Keywords
Peripheral nerve regeneration, Digital nerve injury, Transcutaneous electrical stimulation, TENS, Randomized controlled trial, Digital nerve
Sensory recovery following digital nerve neurorrhaphy is often incomplete, and strategies to enhance regeneration remain under investigation. Low-frequency transcutaneous electrical stimulation has been proposed as a potential adjunctive therapy, but its efficacy in clinical settings is uncertain.
In this randomized controlled trial, 32 patients with isolated traumatic digital nerve injuries underwent surgical neurorrhaphy at a tertiary care hospital. Participants were randomly allocated to an intervention group (n = 16) or sham group (n = 16). The intervention consisted of a single postoperative session of square-pulsed, biphasic transcutaneous electrical stimulation at 20 Hz for 1 hour. The sham group received identical conditions without active stimulation. After stimulation, patients underwent physiotherapy sessions for three months. Sensory recovery was assessed using Semmes-Weinstein monofilament testing and two-point discrimination at baseline, 1 week, 1 month, and 3 months postoperatively.
Both groups showed progressive sensory improvement throughout follow-up, approaching normal values at 3 months. No statistically significant differences were observed between groups in any outcome measure. Confidence intervals for group comparisons overlapped, and no clinically meaningful differences were detected. No adverse effects were reported.
A single postoperative session of low-frequency transcutaneous electrical stimulation did not significantly enhance sensory recovery after digital nerve repair. Further research with varied stimulation protocols, repeated sessions, or extended follow-up may be warranted to clarify its potential role in peripheral nerve regeneration.
Therapeutic Level I.
Peripheral nerve regeneration, Digital nerve injury, Transcutaneous electrical stimulation, TENS, Randomized controlled trial, Digital nerve
The human hand is a rich sensory and motor multifunctional tool with dexterous control to perform essential manipulation tasks.1 Peripheral nerve injuries, especially of the upper limb, can result in severe disability and reduced quality of life.2–4 Several strategies5–8 including the use of neurotrophic factors, stem cell therapy,9,10 and electrical stimulation,11 have been investigated to promote peripheral nerve regeneration as well as functional recovery after these traumas.12 Electrical stimulation has also been considered as an ancillary to surgical repair, and its effects on nerve recovery has been the focus of several studies.13–25
It is to be noted that the characteristics and regenerative potential of peripheral nerves differ markedly depending on the location and type of lesion.26 Differences in digital nerve lesions compared with more proximal and mixed lesions are described.26,27 Digital nerves are almost exclusively sensory, and injuries to these nerves, properly repaired, generally have shorter regeneration distances and can serve as a model for evaluating the effects of transcutaneous electrical nerve stimulation (TENS).27 By contrast, proximal nerve injuries, or nerve injuries with larger gaps to overcome, may be more difficult to completely regenerate, given the increased length for axonal growth and the complexity of motor and sensory functional recovery.28
There are different ways to deliver the PES such as implanted electrodes,15,16 percutaneous electrostimulation17,18 (acupuncture needles inserted into the skin and connected to an electric current generator), intraoperative electrostimulation,19–23 thin-film wireless implantable nerve stimulators,24 and surface electrodes.25 The use of transcutaneous surface electrodes is a non-invasive, practical, and simple option, avoiding the reactions provoked by implant surgery or percutaneous stimulation.29
Based on these features, we hypothesized that PES may influence digital nerve regeneration in humans. We conducted a randomized clinical trial to study the influence of surface PES on the recovery of sensory function, cold sensitivity, and pain disability on the social participation of patients undergoing neurorrhaphy of digital nerves of the hand.
This double-blind, randomized, controlled clinical trial was conducted at a general hospital in Bahia, Brazil, from December 19, 2020, to June 10, 2022. The study was prospectively registered in the Brazilian Clinical Trials Registry (ReBEC) on December 18, 2020 (registration number: U1111-1259-1998; available at: https://ensaiosclinicos.gov.br/rg/RBR-8xn3qq5). Ethical approval was obtained from the Research Ethics Committee of the Faculty of Medicine of Bahia, and the study protocol was published30 in advance to ensure methodological transparency and compliance with the Declaration of Helsinki.31
Adult patients aged 18 to 60 years with an acute, non-segmental digital nerve injury of the hand were eligible for inclusion if surgical repair was successfully performed within two weeks of injury. Exclusion criteria comprised the presence of metal implants at the surgical site, history of seizures, use of a cardiac pacemaker, local infection or skin lesions at the intervention site, associated bone or tendon injuries, and any pre-existing neuropathies.
All patients underwent standardized microsurgical neurorrhaphy under ultrasound-guided axillary block, with epineural approximation using 2 to 4 nylon 8-0 sutures to align nerve fascicles and minimize trauma. Within 24 hours after surgery, participants were randomly allocated to one of two groups.
The stimulation parameters were chosen based on previous studies related to nerve regeneration and patient safety.11,14,15,19 Group A (Surgery + PES) received one hour of transcutaneous electrical stimulation using the Neurodyn II device (Ibramed, Brazil), delivering a square-pulsed, biphasic, symmetrical current at 20 Hz with a 0.4 ms pulse width, at the motor threshold of the median nerve. Group B (Surgery + sham PES) underwent an identical setup with the same device and electrode positioning, but after an initial perceptible activation, the device output was reduced to zero for the remainder of the session. In both groups, two 1 x1 cm silicone-carbon gel electrodes were positioned proximally and distally to the surgical site ensuring identical placement to maintain blinding of both participants and the administering physiotherapist ( Figure 2). A certified physiotherapist, blinded to the group allocation, supervised the rehabilitation protocol. Sessions were remotely monitored via electronic platforms such as WhatsApp or Skype. Patients underwent a hand sensory re-education program based on the approach proposed by Dellon & Jabaley (1982),32 focused on hand sensory re-education over 3-month period. Participants were also encouraged to perform complementary exercises in a home-based program.
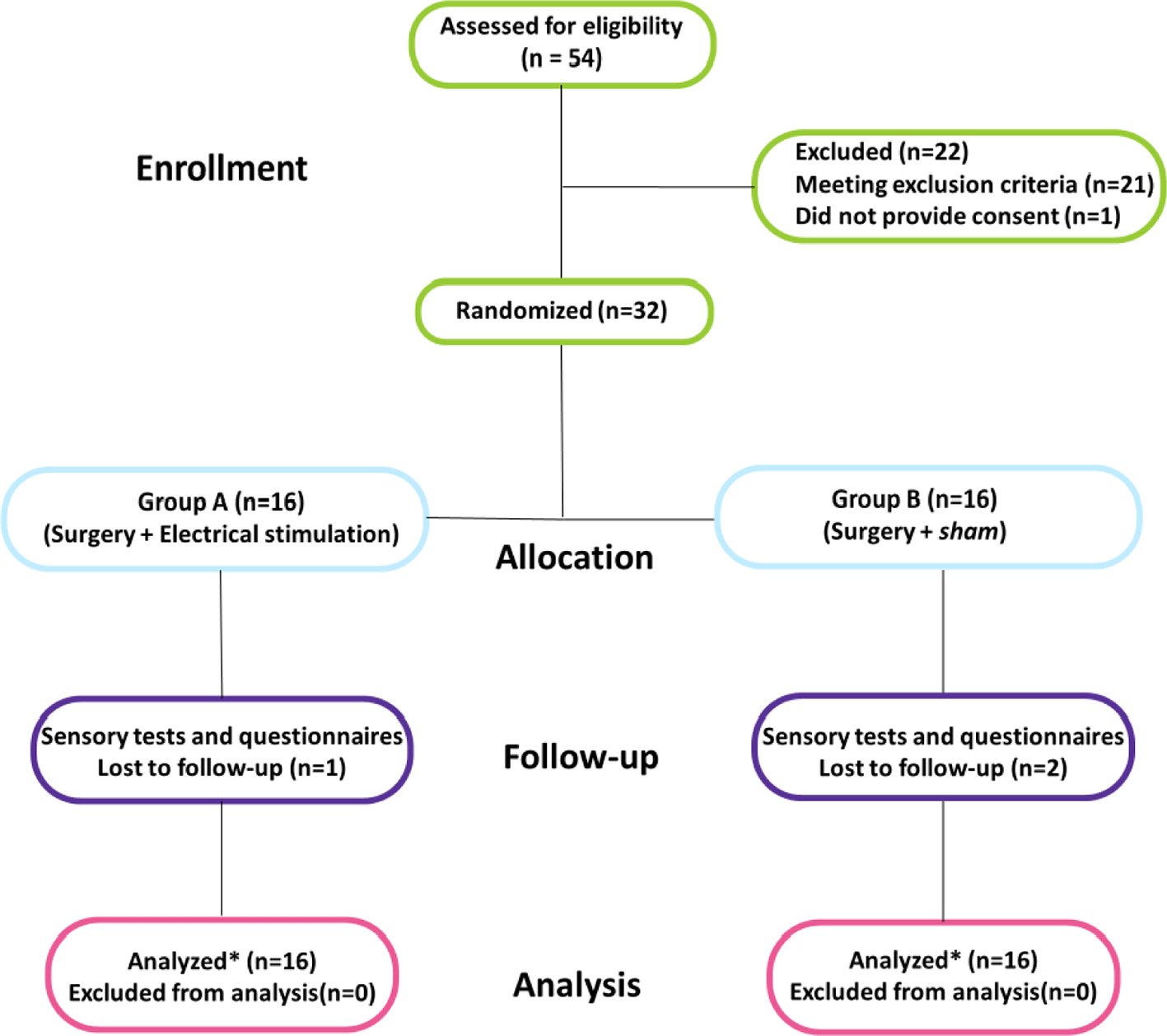
*Intention-to-treat (ITT) analysis.
Patients were randomly assigned in a 1:1 ratio to Group A (surgery + PES) or Group B (surgery + sham) using an electronic randomization sequence generated with the website randomization.com (available at the time of study planning). Allocation concealment was ensured through centralized management by an independent researcher who was the only person with access to the randomization list. A physiotherapist, blinded to group assignment, administered all stimulation sessions using identical devices with the same electrode placement and duration. For sham sessions, the device was initially activated to produce perceptible stimulation cues before being set to zero output. Although real PES could induce subtle muscle contractions, the identical device design and protocol helped maintain blinding for both participants and the administering physiotherapist.
All patients were evaluated in person by the same surgeon responsible for both the surgical procedure and postoperative follow-up. Assessments were scheduled at four time points: (1) pre-intervention; (2) one-week post-intervention; (3) one-month post-intervention (including ongoing rehabilitation sessions); (4) three months post-intervention (upon completion of all 20 rehabilitation sessions). The three-month follow-up period was selected based on the expected timeframe for peripheral nerve regeneration over short distances (2 to 6 cm), assuming an average axonal growth rate of 1 to 3 mm per day.33
The primary outcome was sensory recovery of digital nerves following microsurgical neurorrhaphy, assessed using quantitative sensory tests. Specifically, the Semmes-Weinstein Monofilament (SWM) test and the static two-point discrimination (s2PD) test were applied during four scheduled in-person evaluations. Outcome differences between the two groups (intervention vs. sham) were analyzed post-randomization.
The SWM test, a crucial marker of functional recovery, assesses perception of pressure thresholds related to peripheral reinnervation.34 During the test, participants rested their hands on a table and closed their eyes. In three trials, we applied scored probes perpendicularly to the pulp side of the affected finger for 1 to 1.5 seconds. A positive response in at least two of three trials indicated the sensory threshold.23
The secondary outcome included self-reported measures of cold sensitivity and pain-related functional disability. These were evaluated using the Cold Sensitivity Severity Scale (CSS)35 and the Pain Disability Index (PDI),36 both validated tools for assessing postoperative sensory complaints and pain impact on daily life, aimed to measure improvements in terms of cold sensitivity and pain disability in social functions for individuals who underwent neurorrhaphy of digital nerves in the hand. We used two patient-reported outcome questionnaires: the Cold Sensitivity Severity Scale (CSS)35 and the Pain Disability Index (PDI).36
The s2PD test serves as an established assessment tool for evaluating tactile gnosis.2,37 It measures the ability to distinguish between two nearby points touching the skin, ensuring they are truly distinct rather than perceived as a single point. The test estimates the minimum distance necessary for the patient to perceive the two pressure points as separate contacts.38 It reflects the degree of innervation in a specific skin area. The Medical Research Council classification, modified by Mackinnon & Dellon, allows grouping based on different value ranges related to the sensitive recovery threshold34,39 ( Table 2).
The CSS offers a reliable way to assess cold sensitivity. In cases like amputation or nerve damage, hypersensitivity can occur and lead to significant disability. The CSS consists of four questions related to cold-induced symptoms. The total score provides the cold-sensitivity severity score.
The PDI comprises a seven-item questionnaire evaluating how pain affects various aspects of daily life. Each item is rated from 0 (no disability) to 10 (total disability), and the final score (ranging from 0 to 70) reflects the level of disability due to pain. The PDI has demonstrated consistency, validity, and reliability in studies related to nerve damage.36
The sample size was estimated based on effect size data reported by Gordon et al. (2010).23 We calculated the sample size considering a repeated measures analysis of variance (ANOVA) test, accounting for interactions between and within factors. The effect size, as reported by Gordon et al. (2010),23 was 0.26. Additionally, we set an alpha-type error of 5%, a statistical power of 80%, and worked with two groups and three measures.
Adjustments were applied to account for correlations among repeated measures (correlation coefficient = 0.5) and a non-sphericity correction factor of 1.0 (assuming compound symmetry). Based on these assumptions, the minimum required sample was calculated to be 26 participants.
To account for potential losses, we increased the sample size by 20%, resulting in a final sample of 32 patients (16 per group).
The data were evaluated in a paired and non-paired way through within and between-group comparisons. For within-group evaluations, repeated measures ANOVA or the Friedman test was applied, followed by the Student–Newman–Keuls post hoc test. For between-group comparisons, one-way ANOVA or the Kruskal-Wallis test was used, followed by the Student-Newman-Keuls post hoc test.
The choice of statistical tests was based on the distribution characteristics of the data, and normality was assessed using the Shapiro-Wilk test or the nature of the data. A 95% confidence interval was considered for statistical analysis, with statistical significance set ai p < 0.05 an alpha of 5% (P < 0.05) and a power of 80%.
Descriptive analysis was conducted using means and standard errors or medians and interquartile range (25th/75th percentiles), as appropriate to data distribution. Both measurements of the variables and the statistical analysis were performed under blinded conditions by assessors unaware of group allocation. The independent variable for both groups was the use of electric current. The dependent variables were derived from the pre- and post-treatment assessments (SWM, s2PD, CSS, and PDI). All statistical tests were performed using JASP (V0.18.3).
Eligibility was evaluated in a total of 54 patients. Of these, 21 did not meet the inclusion criteria, and one declined to participate. Thus, 32 patients were randomized, with 16 allocated to the PES group and 16 to the sham group ( Figure 1). Baseline characteristics were comparable between groups, and all participants presented with severe sensory impairment on preoperative evaluation, assessed by MSW and s2PD tests ( Table 1). No significant differences between groups were observed during the immediate postoperative period (up to one week; p > 0.05), so subsequent statistical analyses focused on the 1- and 3-month follow-up data.
Sensory outcomes improved over time in both groups, with significant changes observed between 1 and 3 months postoperatively. For SWM values, there was a significant effect of time (p = 0.012), indicating overall sensory recovery. Age showed a marginal influence on SWM outcomes (p = 0.082), becoming significant when scores were converted to needle size (p = 0.014) ( Figure 3). However, no significant differences were found between the PES and sham groups at any time point (p > 0.05). Simillarly, s2PD values demonstrated significant improvement over time (p = 0.002), with age showing a trend toward significance (p = 0.083), but again without significant differences between groups.
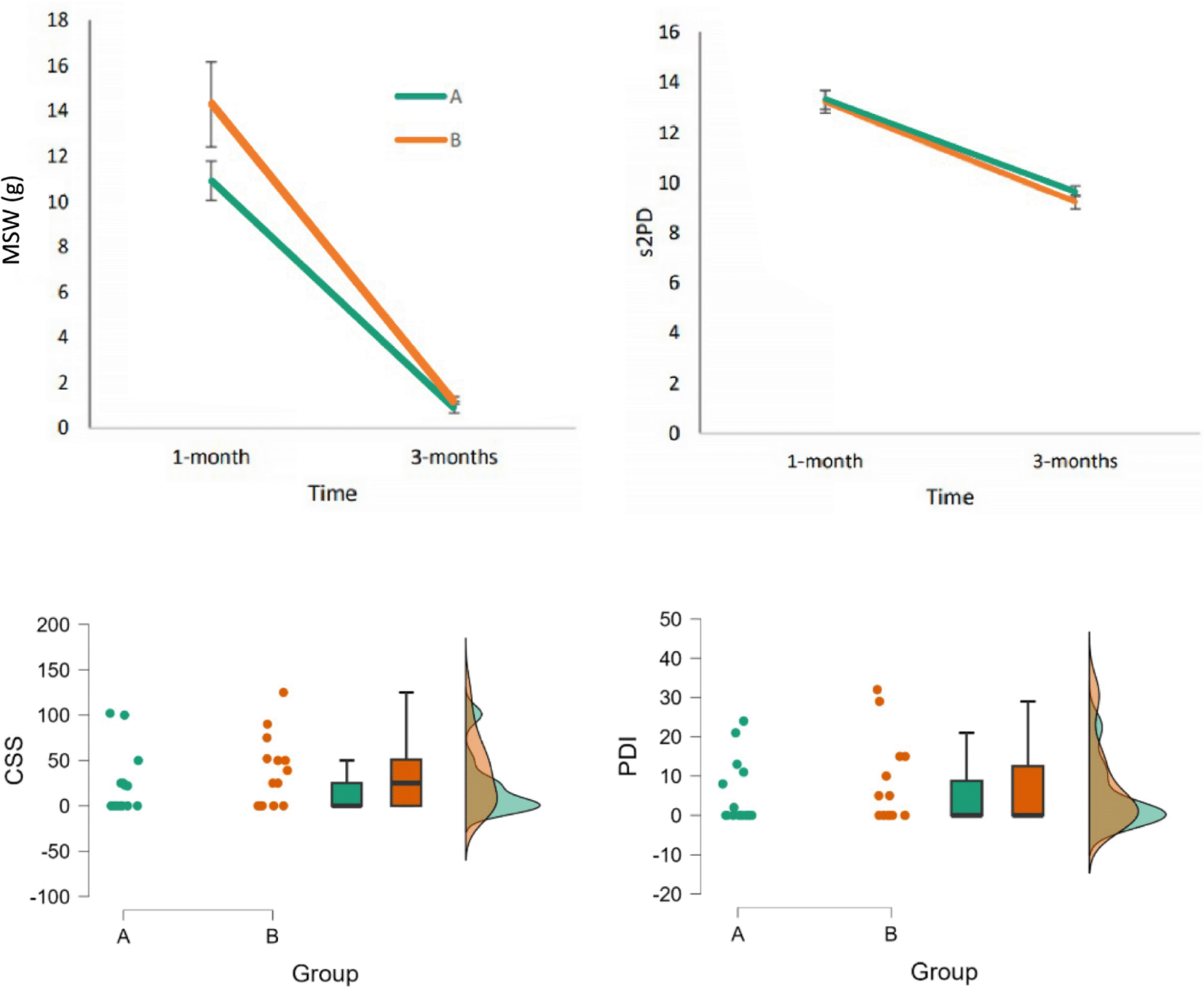
Bottom-panels: Boxplot of CSS (left panel) and PDI (right panel) for Groups A and B. Error bars represent standard error of the mean.
For secondary outcomes, CSS and PDI scores at the 3-month follow-up were compared between groups using an independent samples t-test. No statistically significant differences were found. However, the two scales were highly correlated (r = 0.819, p < 0.001), suggesting consistent subjective perception of disability and cold sensitivity among patients. To assess the robustness of the results, the analyses were repeated under two conditions: (1) excluding age as a covariate, and (2) removing an outlier – a 57-year-old participant from the PES group with discrepant SWM values (z-score ≈ 5). Exclusion of age as a covariate did not affect the main findings. However, removal of the outlier nullified the previously observed significance of age, indicating sensitivity of the result to the outlier.
In summary, the results show that patients from both groups recovered gradually their sensitivity as measured by MSW and s2PD tests, reaching a satisfactory level at the last measurement. However, no significant effect was found for the treatment at the end of the three measurement moments after the operation.
This randomized controlled trial investigated the effectiveness of surface PES in promoting sensory recovery following digital nerve neurorrhaphy. Both groups exhibited a gradual recovery of sensory function over the 3-month follow-up, as measured by SWM and s2PD tests. However, no statistically significant effect of PES was observed at either follow-up time point. It is unlikely that the hypothesized treatment effect was masked by the good recovery of the patients, as after 1 month, sensitivity was still highly affected. Although age showed a near-significant effect in some models, its overall influence appears limited. Cold sensitivity and pain-related disability were assessed only at the final follow-up and showed high inter-individual variability, limiting the ability to draw definitive conclusions regarding these secondary outcomes. Transcutaneous electrical stimulation holds promise in nerve regeneration, offering a non-invasive approach with potential practical benefits.40 It can be utilized to circumvent the complications of surgical implantation or percutaneous stimulation.41,42 Some research indicates that it may take up to 8 weeks for the regenerating axons to cover a distance of 25mm, and the use of PES may reduce this period.20,26 Previous results demonstrated that subjects who received stimulation exhibited earlier and better outcomes around 3 months post-surgery.26 Gordon at al. (2010)23 conducted an innovative randomized controlled trial (RCT) of 21 patients undergoing carpal tunnel decompression surgery. Postoperative direct nerve stimulation using implanted wires (20 Hz, 4-6 volts 0.1-0.8 ms) for one hour led to earlier improvements in electrophysiological parameters when compared to controls. Simillarly, Wong et al. (2015)26 conducted a double-blind RCT involving 31 patients with transected digital nerves and observed significantly improved sensory outcomes with PES (20 Hz, <30 V, 0.1–0.4 ms), although no differences in overall functional recovery were found. In another trial, Power et al. (2020)43 evaluated PES following cubital tunnel decompression in 31 patients. The intervention group received a single 1-hour session of PES (20 Hz, <30 V, 0.1 ms) and demonstrated greater improvements in Motor Unit Number Estimation (MUNE) over time compared to the control group.
Some studies reported adverse findings that contradict prior research that has highlighted the advantageous impact of direct current electric fields on the regeneration of peripheral nerves.44,45 In the present trial, neither the s2PD nor the SWM tests demonstrated a significant enhancement in tactile receptor reinnervation in the digital pulp among patients who received PES. Given these results, the effectiveness of transcutaneous applied electrical fields in promoting in vivo peripheral nerve regeneration remains uncertain.
Cold intolerance46 and pain47 are commonly reported sources of substantial morbidity following nerve injuries in the hand. Previous studies have associated the severity of these symptoms with the degree of sensory recovery, with poorer reinnervation correlating with more pronounced functional impairment and discomfort.47,48 In the present sample, isolated digital nerve injuries were not associated with worse outcomes in terms of pain or cold intolerance, as measured by the CSS and PDI. These symptoms appear to be more commonly linked to complex cases involving finger replantation, severe vascular compromise, or proximal nerve injuries of the median or ulnar nerves.
Postoperative rehabilitation following hand neurorrhaphy is considered standard of care49 and was prescribed for all participants in this study. Withholding rehabilitation would be ethically inappropriate, as hand therapy is routinely recommended after surgery in real-world clinical settings. Omission of such care could also compromise the study’s external validity. Nevertheless, it is important to acknowledge the potential for interaction effects between the rehabilitation protocol and the electrical stimulation intervention,50 which may have limited the ability to isolate the specific contribution of PES to sensory recovery.
The use of a digital nerve injury model in this study presents inherent limitations regarding the generalizability of the findings. Digital nerve injuries differ substantially from proximal or mixed nerve lesions, particularly due to the shorter axonal regeneration distances and the absence of motor components. These factors may have contributed to a ceiling effect, whereby the sensitivity of the outcome measures is insufficient to detect additional improvements potentially attributable to PES.51–53 Consequently, although our findings provide insight into the effects of PES on sensory nerve regeneration in the hand, they may not be directly applicable to more severe or complex nerve injuries requiring longer regenerative distances. Future studies should consider the use of more complex clinical models, such as proximal or mixed nerve injuries, to better assess the potential of electrical stimulation in promoting meaningful functional recovery.
One limitation of PES is the potential interference of the anesthetic technique with its effectiveness. Ideally, surgery should be performed under general anesthesia. A recent study in rats54 demonstrated that the perioperative use of lidocaine significantly reduced the beneficial effects of electrical stimulation on nerve regeneration. In our study, an axillary block (far from the stimulation site) was used, and PES was applied after post-anesthetic recovery. However, a potential attenuating effect on nerve due to anesthesia cannot be completely ruled out. Current trends in hand surgery increasingly favor local or regional anesthesia, given their advantages of lower perioperative risk, faster recovery, and superior postoperative analgesia. Therefore, proposing PES as an adjunct treatment in digital nerve repair under general anesthesia—without first evaluating its use under standard anesthetic conditions — may limit its clinical applicability. Another limitation of this study is that part of the functional evaluation relied on subjective, patient-reported data. Although neurophysiological assessments can serve as sensitive tools for evaluating the severity and progression of nerve injuries in adults, their application was limited in this study. All patients had isolated digital nerve lacerations, which complicates electrophysiological interpretation due to signal contamination through volume conduction from the intact digital nerve branch on the opposite side of the finger.26 Nevertheless, recent outcome research in the field of peripheral nerve injury has increasingly emphasized the importance of combining functional assessments with patient-reported outcomes.55
In this study, the use of surface electrical stimulation following neurorrhaphy in the hand did not result in improved postoperative sensory recovery, as assessed by the SWM and s2PD tests. No significant differences were observed in cold sensitivity or pain-related disability outcomes between the intervention and control groups. These findings suggest that, within the context of isolated digital nerve injuries, surface PES may not confer additional clinical benefit beyond standard surgical repair and rehabilitation. Further research is warranted to explore the efficacy and safety of electrical stimulation protocols in more complex nerve injury models and under varying clinical conditions.
This study was approved by the Research Ethics Committee of the Faculty of Medicine of Bahia (Federal University of Bahia), under protocol number 4.430.230. All procedures were conducted in accordance with the Declaration of Helsinki. The study was prospectively registered in the Brazilian Clinical Trials Registry (ReBEC) under registration number U1111-1259-1998.
All participants provided written informed consent prior to enrollment in the study, including consent for surgical intervention, application of surface electrical stimulation, and participation in follow-up assessments.
All data underlying the results of this study are available within the article itself. The CONSORT 2010 checklist and flowchart associated with this trial are openly available in Figshare at https://doi.org/10.6084/m9.figshare.30120691, under the Creative Commons CC0 license.56 The database and project analysis are also openly available in Figshare under the Creative Commons CC0 license at https://doi.org/10.6084/m9.figshare.30350653.57 Additional study resources are available in Figshare, including the list of study materials https://doi.org/10.6084/m9.figshare.13636685.v1,58 the available facilities https://doi.org/10.6084/m9.figshare.13636694.v1,59 and the participant information sheet and consent form. Reporting guidelines of protocol are also available in Figshare, specifically the SPIRIT checklist for “Influence of surface peripheral electrical stimulation on nerve regeneration after digital nerve neurorrhaphy: study protocol for a randomized clinical trial”.60 All data and materials are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: TENS, sensorimotor control, Sensory perception, Motor control, Motor unit physiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 06 Nov 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)