Keywords
Malang green apple juice, fermentation, anti-aging, MMP-1, COL1A1, Lactobacillus plantarum, Saccharomyces cerevisiae
This article is included in the Plant Science gateway.
This article is included in the Advances in Fibroblast Research collection.
Continuous exposure to sunlight and pollution without adequate protection can damage skin, making it prone to premature aging. One of the natural ingredients for anti-aging is antioxidant. Malang green apple (Malus sylvestris Mill) contains bioactive compounds that can act as antioxidants, but its use is still limited to only as food ingredient. Furthermore, some studies show the potency of fruit fermentation to increase these bioactive compounds. Thus, this study is a preliminary study to examine the anti-aging potency of fermented Malang green apples.
Human foreskin fibroblasts were treated with fermented Malang green apple juice extract (FAJ) by Lactobacillus plantarum DAD-13 and Saccharomyces cerevisiae Fermipan and its unfermented (AJ) for 24, 48, and 72 hours. Cell viability was measured using the MTT assay and collagen deposition was measured using the Sirius red staining method. The expression of matrix metalloproteinase 1 (MMP-1) and collagen type 1 alpha 1 chain (COL1A1) was measured in fibroblasts after treatment with extracts.
Cell viability and collagen deposition of fibroblasts tended to be higher after 48 h of extract exposure. Fibroblasts that received FAJ 62.5 μg/mL showed higher cell viability than negative control (p <0.05) and FAJ 31.25 μg/mL showed higher collagen deposition than negative control (p <0.05) at 48 hours of extract exposure. AJ and FAJ also decreased MMP-1 gene expression. They showed a significant decrease in MMP-1 levels (p <0.01) compared to the untreated group, whereas they showed increased expression of COL1A1 at low concentrations. There was no significant difference in the gene expression between FAJ and AJ.
Our results indicated that Malang green apple juice extract affected fibroblast cell viability, collagen deposition, MMP-1, and COL1A1 gene expression. Exposure of lower concentrations of FAJ to skin fibroblasts for 48 hours showed better results.
Malang green apple juice, fermentation, anti-aging, MMP-1, COL1A1, Lactobacillus plantarum, Saccharomyces cerevisiae
For version 1, we decided to change the authorship order between the second author and the third author based on their roles in this research. We also have made some changes regarding the methodology to explain the component of media culture and give more details about the volume of reagents used in this research.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Aging is a naturally occurring biological process that differs among individuals.1 Given its feature as the outermost part of the human body, signs of aging in the skin are more noticeable by the human eye than in other organs.1,2 Skin aging can be divided into intrinsic and extrinsic aging.3,4 Intrinsic aging is a physiological process characterized by a progressive decline in body functions.3,5 On the other hand, extrinsic aging is estimated to be the cause of 80% of all signs of aging, and is caused by a multitude of factors, including UV exposure, air pollution, and lifestyle factors such as diet and smoking.6
Ultraviolet irradiation causes damage to fibroblasts by reducing collagen synthesis, increasing the rate of its breakdown, promoting oxidative stress, and increasing the content of matrix metalloproteinases (MMP) that help break down collagen.7 Various types of MMPs are responsible for pathophysiological processes, not only limited to aging; however, MMP-1 degrades type I and III collagen, where collagen is responsible for the elasticity and strength of the skin.8
Ingredients with anti-aging effects can inhibit the aging process. The use of materials with antioxidants is one way to prevent aging by reducing and neutralizing reactive oxygen species (ROS).4,5,9,10 Antioxidants can be obtained from natural ingredients such as fruits and vegetables, including apples.11 Green apples contain catechins, vitamin C, quercetin, tocopherols, and carotenoids.12,13 The Malang green apple (Malus sylvestris mill) contains a high amount of quercetin, a natural antioxidant.14 Thus, Malang green apple has the potential to be developed as an anti-aging ingredient. Unfortunately, Malang green apple is limited to food processing. On the other hand, fermentation can increase a material’s bioactive compounds. Fermentation by lactic acid bacteria in fruits has been shown to increase their antioxidant activity.15 Thus, in this study, we investigated the effect of the fermentation process in Malang green apple juice, especially the green apple variety from Malang, Indonesia on cell viability and collagen deposition in skin fibroblasts. We also assessed MMP-1 expression in treated fibroblast cells. We hope that this research can add more benefit value to Malang green apple as a potential anti-aging ingredient.
This was an in vitro experimental study. This study was conducted by the Department of Dermatology and Venereology in collaboration with the Department of Biochemistry, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University.
1. Green apple juice making and pasteurization
Malang green apple were obtained from a traditional market in Malang, Indonesia. Green apple juice 70% (w:v) was prepared by adding 10 mL of 0.05 M citric acid and 10 ml of 100 mg/mL ascorbic acid to green apple slices that had previously been sprayed with 0.05 M citric acid. The addition of an acidifying agent can prevent enzymatic browning by reducing pH.13 It was crushed with a blender, filtered, and pasteurized at 105 °C in an autoclave for 1 minute.
2. Green apple juice fermentation
Pasteurized Malang green apple juice was fermented with 1.8% (v:v) Lactobacillus plantarum DAD-13 + 1.8% (v:v) Saccharomyces cerevisiae Fermipan for 1 day at 37 °C.
3. Green apple juice extraction
Fermented and non-fermented Malang green apple juice is added with ethyl acetate at a ratio of 1:1 (v:v) and macerated for 1 day. The ethyl acetate layer was extracted again using ethyl acetate. The filtrate was evaporated on evaporation porcelain and then transferred to a glass vial (dried in an oven at 45 °C) to obtain fermented and non-fermented Malang green apple juice extracts.
4. Cell culture
Fibroblast cell lines were obtained from human foreskin. The growth process of fibroblast cell lines was conducted in the Laboratory of Dermatology Venerology, Department of Dermatology and Venereology, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University. Fibroblast cell lines were grown in Dulbecco’s modification of Eagle’s medium (DMEM, Gibco®, USA) with 10 mL of fetal bovine serum (FBS), 1 mL of 1% penicillin-streptomycin (Gibco®, USA), and 1 mL of amphotericin B. Cells were incubated in an incubator at 37 °C and 5% CO2.
5. Cell viability with MTT assay
To conduct a cytotoxicity test on the fibroblast cell lines, the medium was aspirated and the cells were washed with phosphate-buffered saline (PBS) twice. The cells were then separated by trypsin (Gibco®, USA). Trypsin was diluted in DMEM after 4 minutes, and the detached cells were centrifuged for 10 minutes at 2000 rpm. The cells were counted and plated the cells in 96-well plates (100 μL solution per well contained 10.000 cells/well). The fibroblast cell lines were incubated for 24 hours (37 °C, 5% CO2). The next day, the cells were treated with fermented green apple juice extract and unfermented green apple extract at various concentrations ranging from 15.826 μg/mL to 1000 μg/mL. The 96-well plates were then incubated overnight. After incubation, the medium in each well was aspirated, and 100 μL of MTT was added to create formazan.16 After 4 hours of incubation and formation of formazan, a stopper of 100 L SDS 10% in 0.1 N HCl was added and incubated overnight in the dark. The intensity of the purple color was measured using an ELISA reader at a wavelength of 595 nm.
6. Collagen deposition
Collagen deposition in fibroblasts was determined according to the method proposed by Benitez et al.17 with some modifications. Fibroblasts (10.000 cells/well) treated with the extracts were incubated for 24, 48, or 72 hours. After incubation, the medium was aspirated, and the cells were washed twice with PBS. Bouin’s solution (100 μL) was added to each well and allowed to stand for 1 hour in the dark. Bouin’s solution was rinsed with deionized water until the yellow color disappeared and was left overnight. The wells were filled with Sirius red stain (100 μL per well), and the 96-well plate was shaken for 1 hour. Give 0.01 N HCl, followed by 0.5 N HCl, and then shake the well for 30 minutes. The absorbance was read using an ELISA reader at 550 nm.
7. MMP-1 and COL1A1 quantification
Ribonucleic acid (RNA) was isolated from fibroblast cells using PRImeZOLTM reagent (RNA isolation reagent) according to the manufacturer’s instructions (Canvax, America). The cells were homogenized with the reagent. The supernatant from the sample was separated by adding 0.2 mL of chloroform per 1 mL of the reagent. After that, it was centrifuged for 15 minutes at 12000 rpm. The aqueous layer, containing RNA, was transferred to a different tube and precipitated with cold 70% isopropyl alcohol, followed by incubation at room temperature. Then, the sample was centrifuged at 12000 rpm for 10 minutes at 4°C to obtain RNA pellet. Pellet was washed with 75% ethanol and then vortexed and centrifuged at 7500 rpm for 5 minutes at 4 °C. The RNA pellet was dissolved with nuclease-free water (NFW) and incubated at 60°C for 5 minutes. Isolated RNA was stored at -20 °C. The isolated RNA was then subjected to complementary deoxyribonucleic acid (cDNA) preparation process according to the manufacturer’s instructions (SMOBio). Isolated RNA was mixed with 1μL of oligo (dT), and 10μL Diethyl pyrocarbonate (DEPC)-treated H2O, spun down, and incubated at 70 °C for 5 minutes, mixed again with a cDNA synthesis mixture consists of 4μL 5-RTX buffer, 5μL DEPC treated H2O, and 1μL RT-ase, and then incubated to make cDNA. The cDNA samples were diluted from 100ng/mL to 50ng/mL, 20μL of the sample was taken and added into 20μL of NFW, mixed by up and down method. The polymerase chain reaction (PCR) master mix was prepared by adding 10μL of the master mix, 0.8μL of the forward primer, 0.8μL of the reverse primer, 4.4 μL of NFW. Primers used were obtained from Integrated DNA Technologies (IDT), and the primer sequences are listed in Table 1. The 16μL of the PCR mix was mixed with 4μL of the sample. The results of the samples were read using a Bio-Rad thermocycler.
Cell viability and collagen deposition data were analyzed using two-way analysis of variance (ANOVA) and Dunnett’s test for post hoc analysis. MMP-1 gene expression was analyzed using one-way ANOVA. Data analysis was done using SPSS while GraphPad Prism 9.3.0 were utilized to make a graph. For all tests, statistical significance was defined as p < 0.05.
The effect of FAJ on fibroblast cell line viability was compared with that of the fibroblast cell line that received AJ after cells were exposed to the extract for 24, 48, and 72 hours (Figure 1). The analysis of the fibroblast cell line showed a significant effect between different types of extracts (FAJ and AJ) and cell viability at 48 and 72 hours of extract exposure, while the variation of extract concentration from 15.625 μg/mL to 1000 μg/mL had a significant effect on cell viability after 24 and 72 hours of extract exposure (p < 0.05). The viability of fibroblasts receiving FAJ was higher than that of fibroblasts receiving AJ on 24-hour extract exposure, except at the concentration of 62.5 μg/mL, but it was not statistically significant. In the 24-hour extract exposure group, fibroblasts receiving FAJ and AJ 1000 μg/mL showed higher cell viability than the negative control. This pattern changed on 48-hour extract exposure. Fibroblasts receiving AJ at 125-500 μg/mL concentration and FAJ at 15.625 and 62.5 μg/mL concentration showed higher cell viability than the negative control (p < 0.05). The cell viability in FAJ was higher than that in AJ at 15.625 and 62.5 μg/mL. After receiving its peak at 48-hour extract exposure, cell viability decreased at 72-hour extract exposure. Cell viability in the fibroblast group of FAJ at the concentration of 15.625 – 31.25 μg/mL and 1000 μg/mL (72 hours) was lower than negative control (p < 0.05).
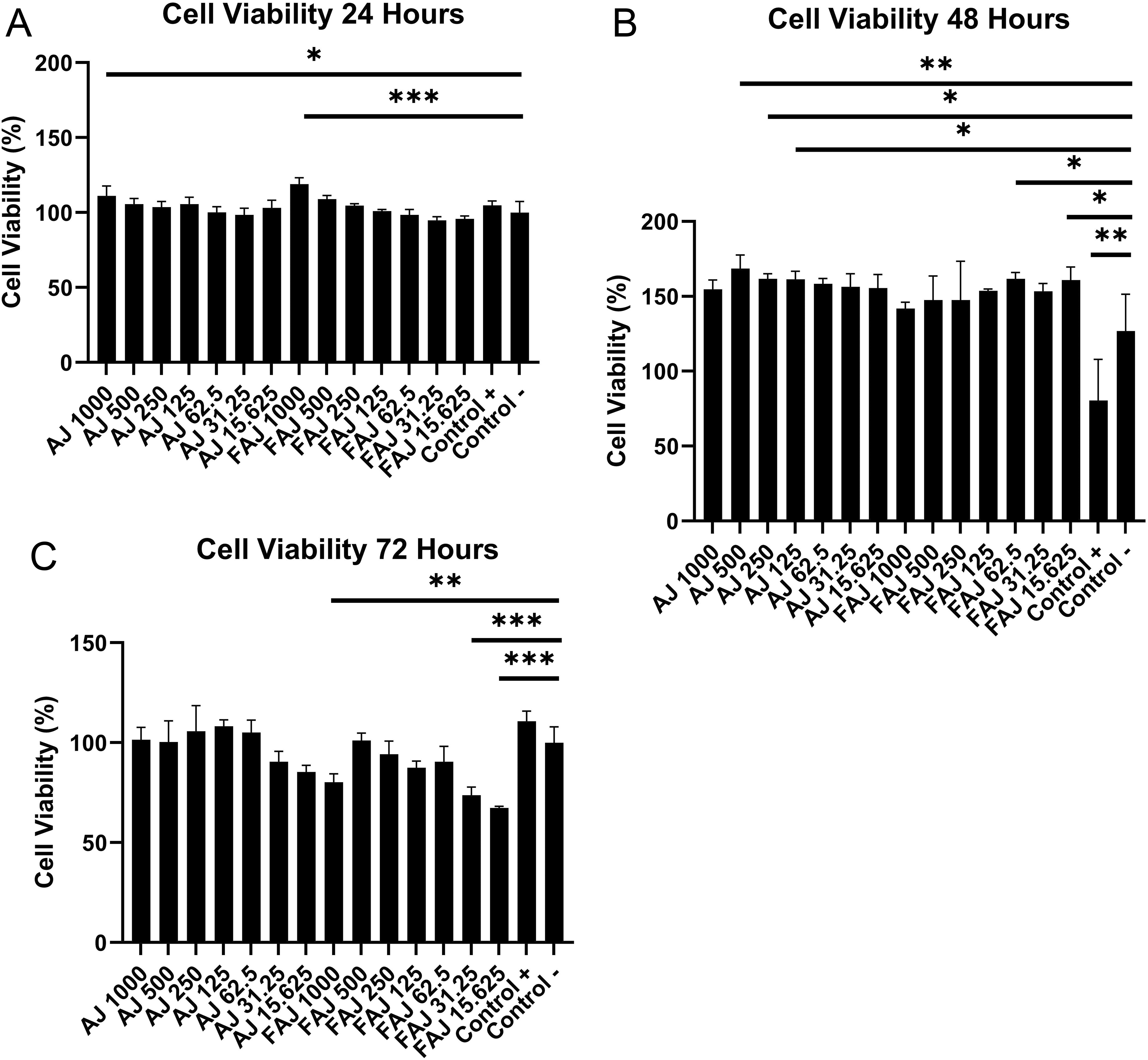
Cell line received various concentrations of AJ and FAJ extracts for (A) 24 hours; (B) 48 hours; (C) 72 hours determined by MTT assay. The error bars depict mean values ± SD from triplicate experiments. The significance of difference was set to *p<0.05, **p<0.01, and ***p<0.001 compared with non-treated fibroblasts cell line.
The effect of FAJ on fibroblast collagen deposition was compared with that of the fibroblast cell line that received AJ after cells were exposed to the extract for 24, 48, and 72 hours. Overall, the collagen deposition ratio (Figure 2) showed the same pattern as cell viability, which increased up to 48 hours and decreased after 72 hours of extract exposure, except in the fibroblasts group that received AJ 250-1000 μg/mL. The analysis carried out on the data showed that the duration of extract exposure (24, 48, and 72 hours) and variation of extract from 15.625 to 1000 μg/mL had a significant effect on the collagen deposition ratio, while different types of extracts had a significant effect on the collagen deposition ratio after 24 and 72 hours extract exposure. The collagen deposition ratio in fibroblasts receiving FAJ at a concentration of 31.25 μg/mL and AJ at 62.5 μg/mL for 24 and 48 hours was higher than that in the negative control (p < 0.05).
Cell line received various concentrations of AJ and FAJ extracts for (A) 24 hours; (B) 48 hours; (C) 72 hours. The error bars depict mean values ± SD from triplicate experiments. The significance was set to *p<0.05, **p<0.01, and ***p<0.001, compared with non-treated fibroblasts cell line.
MMP-1 gene expression (Figure 3) in fibroblasts treated with FAJ and AJ at concentrations of 31.25, 62.5, and 125 μg/mL for 48 hours showed significant downregulation relative to that in the control (p < 0.05). MMP-1 levels in the FAJ group at concentrations of 31.25, 62.5, and 125 μg/mL decreased by 89.02%, 89.63%, and 94.6%, respectively, compared to the control group, whereas in the AJ group, it decreased by 88.62%, 91.2%, and 86.6%, respectively, compared to the levels of MMP-1 in fibroblasts not treated with any extract. There were no significant differences in MMP-1 levels between AJ and FAJ. COL1A1 expression (Figure 4) also exhibited a dose-dependent pattern, with higher concentrations of AJ and FAJ showing lower COL1A1 expression. COL1A1 levels in the FAJ group at concentrations of 31.25 and 62.5 μg/mL increased by 30% and 4%, respectively, compared to the control, whereas it decreased by 17% at a concentration of 125 μg/mL. In the AJ group, COL1A1 levels at concentrations of 31.25 and 62.5 μg/mL increased by 47% and 42%, respectively, compared to the control group, whereas it decreased by 12% at a concentration of 125 μg/mL. The changes in COL1A1 levels in both the AJ and FAJ groups were not statistically significant (p > 0.05).
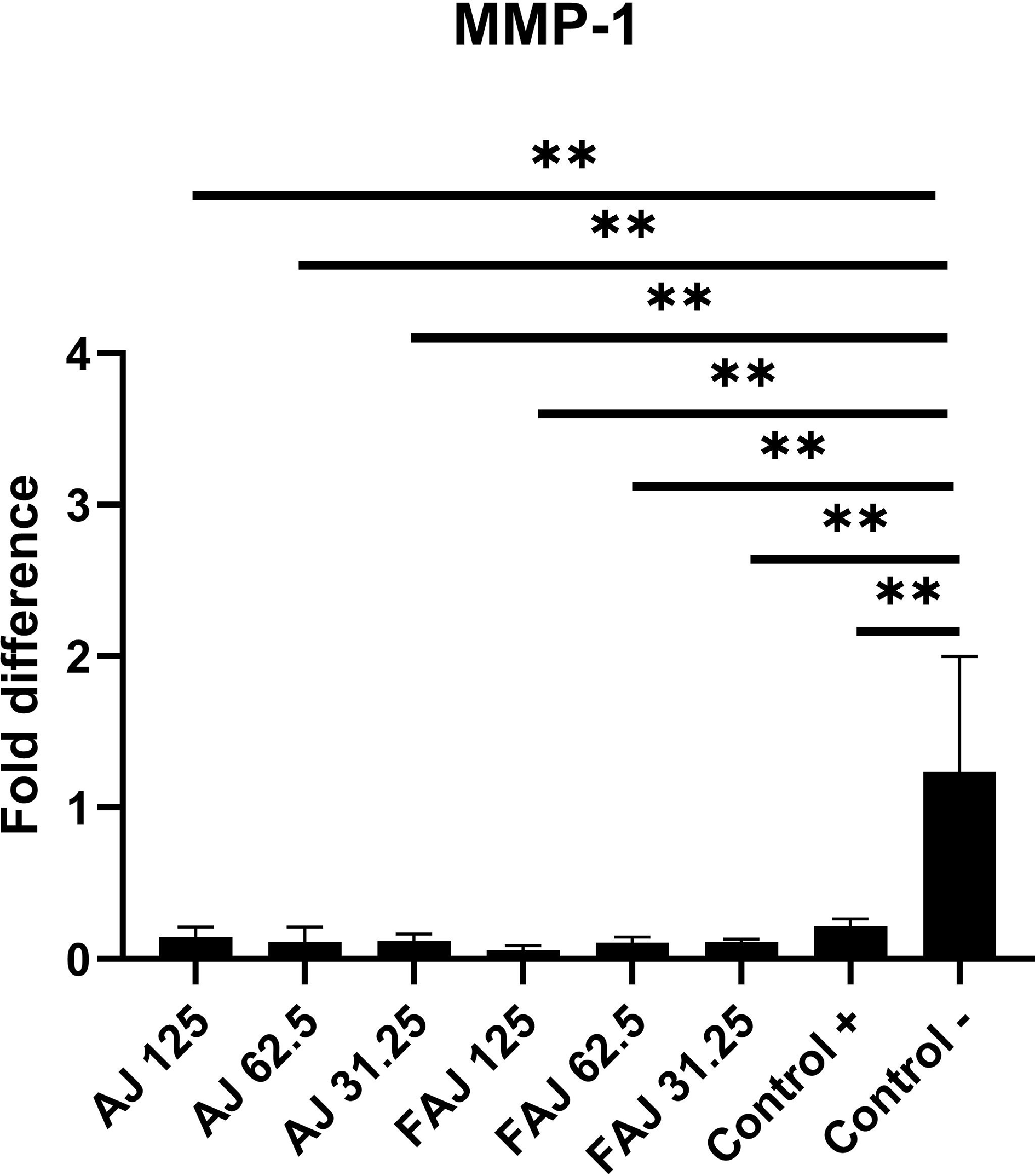
The significance was set to *p<0.05, **p<0.01, and ***p<0.001, compared with control.
Our study conducted a toxicity study on fibroblasts receiving extracts from fermented and unfermented Malang green apple juice. We also conduct a study to know the expression of MMP-1 and COL1A1. In our study, exposure of human skin fibroblast to FAJ and AJ affected cell viability and collagen deposition. Fibroblasts that received FAJ and AJ showed an increase in cell viability along with increasing extract concentration at 24 hours exposure to FAJ 1000 μg/mL, which showed the highest increase compared to the negative control. However, this pattern changed after 48 hours of exposure. Fibroblasts that received low concentration of FAJ showed higher cell viability than those that received high concentration of FAJ, with a decrease starting at 62.5 μg/mL. In the AJ group, the pattern after 48 hours of exposure remained the same with 24 hours exposure group. Fibroblasts that received a low concentration of FAJ also showed higher collagen proliferation than those that received high concentrations.
The results of cell viability and collagen deposition were also reflected by MMP-1 and COL1A1 expression. Low concentrations of FAJ and AJ increased COL1A1 expression compared to untreated cells one and decreased COL1A1 expression at 125 μg/mL, although the change was not statistically significant. FAJ and AJ also decreased MMP-1 expression in a dose-dependent pattern, indicating that this extract can inhibit one of the genes that control collagen degradation. However, the decrease in MMP-1 levels at 125 μg/mL, which is too strong, and the decrease in COL1A1 levels at the same concentration might indicate a decrease in living fibroblasts.
Fibroblasts dominate the dermis.18 These cells play important roles in the formation and repair of skin components by producing collagen and extracellular matrix.19 Fibroblasts are skin cell types that have been widely used in in vitro research to determine the effect of exposure to certain extracts exposure before being tested on skin tissue.20 The fermentation process carried out on green apple juice could increase the bioactive compounds contained in apple juice, especially compounds that with antioxidant activity. Antioxidants play a role in neutralizing the effects of reactive oxygen species (ROS) such that they do not cause damage and aging in cells.21 Several previous studies have demonstrated the effect of fermentation on increasing antioxidant activity by increasing total phenol, anthocyanin, and flavonoid content, especially myricetin and quercetin22,23 as well as increasing the biological activity of polyphenols.24 Quercetin present in green apples has antioxidant activity that increases the lifetime and viability of fibroblasts.25 Flavonoids and polyphenols contained in apples are also predicted to protect cells from oxidative stress and increase cell viability.26 In addition, polysaccharides produced by lactobacilli can increase cell proliferation because of their effect on fibroblast growth factors.27
The higher collagen deposition ratio in the extract group than in the negative control was in accordance with previous studies. Fermentation of fruit and vegetable drinks using Saccharomyces cerevisiae and Streptococcus thermophilus TCI125 could increase collagen levels.28 The fermented extract can increase collagen levels, especially collagen type 1, by inhibiting mRNA expression and collagenase production.29 Phenolic compounds found in apples can inhibit collagen degradation by inhibiting matrix metalloproteinase 1 (MMP-1) and these compounds also prevent cell damage from free radicals.30
Collagen, especially type I collagen, is the most abundant protein in skin connective tissue and is responsible for maintaining skin structure.31 Reduction in fibroblasts and collagen fragmentation due to chronological age or ultraviolet radiation can disturb skin elasticity and cause damage to the skin.32,33 Increased production of MMP-1 caused by exposure to intrinsic and extrinsic factors is strongly related to collagen fragmentation, therefore, a decrease in its production may correlate with collagen increase and later have an anti-aging effect.32,34 Several previous studies have shown that fermented extract can decrease the expression of MMP-1.35,36 In our study, both Green Malang apple juice extracts significantly inhibited the expression of MMP-1. This indicates that FAJ and AJ exert anti-aging properties by decreasing one of the main causes of collagen degradation.
Our study showed better results with low FAJ concentrations. This may have been caused by some factors. First, the antioxidant activity of the FAJ extract reached its maximum activity, thus reducing the antioxidant efficacy.37 The decrease in antioxidant activity due to the fermentation process has been shown by a decrease in total phenol content in strawberry fermentation due to anthocyanin condensation.38 Red cabbage fermentation using Lactobacillus.acidophilus and Lactobacillus plantarum caused a decrease in anthocyanin content and total phenolic content after 7 days of fermentation.39 Two, the fermentation process of green apple juice increased the acidity of the extract, which can negatively impact the fibroblasts. Saccharomyces cerevisiae and Lactobacillus plantarum can further decrease the pH of the juice more than a single fermentation.40 Fibroblasts in an acidic environment (pH 5.5) showed lower proliferation activity than those in a neutral or base environment.41 Three, the formation of pro-oxidant activity. A previous study showed that papierowka apple peel extract had a high polyphenol content and high pro-oxidant activity because phenolic components could also initiate the formation of ROS.23 Overall, changes in the phenolic components that play a role in antioxidant components and activity are influenced by the probiotic strain, fermentation temperature, fermentation substrate, and fermentation period.42–44
This study provides preliminary information on the effects of the fermentation process of local green apple juice from Malang, Indonesia on normal skin fibroblasts. Malang green apple juice (fermented and unfermented) affected cell viability, collagen deposition, and MMP-1 and COL1A1 levels in the skin fibroblasts. Exposure of skin fibroblast to fermented Malang green apple juice at low concentrations for 48 hours gave better results than exposure to high concentrations, given that the high concentration might undergo increased pH level, decreased antioxidant activity over time, or pro-oxidant activity.
Our study was limited by the absence of high-performance liquid chromatography (HPLC) data to analyze the type and amount of compounds in FAJ, the pH value before and after fermentation, antioxidant activity, and the effect of different probiotic strains, temperature, and fermentation time on cell viability and collagen deposition in normal fibroblasts.
Ethical approval for this study was approved by the Ethics Committee of the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, with reference number KE/FK/0143/EC/2022 on February 9th 2022.
Figshare: Toxicology Study and MMP-1 and COL1A1 Expression of Malang Green Apple Juice Fermented by Lactobacillus plantarum DAD-13 and Saccharomyces cerevisiae Fermipan on Human Fibroblast: Potential Modality of Anti-aging. https://doi.org/10.6084/m9.figshare.28233320.v3.45
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Dataset for ‘Toxicology Study and MMP-1 and COL1A1 Expression of Malang Green Apple Juice Fermented by Lactobacillus plantarum DAD-13 and Saccharomyces cerevisiae Fermipan on Human Fibroblast: Potential Modality of Anti-aging’, https://doi.org/10.6084/m9.figshare.28233320.v3.45
The authors extend their gratitude to the Department of Dermatology and Venereology and the Department of Biochemistry at the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta for making this study a success.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioactive compounds on aging and colon cancer.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cellular senescence and aging
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 22 Dec 25 |
read | read |
|
Version 1 07 Nov 25 |
||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)