one minute for the development of a red color, which indicated a positive reaction. Escherichia coli (ATCC 25922) was used as the positive control.
b. Coagulase test: The coagulase test was performed using the sliding method. A drop of normal saline was applied to each end of a clean grease-free slide. A colony of test isolates was collected using a sterile wire loop and emulsified with a drop of normal saline at each end to create two thick suspensions. A loopful of fresh human plasma was added to one end of the suspension, which was gently stirred using a sterile wire loop. In contrast, the suspension remained under the control. This was verified for clumping. Staphylococcus aureus (ATCC 25923) was used as a positive control was established using Staphylococcus aureus.
c. Catalase test: The catalase test was performed as previously described (Maori et al., 2013). A colony of each gram-positive isolate was emulsified in distilled water on a clean grease-free slide on a petri plate. Two drops of hydrogen peroxide solution (Merck, Cat. No. H1009) were added, and effervescence was checked. Effervescence confirmed the presence of the tested organisms.
d. Oxidase test: This assay was carried out using the oxidase reagent described previously (Carrillo et al., 2015). Two drops of freshly (Sigma-Aldrich, Cat. No. T3134) prepared oxidase reagent (1% aqueous tetramethyl-p-phenylenediamine hydrochloride solution) were placed on a piece of filter paper. A part of the colony of the bacterial isolate was collected using one end of sterile grease-free glass slide, smeared across the filter paper impregnated with the oxidase reagent, and observed for deep purple color within 10 seconds.
e. Citrate utilization test: This was carried out with Simmon’s citrate agar (HiMedia, Cat. No. M099), as described previously (Maori et al., 2013). A light suspension of the bacterial isolate was prepared in normal saline (NS). Using a straight sterile wire, the suspension was stab-inoculated into Simmon’s citrate agar and incubated overnight. This was examined for characteristic blue color, indicating growth. Positive and negative controls were Klebsiella pneumonia (ATCC 13883) and Escherichia coli (ATCC 25922).
f. Urease test: This test was conducted using the Christensen’s medium (Oxoid™, CM0053). The entire surface of the Christensen’s urea slope was inoculated with the suspected bacterial isolate suspension, cultured overnight, and then tested for red and pink colors. Proteus vulgaris (ATCC 6380) and Escherichia coli, positive controls.
The number of isolated bacteria was counted based on the number of urine samples processed and the frequencies were recorded. The percentage of bacterial isolates was evaluated accordingly.
The data were analyzed using IBM SPSS Statistics version 28. Descriptive statistics, including frequencies and percentages, were used to summarize sociodemographic characteristics, ASB awareness, hygiene practices, and bacterial isolate distribution. Categorical variables were coded and entered in the SPSS database to ensure data integrity. Laboratory results from the culture and biochemical tests were analyzed to determine the frequency of each isolate. Data are presented using tables and charts to enhance the clarity and interpretation of the key findings.
Ethical approval for this study was obtained from the Ethics Committee of the Department of Science Laboratory Technology, Federal University of Technology Owerri (FUTO), Nigeria. The committee reviewed and approved the research protocol in accordance with institutional requirements for studies involving human participants. We clarify that the committee does not issue reference or approval numbers for departmental-level approvals; therefore, no formal number is available. All study procedures adhered strictly to the principles of the Declaration of Helsinki (2013 revision) on ethical conduct for research involving human participants. Prior to data collection, residents of the selected public compounds in Obinze were informed about the objectives, scope, and procedures of the study one week in advance. Verbal informed consent was obtained from all participants before the administration of questionnaires and urine sample collection, taking into account the low literacy level among some residents. Participants were assured of confidentiality, anonymity in reporting, and the voluntary nature of their participation, with the right to withdraw at any time without penalty. No minors were included in this study; therefore, parental consent and child assent were not applicable. Urine samples were collected using sterile universal containers, properly labeled with anonymized study codes, and transported in ice-cooled boxes to the Microbiology Laboratory, FUTO, within two hours of collection for analysis. All steps were conducted in accordance with biosafety and ethical standards, ensuring respect for participants’ autonomy, privacy, and safety.
All 150 screened participants met the diagnostic criteria for asymptomatic bacteriuria, defined as a urine culture yielding ≥10 colony-forming units (CFU)/mL in the absence of clinical symptoms. This represents a 100% prevalence rate, indicating a substantial and silent burden of ASB within the studied community. This finding reflects a likely underestimation of ASB in public health planning, and supports the need for targeted surveillance.
The sociodemographic characteristics of the respondents whose urine samples were used for bacteriuria examination are presented in Table 1. The age distribution ranged from 20 to 45 years, with the majority (62%) aged between 31 and 35 years, followed by 20% aged 26–30 years. The mean age was 32.8 ± 4.9 years. Females constituted a higher proportion (60%) than males (40%), reflecting the gender-based population dynamics in the study setting. Regarding educational attainment, 68% had no formal education, while only 4% had attained secondary or tertiary education, highlighting a low literacy level. Most of the participants were married (80%) and practiced Christianity (90%). Household sizes were relatively small in most cases, with 42% reporting 1–3 members and 40% reporting 4–6 members. These sociodemographic factors may influence hygiene behaviors and risk perceptions of tract infections.
This result represents the distribution of participants based on their awareness of bacteriuria, personal hygiene behaviors, and related health practices, as presented in Table 2. The responses reflected limited knowledge of asymptomatic bacteriuria, moderate understanding of personal hygiene, and generally positive practices in preventing infection transmission.
Almost all participants’ urine test results were markedly normal, as shown in Table 3. Normal urine should be yellow light to deep amber in color, free of blood, proteins, ketones, nitrites, glucose, bilirubin, and urobilirubin, with a pH between 4.5-8.
Biochemical and Gram staining characteristics were used to differentiate the five major bacterial isolates from urine samples, as presented in Table 4. Escherichia coli was identified by positive indole and catalase tests and negative citrate, urease, and Gram staining. Klebsiella spp. were positive for catalase, citrate, and urease, but negative for indole, coagulase, and gram-negative bacteria.
The total number of bacteria isolated from the urine samples collected from respondents in Obinze was seventy-five and presented in Table 5.
| Isolates | Bacterial isolates | Frequency | Percentage (%) |
|---|---|---|---|
| 1 | Escherichia coli | 84 | 56.0 |
| 2 | Staphylococcus aureus | 42 | 28.0 |
| 3 | Enterococcus spp. | 10 | 6.7 |
| 4 | Pseudomonas aeruginosa | 10 | 6.7 |
| 5 | Klebsiella spp. | 4 | 2.7 |
| Total isolates | 150 | 100.0 |
The distribution of bacterial isolates obtained from the urine samples of asymptomatic individuals is presented in Figure 1. Escherichia coli was the most prevalent organism, accounting for approximately 60% of the isolates, followed by Staphylococcus aureus (32%). Other bacteria detected included Enterococcus spp. (9%), Pseudomonas aeruginosa (10%), and Klebsiella spp. (5%). This distribution highlights the predominance of E. coli as the leading cause of asymptomatic bacteriuria while also demonstrating the presence of other clinically relevant uropathogens at lower frequencies. These findings underscore the microbial diversity among carriers despite the absence of overt urinary tract infection symptoms.
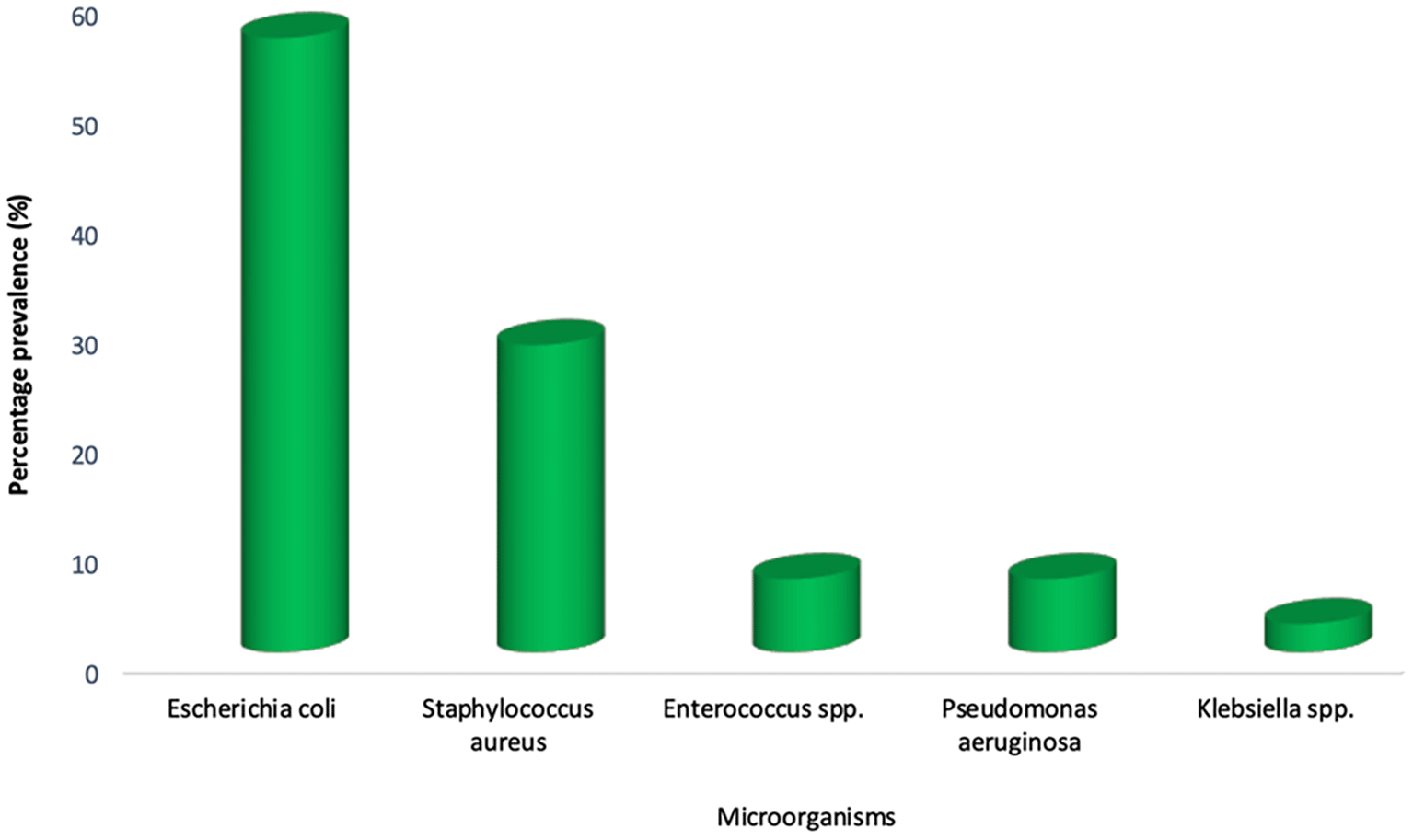
Notably, all individuals from whom these bacteria were isolated remained asymptomatic, indicating the presence of asymptomatic bacteriuria. The distribution of bacterial isolates among respondents is further illustrated in Figure 1, which presents a bar chart detailing the relative frequency of each organism detected in the urine samples collected. This visual representation highlights the predominance of E. coli in the study population and underscores the microbial diversity present even in the absence of clinical symptoms.
This study investigated the prevalence, microbiological profile, and antibiotic susceptibility patterns of asymptomatic bacteriuria (ASB) in individuals in Obinze, Imo State, Nigeria. These findings contribute to the growing body of evidence reporting ASB as a clinically relevant but often under-recognized condition, particularly in sub-Saharan Africa.
The results of this study revealed that Escherichia coli was the most frequently isolated uropathogen, accounting for 56% of urine samples. This was followed by Staphylococcus aureus (28%), Enterococcus spp. (6.7%), Pseudomonas aeruginosa (6.7%), and Klebsiella pneumoniae (2.6%). The prevalence of E. coli is consistent with the substantial body of literature that identifies it as the principal etiological agent of urinary tract infections (UTIs) globally (Zhou et al., 2023). Studies from Uganda, Somalia, South Korea, and other low- and middle-income countries have consistently documented E. coli as the leading uropathogen owing to its uropathogenic virulence (Chowdhury et al., 2024; Mohamed et al., 2023; Odongo et al., 2020). The ability of an organism to express adhesins (P fimbriae), siderophores, and toxins, such as hemolysins, promotes its persistence in the urinary tract, even in asymptomatic individuals. These findings indicated that E. coli may serve as a silent reservoir for antimicrobial resistance genes within the community ( Washington et al., 2021). Its detection underscores the importance of integrating ASB surveillance into Nigeria’s National Action Plan for Antimicrobial Resistance (NAP-AMR), especially in primary care and community health settings.
Additionally, the detection of Staphylococcus aureus, Klebsiella spp., Enterococcus spp., and Pseudomonas aeruginosa in the urine isolates indicated the polymicrobial nature of asymptomatic bacteriuria (ASB). This microbial diversity suggests the potential influence of various host and environmental factors on colonization of the urinary tract. Notably, the relatively high frequency of S. aureus (28%) was higher than that typically reported in hospital-based studies, where gram-negative rods predominate, which may reflect unique local environmental exposure. This includes poor personal hygiene, shared or inadequate sanitation infrastructure, and possible contamination from the skin flora during sample collection. Similar patterns have been observed in some community-based studies, where S. aureus has emerged as a significant uropathogen, particularly among women and individuals with recurrent or untreated infections (Gehrke et al., 2023; Wilkinson et al., 2025). The presence of Staphylococcus aureus, Klebsiella spp., Enterococcus spp., and Pseudomonas aeruginosa among the isolates highlights the polymicrobial nature of ASB. Notably, the relatively high frequency of S. aureus isolation, more than is typically reported in hospital-based studies, likely reflects environmental factors, such as shared sanitation infrastructure and skin contamination (Nkuwi et al., 2018; van der Schoor et al., 2023). This finding reinforces the urgency of implementing public health policies aligned with Nigeria’s National Hygiene Promotion Strategy and the United Nations Sustainable Development Goal (SDG) 6, which emphasizes universal access to clean water and adequate sanitation (Jurnal Kajian Bimbingan Dan Konseling & Ifabiyi, 2025).
Furthermore, this study revealed a markedly low level of awareness regarding asymptomatic bacteriuria (ASB) among participants, with only 2% accurately identifying the diagnostic threshold for the condition. This finding underscores the widespread lack of knowledge regarding asymptomatic infections, particularly those not associated with overt symptoms (Nikolai et al., 2020). The implication is that significant individuals, even those at elevated risk, such as pregnant women, diabetics, and the elderly, are unlikely to seek screening or preventive care if they are unaware of the existence or potential complications of asymptomatic infections. This knowledge gap highlights the urgent need for targeted health education interventions in communities. Specifically, it reinforces the importance of integrating behavior change communication (BCC) strategies into Nigeria’s primary healthcare framework, in line with the National Health Promotion Policy (Idriss-Wheeler et al., 2024; UNICEF, 2017). A mixed personal hygiene practice was reported, with most respondents reporting regular cleaning of the genital areas. The use of soap and water, especially among women, poses a risk to the vaginal microbiome. Similar results have been observed in Kenya (Czapar et al., 2024), where inappropriate vaginal cleansing practices have been linked to a higher UTI incidence. A study from India (Das Purkayastha et al., 2019) reported such practices to increase colonization by pathogenic flora and reduce protective lactobacilli. This implies that hygiene promotion must be culturally sensitive and evidence-based to avoid inadvertently encouraging harmful behaviors. This supports the WHO WASH (Water, Sanitation, and Hygiene) recommendations and Nigeria’s sanitation investment plans under the Clean Nigeria Campaign (Okesanya et al., 2024). Environmental and infrastructural challenges were also evident, with the majority of participants reporting urination in public places due to a lack of functional toilets. This pattern is common in peri-urban areas, as shown in studies from Uganda, (Dickson-Gomez et al., 2023) Ethiopia, (Berihun et al., 2025) and parts of Nigeria (Ekanem et al., 2025), where poor sanitation has been linked to increased exposure to uropathogens. Advocating the need to improve community infrastructure and parallel emphasis on behavioral interventions to minimize environmental transmission routes of infection.
Interestingly, the respondents demonstrated responsible sexual behaviors, with the majority reporting using condoms and maintaining monogamous relationships. This contrasts with earlier reports in similar communities, where multiple sexual partners and inconsistent condom use were prevalent (Muleia et al., 2024). Comparable improvements in sexual behavior have been reported in Nigeria, Kenya, and Uganda (Camlin et al., 2025; Ukaegbu et al., 2022) owing to intensified HIV awareness campaigns. These protective behaviors have the potential to reduce exposure to sexually transmitted pathogens, including those that may ascend the urinary tract. However, this alone cannot mitigate the risk of ASB if environmental and hygienic factors are not addressed.
Collectively, these findings provide insights into the multifactorial nature of ASB in low-resource communities. They highlighted the need for integrated interventions combining microbiological surveillance, public education, environmental sanitation, and antibiotic stewardship. These efforts must align with Nigeria’s National Strategic Health Development Plan II (2018–2022) and global frameworks, such as the Global Health Security Agenda (GHSA), which prioritize early detection and prevention of infectious threats in communities.
The findings of this study underscore the urgent need for integrated community-focused interventions to address asymptomatic bacteriuria (ASB) in low-resource settings. Strengthening public awareness through targeted health education is critical to overcoming the widespread ignorance surrounding ASB and its complications. Efforts must also prioritize the improvement of water, sanitation, and hygiene (WASH) infrastructure to minimize the environmental risk factors that contribute to silent uropathogen transmission. Furthermore, culturally sensitive hygiene promotion and behavior change strategies should be embedded within Nigeria’s primary healthcare framework to prevent harmful practices. Importantly, routine screening and microbiological surveillance should be incorporated into public health programs to enable the early detection and prevent antimicrobial resistance. Aligning these actions with national health policies and global frameworks, such as the SDGs and Global Health Security Agenda, will not only mitigate the burden of ASB but also contribute to the broader goal of strengthening community health resilience in underserved populations.
Based on the findings of this study, the following recommendations are proposed to effectively address asymptomatic bacteriuria (ASB) and its associated public health challenges in low-resource settings:
1. Integrating ASB screening into primary healthcare services: Routine urine screening, especially for high-risk groups such as pregnant women, the elderly, and diabetics, should be included in community health outreach and antenatal care programs.
2. Enhanced Public Health Education: Health promotion campaigns should be implemented to raise awareness about ASB, its risks, and the importance of early detection. These campaigns must be culturally sensitive and community specific.
3. Improve WASH Infrastructure: Government and stakeholders should invest in the provision of clean, accessible public sanitation facilities to reduce environmental exposure to uropathogens.
4. Promote Safe and Evidence-Based Hygiene Practices: Behavior change communication (BCC) strategies should discourage harmful genital hygiene practices and encourage safe, microbiome-friendly methods, especially among women.
5. Additional studies are needed to explore regional variations in the prevalence of ASBs, antibiotic resistance patterns, and socio-behavioral determinants to guide more tailored interventions.
Ethical approval was obtained from the Department of Science Laboratory Technology, Federal University of Technology Owerri (FUTO), Imo State, Nigeria. Informed verbal and written consent were obtained from all participants prior to sample collection and data collection. Participation was voluntary, and the confidentiality of all personal information was maintained.
This study did not involve the publication of any individual participant’s personal data, images, or identifiable information.
The datasets generated and analyzed during this study contain sensitive human participant information and therefore cannot be made publicly available in order to protect participant confidentiality. The Ethics Committee of the Department of Science Laboratory Technology, Federal University of Technology Owerri (FUTO), Nigeria, which reviewed and approved this study, stipulated that participant-level data should not be shared in open repositories to prevent potential identification of individuals. However, de-identified datasets may be made available to qualified researchers upon reasonable request. Requests for access will be considered on a case-by-case basis and must be accompanied by a clear description of the intended use. Researchers seeking access should contact the corresponding author. Any data provided will be stripped of personal identifiers, and access will be conditional on a commitment to use the data solely for research purposes consistent with ethical standards and the Declaration of Helsinki.
The datasets generated and analyzed during this study contain sensitive participant information and are therefore not publicly available to protect confidentiality. However, de-identified data can be made available upon reasonable request from the corresponding author Swase Dominic Terkimbi, princeswasedominic@gmail.com subject to approval by the Federal University of Technology Owerri and completion of a data use agreement.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My research area is microbiology, including its genetic evolution and biological resistance.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmaceutical Microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Nov 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)