Keywords
Complex Lesion, Intravascular Imaging, Invasive Physiological Assessment, Non-Hyperaemic Index, Case Report.
A diffuse and heavily calcified coronary lesion represents a complex clinical scenario, often leading to suboptimal outcomes. The optimal management, whether percutaneous coronary intervention, surgery, or medical treatment, remains debatable. A 64-year-old male was referred to our outpatient clinic with persistent typical chest pain. Coronary angiography revealed diffuse and heavily calcified lesions with 75% stenosis at the proximal to middle LAD. A physiological assessment was performed using a hybrid approach of resting full-cycle ratio (RFR) and fractional flow reserve (FFR). Initial RFR was inconclusive (0.91). Follow-up FFR measurement supported the indication for stenting. Lesion characterization using intracoronary optical coherence tomography (OCT) showed a thick, long, and heavily calcified lesion with an OCT Calcium score of 4. The minimal lumen area (MLA) was only 1.78 cm2 and indicative of stent deployment since the MLA was less than 3 mm2. Three runs of rotational atherectomy were performed, followed by non-compliant balloon dilatation and two DES implantations. The procedure was successful with TIMI flow grade 3 and optimal OCT evaluation. The combination of hybrid physiological assessment and intravascular imaging is an effective and efficient strategy for managing complex diffuse-calcified coronary lesions.
Complex Lesion, Intravascular Imaging, Invasive Physiological Assessment, Non-Hyperaemic Index, Case Report.
Complex coronary lesions, particularly those involving calcification and diffuse lesions, remain a common challenge in the management of coronary artery disease (CAD). Calcification increases the rigidity of atherosclerotic plaques and adds technical complexity to revascularization procedures. Heavy calcification increases the risk of complications during and after PCI, including stent under-expansion, uncertain landing zones, the use of multiple stents, and vessel dissection or perforation. These complications potentially result in stent thrombosis, restenosis, myocardial infarction, and increased mortality.1
Diffuse lesion also increase the complexity of PCI procedures and are associated with poor prognosis. According to angiography findings, diffuse lesion is defined as a long coronary segment (20 mm or above) with angiographic abnormalities without clear focal stenosis. Dealing with diffuse lesions is very challenging since diffuse lesions have a higher incidence of stent malapposition, edge dissection, and underexpansion.2 Hence PCI with imaging guidance is required with careful consideration of stent usage to avoid over-stenting.3
Achieving optimal PCI outcomes can be challenging, necessitating adequate lesion preparation and modification techniques before intervention. Given these difficulties and associated risks, the optimal treatment approach—whether PCI, surgical revascularization, or medical treatment—remains a topic of debate. We reported a method for managing a complex diffuse-calcified lesion in the LAD using a combination of hybrid physiological assessment with intravascular imaging.
A 64-year-old male was referred to our outpatient clinic with persistent typical chest pain. The patient was diagnosed with unstable angina 3 months before and had a history of coronary artery disease with hypertension for 5 years. Physical and laboratory findings were unremarkable. The ECG showed normal sinus rhythm with poor R wave progression. Left ventricular ejection fraction (LVEF) was 68%.
The coronary angiography revealed diffuse and heavily calcified lesions extending from the proximal to middle left anterior descending (LAD) artery with 75% stenosis at the proximal segment. The left circumflex artery (LCx) showed a non-significant lesion and the right coronary artery (RCA) appeared normal. We proceeded with a physiological assessment using the resting full-cycle ratio (RFR) and showed a negative result of 0.91. While the confirmatory fractional flow reserve (FFR) showed a significant lesion of 0.76 ( Figure 1).
Intracoronary OCT showed a heavily calcified lesion with a total length of 64 mm, a proximal diameter of 3.5 mm, a distal diameter of 3.0 mm, a percentage area stenosis (%AS) of 79.7%, and a minimal lumen area (MLA) of 1.78 mm2 ( Figure 2). The calcification was thick (0.78 mm) and circumferential with an arc of almost 270 °. The overall OCT Calcium score was 4.
Based on these findings, we planned to perform an atherectomy and implant two stents. Transradial access was obtained using a 6F sheath and an EBU 3.5 6F guiding catheter. The LAD lesion was crossed with a Runthrough Hypercoat, followed by Microcatheter and RotaWire Extra Support. Rotational atherectomy was performed at the proximal and mid-LAD using 1.75 mm RotaBurr at 160.000-190.000 rpm. After three runs, the lesion was expanded using Sapphire NC Balloon 2.75 x 18 mm.
The first stent was deployed to mid-distal LAD using DES Xlimus 3.0 x 32 mm at 14 atm, followed by the second stent to proximal-mid LAD using DES Angiolite 3.5 x 34 mm at 18 atm. Evaluation angiography was adequate with TIMI flow grade 3. The evaluation OCT confirmed optimal stent apposition and expansion (MLA of 6.45 mm2) without any medial edge dissection ( Figure 3).
This case illustrates the clinical dilemmas of revascularization decisions and strategies in moderate lesions with diffuse and heavy calcification at the LAD. The first dilemma was the decision to revascularize. The second dilemma was the discordance of the physiological assessment results using RFR and FFR, making the decision more problematic.
The decision to revascularize for CAD is based on symptoms and survival improvement. Revascularization is indicated for those with refractory angina despite optimal medical treatment and suitable anatomic conditions, including left main disease or LV dysfunction. The current guideline on coronary revascularization highlighted the uncertain benefit of revascularization in stable ischemic heart disease with preserved LVEF, even when significant proximal LAD stenosis is present.4 Based on these recommendations, the indication and decision for revascularization for our case is unclear and doubtful. However, medical therapy alone is often insufficient to optimally treat calcified lesions.
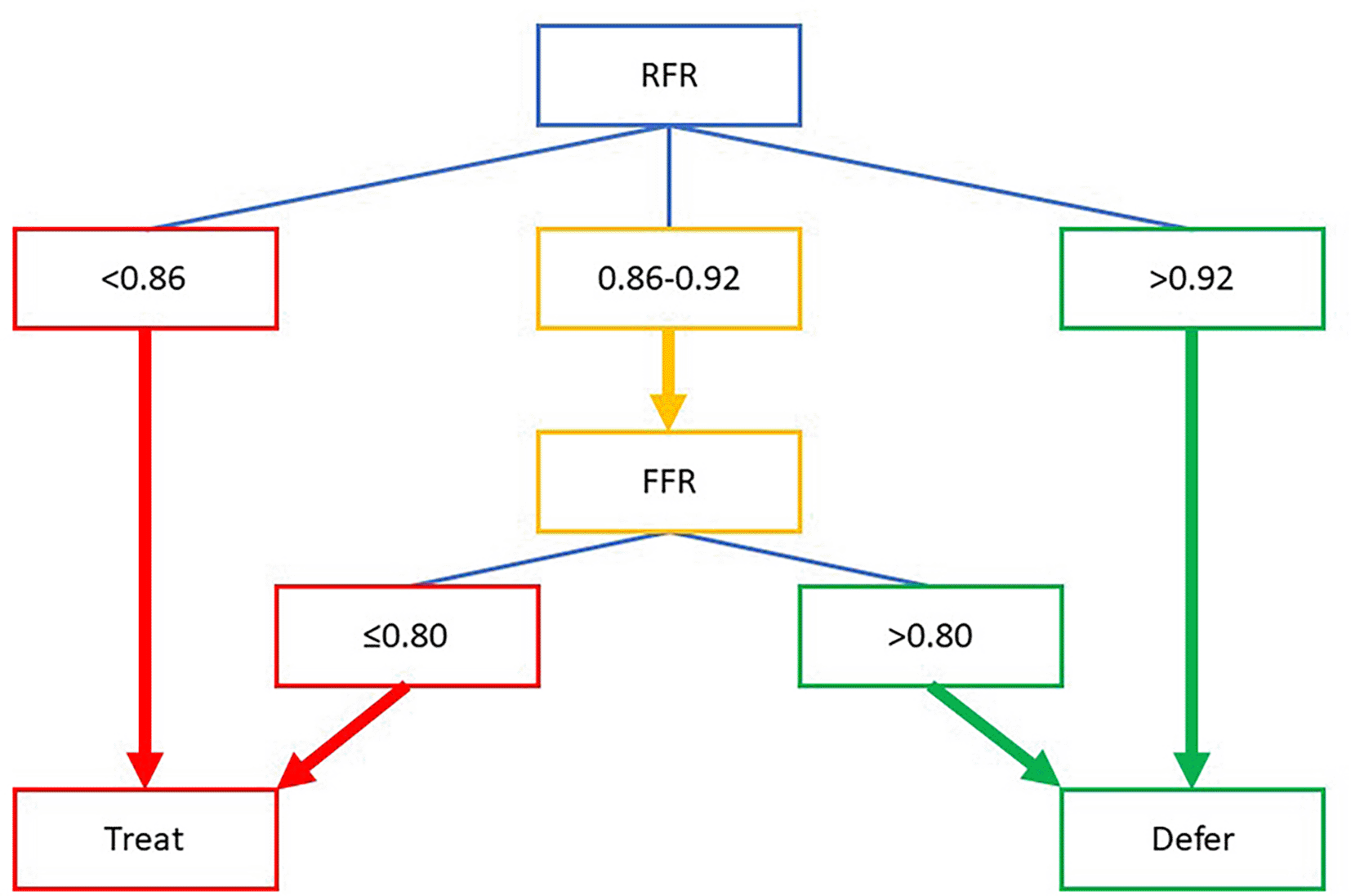
Standard coronary angiography frequently underestimates the extent and severity of coronary calcification, thereby limiting its reliability for PCI planning. Physiological assessment can aid in determining the necessity of stenting in such lesions by evaluating the hemodynamic condition. Two major coronary physiological indexes are hyperemic and non-hyperemic pressure ratios (NPHR). The use of hyperemic index, FFR, obligates the use of vasodilators such as adenosine. This can lead to additional side effects, complications, and patient discomfort. Performing FFR also requires extra procedural steps and dedicated pressure-sensing guidewires, which increase both procedural time and cost. Hence, more NPHR indexes have been developed and showed non-inferior results to FFR. In 2018, Resting Full-cycle Ratio (RFR) was introduced and validated as a novel non-hyperaemic physiological index. Unlike FFR, RFR does not require vasodilators and limits procedural time with maintained high diagnostic equivalence to FFR (85%). RFR also measures the entire cardiac cycle to determine the lowest distal-to-proximal pressure ratio, which may reduce the operator-dependent bias.5,6
Despite its potential, a study showed that RFR and FFR had discordance for up to 21%. Two major variables associated with this discordance include clinical-anatomical variations and physiological problems ( Table 1).7 In this case, we considered the diffuse lesion at the proximal LAD might be the cause of the discordance. The discordance was more prevalent in the lesion at the LAD (70.5% vs 53.1%, p < 0.001). Under univariate analysis, lesions at the LAD were also associated with discordance (OR = 2.11, 95%CI = 1.51-3.02).8 Cut-off value for RFR might also cause the discordance. Since there was no universal cut-off value for RFR, we adopted the cut-off value from the validation study (VALIDATE RFR Study) with the optimal cut-off value of ≤0.89. This cut-off had a diagnostic accuracy of 78%, a sensitivity of 85%, and a specificity of 69% compared to FFR with a cut-off value of ≤0.80.5
The discordance between the FFR and NPHR index may complicate clinical decisions to do PCI or not. Deferring intervention in such cases is associated with a worse risk of 5-year vessel-oriented composite outcomes. In our case, we decided to adopt a hybrid approach of RFR and FFR by Casanova et al. ( Figure 4), which increased the agreement with FFR to 95% and reduced the need for vasodilators by 58%.9 According to this strategy, the RFR value of our patient was indeterminate (0.91), yet the follow-up FFR measurement was 0.76 and indicated for intervention.
OCT has become more essential in PCI planning due to its ability to produce high-resolution visualization of calcium morphology, including calcium circumferential arc, thickness, and length. A study concluded that calcified lesions with a circumferential arc >180°, maximum thickness >0.5 mm, and length >5 mm are associated with poor stent expansion.10 Our patient had a lesion arc of 270°, a maximum thickness of 0.78 mm, and a length of 64 mm. Based on these characteristics, the stent expansion would be suboptimal. Therefore, aggressive calcium modification techniques such as atherectomy are required.
Besides lesion characterization, OCT can also support the decision to revascularize by measuring MLA and %AS. An MLA less than 3.1 mm2 or %AS above 61% has been associated with an FFR below 0.80 with good sensitivity and specificity. A combination of these parameters was also associated with a significantly higher risk of major adverse cardiac events among patients with FFR-negative who did not undergo revascularization (33.3% vs 9.8%, p = 0.040).11 Based on this data, the MLA and %AS of our patient were indicative of revascularization. This highlights the expanded utility of OCT, not only for lesion characterization but also as an integrated tool for revascularizing decisions in cases of discordance.
Combining physiological assessment using a hybrid RFR–FFR strategy with OCT imaging is effective and efficient in managing diffuse and heavily calcified coronary lesions. The integrated approach enabled precise lesion assessment, optimal preparation, and successful stent deployment.
Written informed consent for publication of his clinical details and/or clinical images was obtained from the patient.
Figshare: Seeing-is-Believing: Integrating Physiology and Intracoronary Imaging for Resolving the Revascularization Conundrum of Diffuse and Calcified Coronary Lesions. https://doi.org/10.6084/m9.figshare.30154813.v212
The project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Coronary artery disease interventions, imaging modules and physiology
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Coronary Artery Intervention Imaging
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 14 Nov 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)