Keywords
Abnormal uterine bleeding; endometrial cancer; premenopause; risk prediction
This article is included in the Oncology gateway.
Endometrial malignancy in premenopausal women with abnormal uterine bleeding (AUB) is rare but clinically challenging. Current diagnostic strategies rely on endometrial sampling, which is invasive and often unnecessary. This study aimed to develop and internally validate a novel risk prediction score for endometrial malignancy in premenopausal women with AUB.
A retrospective longitudinal analytical study was conducted over 8 years and 11 months (January 2016–November 2024) at Charles Nicolle Hospital, Tunis, Tunisia. Premenopausal women with AUB who underwent endometrial biopsy followed by hysterectomy were included. Comparative analyses, logistic regression, and ROC curve analysis were performed. Significant variables were weighted according to adjusted odds ratios to construct a risk prediction score.
Among 209 patients, 13 (6.2%) had endometrial malignancy. Independent predictors of endometrial malignancy were: oral contraceptive use (OR 29.9, 95% CI 1.5–587.1, p = 0.025), endometrial thickness >9 mm (OR 25.3, 95% CI 4.3–147.6, p < 0.001), vascularization (OR 98.3, 95% CI 3.7–2594.8, p = 0.006). Protective factors included hemorrhage episode ≤1 (OR 0.20, 95% CI 0.08–0.52, p = 0.001) and lower bleeding abundance (OR 0.30, 95% CI 0.13–0.65, p = 0.002). The final score allocated points as follows: endometrial thickness >9 mm (+3), oral contraceptive use (+3), vascularization (+4), hemorrhage episodes ≤1 (−2), and lower bleeding abundance (−1). A score ≥7 defined high risk. Model discrimination was excellent (AUC 0.901, 95% CI 0.825–0.976, p < 0.001). At a cutoff ≥7, sensitivity was 77%, specificity 90%, positive predictive value 34%, and negative predictive value 98%.
We developed and internally validated a novel risk prediction score for endometrial malignancy in premenopausal women with AUB. With strong diagnostic performance and high negative predictive value, this score may help clinicians better identify women who truly require invasive sampling.
Abnormal uterine bleeding; endometrial cancer; premenopause; risk prediction
Abnormal uterine bleeding (AUB) is one of the most frequent reasons for consultation in gynaecology.1 It can be caused by structural or non-structural disorders of the uterus. According to the PALM-COEIN classification system of the International Federation of Gynecology and Obstetrics (FIGO), the causes include polyps, adenomyosis, leiomyomas, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial disorders, and iatrogenic or not yet classified causes.2
Although most cases of AUB are unrelated to pre-cancerous or cancerous endometrial pathologies, their seriousness should not be underestimated.3 It is established that in postmenopausal women with AUB, the risk of endometrial cancer rises to 10%.4,5 However, this risk drops to less than 1% when transvaginal ultrasound shows an endometrial thickness (ET) of less than 4 mm.6
For premenopausal women, such risk stratification is difficult, as the predictive value of ET assessment has yielded controversial results in the literature.7–10 In this group, other clinical factors are considered to assess the risk of endometrial hyperplasia (EH) or cancer: obesity, nulliparity, age, infertility, intermenstrual bleeding, anovulation, and diabetes.11
Based on these factors, guidelines recommend endometrial biopsy for women over 40 years, and for those under 40 with comorbidities.12,13
Despite these recommendations, many studies have not provided conclusive results regarding the impact of the mentioned risk factors.6,14,15 Consequently, the optimal management of premenopausal patients suffering from AUB remains unclear, hence the interest of our work to develop a predictive model based on clinical variables to assess the risk of pre-cancerous or cancerous endometrial pathologies in cases of AUB in premenopausal women.
Retrospective, longitudinal and analytical study was conducted over a period of 8 years and 11 months, from January 2016 to November 2024, at Gynaecology and Obstetrics Department B, Charles Nicolle Hospital, Tunis, Tunisia.
Premenopausal women presenting with AUB who were referred to our department were identified. An endometrial biopsy was performed during a diagnostic hysteroscopy or a fractional curettage and hemostasis procedure in cases of heavy AUB. All these women subsequently underwent surgical treatment (hysterectomy).
AUB was defined by the presence of bleeding from the uterine corpus that was abnormal in volume, regularity, and/or timing, according to what was reported by women.2
Patients meeting the following criteria were included:
Inclusion criteria:
- Endometrial pathology revealed by endometrial biopsy: atypical endometrial hyperplasia (AEH), endometrial cancer … with a definitive histological diagnosis on the hysterectomy specimen (considered the reference standard).
- Complete and available medical and surgical records.
Exclusion criteria:
- Postmenopausal women (absence of menstruation for at least 12 months after the age of 405).
- Hysterectomy not performed (No definitive histological diagnosis).
- Incomplete or missing data.
A study flowchart detailing case selection and exclusions has been developed ( Figure 1).
Data were retrospectively extracted from electronic medical records and focused on:
• Included women characteristics: Age, family cancer history (mainly endometrial, breast, ovarian, cervical, colon, rectal, and stomach cancer), personal medical history (diabetes, hypertension, obesity, thyroid disorders…), personal surgical history, gynaecological and obstetric history (gravidity, parity, contraception …).
• Bleeding characteristics: Type, abundance, number of haemorrhage episodes, associated symptoms (anorexia, pelvic pain, abdominal mass …).
• Additional examinations: Pelvic ultrasound (endometrial thickness, intra-cavitary image …), method of uterine cavity exploration (hysteroscopy or curettage), date of sampling and abundance of the sample, anatomopathological diagnosis from the biopsy, and definitive anatomopathological diagnosis from the hysterectomy specimen.
Data were entered and analysed with SPSS software (version 26.0, IBM Corp). Microsoft Office Excel was used to create the tables and graphs (https://www.office.com/?omkt=fr-FR ).
For comparative analysis, variables significantly associated with endometrial malignancy were assessed using the chi-square test or Fisher’s exact test for categorical variables and Student’s t test or Mann-Whitney U test for continuous variables.
Multivariate logistic regression models were then constructed to identify independent predictors of endometrial malignancy. Variables with a p value ≤ 0.20 in the univariate analysis were entered into the model. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were reported and a p value ≤ 0.05 was considered statistically significant.16,17
The diagnostic performance of predictors was evaluated using sensitivity, specificity, positive predictive value, and negative predictive value.18
Finally, a risk score for predicting endometrial malignancy was developed based on the variables that remained statistically significant in multivariate analysis.
The study protocol was approved on 6 Mars 2025 by the institutional ethics committee of Charles Nicolle Hospital, Tunis, Tunisia before conducting the study with approval number FWA 00032748- IORG0011243.
As this was a retrospective study using anonymized data, informed consent was waived.
During the study period, a total of 209 patients were included; 13 cases (6.22%) had endometrial malignancy.
The mean age was 45.7 ± 3.2 years (range 38–56). The mean body mass index (BMI) was 26.0 ± 2.2 kg/m2 (range 23–35).
A family cancer history was reported in 6% of cases.
Regarding medical history, diabetes was reported in 6% of patients, hypertension in 15%, thyroid disorders in 8%, and polycystic ovary syndrome (PCOS) in 1%. Other medical conditions included dyslipidemia (13%), lupus (2%), depressive syndrome (1%), and peptic ulcer disease (3%).
Previous surgical history included appendectomy in 7%, cholecystectomy in 12%, and other procedures in 6%.
Tobacco use was found in 3% and coffee consumption in 11%.
Regarding gynaecological history, the mean age at menarche was 12.2 ± 0.7 years. The median gravidity was 3 (range 0–7) and the median parity 3 (range 0–5).
Contraceptive methods included intrauterine device (IUD) use in 29% of patients, oral contraceptives in 3%, and progestin-only pills in 4%. Hormone replacement therapy was reported in 43% of cases. A history of infertility was present in 6%.
Screening procedures included Pap smear (24%) and mammography (18%), colposcopy was rarely performed (0.5%).
The majority (158 cases, 75.6%) had not undergone a Pap smear. Among those tested, 40 cases (19.1%) were normal. Abnormal results were observed in a minority of cases: 6 (2.9%) presented atypical squamous cells of undetermined significance (ASC-US), 2 (1.0%) had low-grade intraepithelial lesions (LIEB), and 3 (1.4%) atypical glandular cells (AGC).
Fibroids were the most frequent gynaecological condition, present in 76% of patients followed by ovarian cysts (10%).
Previous uterine endoscopic procedures were performed in 11% of patients.
The most frequent clinical presentations were pelvic pain (55%) and abdominal mass (35%). Fatigue was reported in 10% of cases. Profuse bleeding was noted in 61%.
The median delay before consultation was 12 months (range 1–72).
On physical and imaging assessment, an increased uterine size was observed in 77% of cases, vascularization in 49%, an abnormal endometrial–myometrial interface in 57%, and an intracavitary image in 23%.
Hemostatic curettage was performed in 10% of cases, while hysteroscopy was carried out in 90%.
Table 1 summarizes family cancer history and medical, surgical and exposure histories of the study population.
| Variable | Category | Benign (n, %) | Malignant (n, %) | Total (n, %) | p-value |
|---|---|---|---|---|---|
| Family cancer history | No | 186 (94) | 11 (6) | 197 (100) | 0.123 |
| Yes | 10 (83) | 2 (17) | 12 (100) | ||
| Tobacco use | No | 189 (94) | 13 (6) | 202 (100) | 0.488 |
| Yes | 7 (100) | 0 (0) | 7 (100) | ||
| Coffee consumption | No | 174 (93) | 13 (7) | 187 (100) | 0.202 |
| Yes | 22 (100) | 0 (0) | 22 (100) | ||
| Diabetes | No | 183 (93) | 13 (7) | 196 (100) | 0.338 |
| Yes | 13 (100) | 0 (0) | 13 (100) | ||
| Hypertension | No | 167 (94) | 11 (6) | 178 (100) | 0.954 |
| Yes | 29 (94) | 2 (6) | 31 (100) | ||
| Thyroid disorder | No | 180 (93) | 13 (7) | 193 (100) | 0.284 |
| Yes | 16 (100) | 0 (0) | 16 (100) | ||
| PCOS * | No | 194 (94) | 12 (6) | 206 (100) | 0.050 |
| Yes | 2 (67) | 1 (33) | 3 (100) | ||
| Other medical history | No | 154 (92) | 13 (8) | 167 (100) | 0.480 |
| Dyslipidemia | 28 (100) | 0 (0) | 28 (100) | ||
| Lupus | 5 (100) | 0 (0) | 5 (100) | ||
| Depressive syndrome | 3 (100) | 0 (0) | 3 (100) | ||
| PUD ** | 6 (100) | 0 (0) | 6 (100) | ||
| Gastrectomy | No | 195 (94) | 13 (6) | 208 (100) | 0.796 |
| Yes | 1 (100) | 0 (0) | 1 (100) | ||
| Colectomy | No | 196 (94) | 13 (6) | 209 (100) | — |
| Appendectomy | No | 182 (93) | 13 (7) | 195 (100) | 0.318 |
| Yes | 14 (100) | 0 (0) | 14 (100) | ||
| Cholecystectomy | No | 173 (94) | 12 (6) | 185 (100) | 0.658 |
| Yes | 23 (96) | 1 (4) | 24 (100) | ||
| Other surgeries | No | 185 (94) | 12 (6) | 197 (100) | 0.350 |
| Cystectomy | 4 (80) | 1 (20) | 5 (100) | ||
| Amygdalectomy | 7 (100) | 0 (0) | 7 (100) |
Most patients had no family cancer history (94% benign vs. 6% malignant, p = 0.123).
Most patients did not use tobacco (94% benign vs. 6% malignant, p = 0.488) or coffee (93% benign vs. 7% malignant, p = 0.202).
Diabetes (93% benign vs. 7% malignant, p = 0.338), hypertension (94% benign vs. 6% malignant, p = 0.954), and thyroid disorders (93% benign vs. 7% malignant, p = 0.284) were uncommon.
Other medical histories such as dyslipidemia, lupus, depressive syndrome, and peptic ulcer disease were rare and not associated with malignancy (all p > 0.05).
Previous surgeries—including gastrectomy, colectomy, appendectomy, cholecystectomy, cystectomy, and amygdalectomy—were infrequent and showed no significant association with malignancy.
Table 2 presents gynaecological history of the study population.
| Variable | Category | Benign (n, %) | Malignant (n, %) | Total (n, %) | Chi-Square p |
|---|---|---|---|---|---|
| Oral contraceptive use | No | 191 (95) | 11 (5) | 202 (100) | 0.013 |
| Yes | 5 (71) | 2 (29) | 7 (100) | ||
| IUD * | No | 137 (93) | 11 (7) | 148 (100) | 0.258 |
| Yes | 59 (97) | 2 (3) | 61 (100) | ||
| Progestin-only pill | No | 188 (94) | 13 (6) | 201 (100) | 0.458 |
| Yes | 8 (100) | 0 (0) | 8 (100) | ||
| Long-term treatment | No | 180 (94) | 12 (6) | 192 (100) | 0.952 |
| Yes | 16 (94) | 1 (6) | 17 (100) | ||
| Hormonal replacement therapy | No | 109 (92) | 10 (8) | 119 (100) | 0.133 |
| Yes | 87 (97) | 3 (3) | 90 (100) | ||
| Infertility | No | 181 (93) | 13 (7) | 194 (100) | 0.628 |
| primary | 9 (100) | 0 (0) | 9 (100) | ||
| secondary | 4 (100) | 0 (0) | 4 (100) | ||
| Pap smear | No | 148 (93.7) | 10 (6.3) | 158 (75.6) | <0.001 |
| Normal | 39 (97.5) | 1 (2.5) | 40 (19.1) | ||
| ASCUS | 6 (100) | 0 | 6 (2.9) | ||
| LIEB | 2 (100) | 0 | 2 (1.0) | ||
| ACG | 1 (33.3) | 2 (66.7) | 3 (1.4) | ||
| Colposcopy | No | 195 (93.8) | 13 (6.3) | 208 (99.5) | 0.796 |
| Yes | 1 (100) | 0 | 1 (0.5) | ||
| Fibroids | No | 43 (86.0) | 7 (14.0) | 50 (23.9) | 0.009 |
| Yes | 153 (96.2) | 6 (3.8) | 159 (76.1) | ||
| Previous uterine endoscopic procedure | No | 177 (95.2) | 9 (4.8) | 186 (89.0) | 0.035 |
| Myomectomy | 7 (87.5) | 1 (12.5) | 8 (3.8) | ||
| Polypectomy | 9 (75.0) | 3 (25.0) | 12 (5.7) | ||
| hemostatic curettage | 3 (100) | 0 | 3 (1.4) | ||
| PCOS ** | No | 194 (94) | 12 (6) | 206 (100) | 0.050 |
| Yes | 2 (67) | 1 (33) | 3 (100) | ||
| Ovarian cyst | No | 176 (93.6) | 12 (6.4) | 188 (90.4) | 0.808 |
| Yes | 19 (95.0) | 1 (5.0) | 20 (9.6) | ||
| Mammogram | No | 162 (93.1) | 12 (6.9) | 174 (83.3) | 0.805 |
| ACR2 | 25 (96.2) | 1 (3.8) | 26 (12.4) | ||
| ACR3 | 7 (100) | 0 | 7 (3.3) | ||
| ACR4 | 2 (100) | 0 | 2 (1.0) | ||
| Interval delay | 1 | 7 (87.5) | 1 (12.5) | 8 (3.8) | 0.040 |
| 2 | 53 (86.9) | 8 (13.1) | 61 (29.3) | ||
| 3 | 105 (97.2) | 3 (2.8) | 108 (51.9) | ||
| 4 | 24 (100) | 0 | 24 (11.5) | ||
| 5 | 6 (85.7) | 1 (14.3) | 7 (3.4) | ||
| Breast neoplasia | No | 196 (94) | 13 (6) | 209 (100) | – |
| Tamoxifen | No | 195 (94) | 13 (6) | 208 (100) | – |
| Pelvic irradiation | No | 196 (94) | 13 (6) | 209 (100) | – |
Oral contraceptive use was significantly associated with malignancy (29% malignant in users vs. 5% in non-users, p = 0.013), whereas use of IUDs, progestin-only pills, long-term treatments, and hormonal replacement therapy showed no significant differences.
Infertility—primary or secondary—was also rare and not associated with malignancy (p > 0.05).
Pap smear results were significantly associated with malignancy (p < 0.001), with the highest proportion of malignant cases observed in patients with ACG (67%). Colposcopy showed no significant association with malignancy (p = 0.796).
Presence of fibroids was associated with a lower risk of malignancy (3.8% malignant in patients with fibroids vs. 14% in those without, p = 0.009).
Previous uterine endoscopic procedures were significantly associated with malignancy (p = 0.035), with higher malignant rates in patients who underwent polypectomy (25%) or myomectomy (12.5%).
Polycystic ovary syndrome (PCOS) was observed in three patients, with one malignant case (p = 0.050).
Ovarian cysts and mammogram findings did not show a significant association with malignancy (p = 0.808 and p = 0.805, respectively).
Breast neoplasia, tamoxifen use, and pelvic irradiation were not associated with malignancy.
Interval delay was significantly related to malignancy (p = 0.04).
Table 3 summarizes the bleeding characteristics and associated symptoms.
Bleeding characteristics—including abundance and type—showed limited association, except for the number of hemorrhage episodes (p = 0.027), where patients with 0–1 episode had higher malignancy rates (25–33%) than those with ≥2 episodes.
Other associated symptoms were significantly related to malignancy (p = 0.001), with anorexia present in 100% of malignant cases. Pelvic pain, fatigue, and uterine size were not significantly associated with malignancy. Presence of an abdominal mass was inversely associated with malignancy (1% malignant if present vs. 9% if absent, p = 0.033).
The complementary examination findings are summarized in Table 4.
Ultrasound features revealed strong associations for vascularization (p < 0.001), with Score 2 showing 71% malignancy, whereas normal vascularization or Score 1 had much lower rates. The endometrial–myometrial interface, intracavitary images, hemostatic curettage, and hysteroscopy did not show significant differences. Quantity of curettage specimen was also significant (p = 0.003), with abundant specimens associated with 10% malignancy compared to 0% for scanty specimens.
▪ Continuous variables ( Table 5)
The median age was 46 years in benign cases and 48 years in malignant cases (p = 0.217).
The median BMI was 25 for benign and 26 for malignant cases (p = 0.576).
Gravidity and parity differed, with median gravidity of 3 versus 2 (p = 0.018) and median parity of 3 versus 2 (p = 0.060).
Median age at menarche was 12 years in both groups (p = 0.603).
The median delay before consultation was significantly shorter in malignant cases (6 months) compared to benign cases (12 months, p = 0.011).
Endometrial thickness was markedly higher in malignant cases (median 12 mm) than in benign cases (median 5 mm, p < 0.001).
Hemoglobin levels were similar between groups (median 10 g/dL, p = 0.487), and the median delay before biopsy was 1 month in both groups (p = 0.756).
The predictive performance of several clinical and ultrasound variables for endometrial malignancy was evaluated using ROC analysis: ( Figure 2) ( Table 6).
▪ Endometrial thickness demonstrated the highest discriminative ability, with an AUC of 0.842 (p < 0.001). A thickness >9 mm was identified as the optimal threshold, yielding a sensitivity of 69%, specificity of 87%, a positive predictive value of 26.5%, and a negative predictive value of 97.7%. This indicates that increased endometrial thickness is a strong predictor of malignancy, particularly useful for ruling out disease when the measurement is below the threshold.
▪ Gravidity showed a moderate predictive value with an AUC of 0.690 (p = 0.022). A gravidity of ≤1 was associated with high specificity (96%) but low sensitivity (23%), suggesting that low gravidity may indicate increased risk of malignancy, though many cases could be missed if this criterion is used alone.
▪ The number of hemorrhage episodes had limited predictive value, with an AUC of 0.576 (p = 0.360). Using ≤2 episodes as a threshold, the sensitivity was 62% and specificity 69%, indicating that this variable alone is a weak predictor of malignancy.
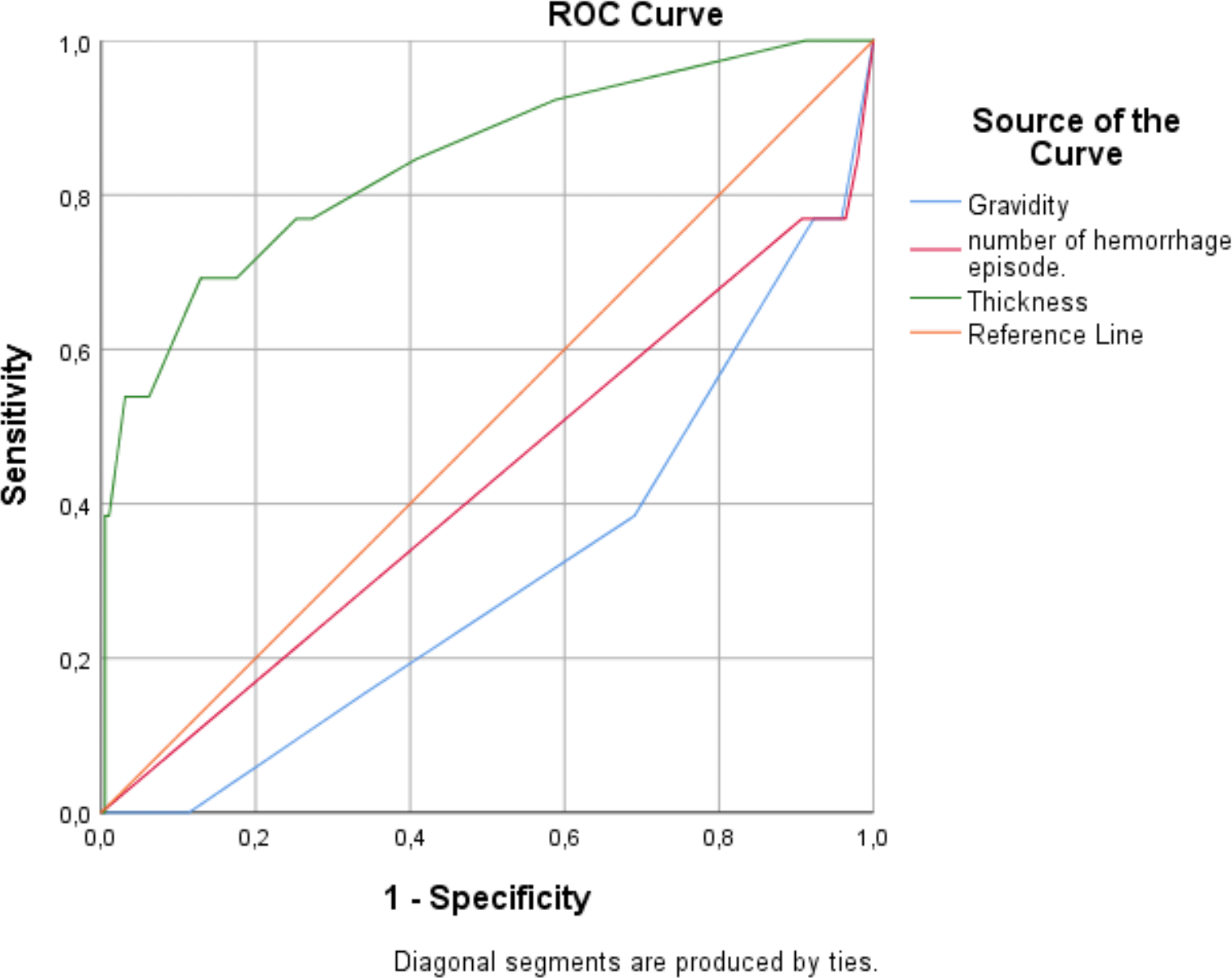
| Variable | AUC | p-value | 95% CI | Criterion | Sensitivity (%) | Specificity (%) | PPV* (%) | NPV** (%) |
|---|---|---|---|---|---|---|---|---|
| Gravidity | 0.690 | 0.022 | 0.542–0.838 | ≤1 | 23.08 | 96.43 | 30 | 95 |
| Number of hemorrhage episodes | 0.576 | 0.360 | 0.398–0.754 | ≤2 | 61.54 | 68.88 | 11.6 | 96.4 |
| Endometrial thickness | 0.842 | <0.001 | 0.715–0.968 | >9 | 69.23 | 87.11 | 26.5 | 97.7 |
Overall, endometrial thickness is the most significant individual predictor of malignancy, while low gravidity adds specificity but is limited by poor sensitivity. The number of hemorrhage episodes does not reliably predict malignancy.
Logistic regression analysis identified several independent predictors of endometrial malignancy ( Table 7).
▪ Oral contraceptive use was significantly associated with increased risk (OR = 29.87, 95% CI: 1.52–587.08, p = 0.025).
▪ Endometrial thickness greater than 9 mm was a strong predictor of malignancy (OR = 25.25, 95% CI: 4.32–147.64, p < 0.001).
▪ The presence of vascularization significantly increased the risk (OR = 98.34, 95% CI: 3.73–2594.79, p = 0.006).
Conversely:
▪ A low number of hemorrhage episodes (≤1) was protective (OR = 0.202, 95% CI: 0.079–0.520, p = 0.001).
▪ Lower bleeding abundance decreased the likelihood of malignancy (OR = 0.295, 95% CI: 0.134–0.648, p = 0.002).
▪ Previous uterine endoscopic procedures showed a borderline association (p = 0.054) but did not reach statistical significance.
A risk score for predicting endometrial malignancy was developed using the variables found to be statistically significant in multivariate analysis.
The included predictors were:
- Endometrial thickness greater than 9 mm,
- Oral contraceptive use,
- Presence of vascularization,
- Low number of hemorrhage episodes (≤1),
- And lower bleeding abundance.
Each predictor was weighted according to its odds ratio:
- Endometrial thickness >9 mm and oral contraceptive use were assigned 3 points each,
- Vascularization was assigned 4 points,
- while a low number of hemorrhage episodes and lower bleeding abundance were considered protective and assigned −2 and −1 points, respectively.
The total score allows stratification of patients into risk categories: scores of 7 or higher indicate a high risk of malignancy.
The predictive score for endometrial malignancy demonstrated excellent discriminative ability ( Figure 3):
- The area under the ROC curve (AUC) was 0.901 (95% CI: 0.825–0.976, p < 0.001), indicating high accuracy in distinguishing malignant from benign cases.
- Using a threshold of >7, the score showed a sensitivity of 77% and a specificity of 90%, with a positive predictive value of 34% and a negative predictive value of 98%, suggesting strong effectiveness in ruling out malignancy among low-risk patients.
The evaluation of women presenting with AUB to exclude endometrial malignancy relies on a combination of clinical risk assessment, imaging, and histopathological sampling.
The presented results, when compared to the broader literature, reveal a consistent emphasis on endometrial thickness as a pivotal triage tool. They also highlight the development and variable utility of integrated risk prediction models.
Evidence consistently highlights the crucial role of transvaginal ultrasound–derived endometrial thickness, which demonstrates robust diagnostic accuracy in predicting endometrial malignancy across studies.
Endometrial thickness among postmenopausal women with AUB below 4 mm seems to be associated with a very low risk of endometrial cancer.19 Unfortunately, there is no established consensus on the threshold for endometrial thickness for premenopausal women. In accordance with the results of other studies, our data indicated that patients with thicker endometrium exhibited a higher risk of endometrial malignancy.20
Our findings, which identified endometrial thickness >9 mm as an independent risk factor for AUB in perimenopausal women, are consistent with previously published evidence. Tian et al.21 similarly reported that an endometrial thickness ≥10 mm was an independent risk factor for AUB, while Sahu et al.22 observed that the majority of perimenopausal women with AUB had an endometrial thickness of 10–12 mm (35.7%), followed by 7–9 mm (27.1%).
In contrast, Getpook et al.23 demonstrated that an endometrial thickness ≤8 mm was unlikely to be associated with malignant pathology in premenopausal AUB.
Collectively, these concordant findings reinforce the clinical significance of transvaginal ultrasonography in assessing endometrial thickness as a key predictor of endometrial pathology in premenopausal women with AUB.
This principle holds strong in clinical guidelines, confirming endometrial thickness as the most robust initial screening parameter.
Several studies have developed integrated risk prediction models to address the limitations of using endometrial thickness alone.
Giannella et al.3 proposed a model including BMI ≥30 kg/m2, diabetes, and endometrial thickness >11 mm, which achieved robust accuracy (AUC 0.854; sensitivity 75.0%, specificity 90.8%; PPV 30.0%, NPV 98.6%).
Similarly, the PAD30 score developed by Bagepalli Srinivas et al.,24 based on anovulatory bleeding pattern, age ≥45 years, BMI ≥30 kg/m2, and diabetes mellitus, showed good diagnostic performance (AUC 0.84; sensitivity 85.7%, specificity 87.6%).
Ruan et al.20 incorporated metabolic diseases, family history, age ≥40 years, resistance index of endometrial vasculature ≤0.5, and endometrial thickness ≥10 mm into a nomogram, which demonstrated good discrimination with an AUC 0.837 (95% CI 0.800-0.874) and calibration in both the development and validation cohorts.
Compared with these models, our predictive score demonstrated even stronger discriminative ability, with an AUC of 0.901 (95% CI 0.825–0.976, p < 0.001). Using a cutoff >7, the model achieved a sensitivity of 77% and specificity of 90%, with a particularly high negative predictive value of 98%, supporting its effectiveness in reliably excluding malignancy in low-risk patients. These results suggest that our score may provide an accurate and clinically useful tool, comparable or superior to previously published models, for guiding the selective use of invasive diagnostic procedures.
The evaluation of patient-specific risk factors shows both consistency and variation.
The divergence underscores that while certain risk factors are epidemiologically important, their utility in a specific predictive algorithm can vary based on the study population and the other variables in the model.
In our study, oral contraceptive use emerged as an independent predictor of endometrial malignancy (OR 29.87, 95% CI 1.52–587.08, p = 0.025). This result contrasts with extensive evidence from large-scale epidemiological studies and meta-analyses, which consistently report a protective effect of oral contraceptives against endometrial cancer. The Collaborative Group on Epidemiological Studies on Endometrial Cancer, through an individual participant meta-analysis including over 27,000 women with endometrial cancer across 36 studies, demonstrated a substantial and sustained risk reduction associated with oral contraceptive use.25 Similarly, Michels et al.26 and Harajka et al.27 confirmed these findings in systematic reviews and meta-analyses, while Karlsson et al.28 further highlighted the time-dependent protective effect, with longer duration of use conferring greater benefit.
The discrepancy with our findings may be explained by the short duration of oral contraceptive use among women in our cohort, which might have been insufficient to exert a protective effect. Additionally, the small sample size and wide confidence interval suggest caution in interpretation. Nevertheless, this unexpected association highlights the importance of considering duration and patterns of oral contraceptive exposure when assessing their relationship with endometrial cancer risk.
Our findings demonstrated that vascularization on transvaginal ultrasound was found to be one of the strongest predictors of endometrial malignancy. Logistic regression analysis showed that the presence of abnormal vascularization significantly increased the risk, with an odds ratio of 98.34 (95% CI: 3.73–2594.79, p = 0.006). Notably, patients with a vascular score of 2 had a malignancy rate of 71%, whereas normal vascularization or a score of 1 was associated with substantially lower rates.
These findings are in line with recent evidence supporting the role of Doppler ultrasound vascular scoring in differentiating benign from malignant endometrial lesions. Tirnovanu et al.29 reported that a vascular score of 1 typically excludes endometrial cancer, with high sensitivity (87.5%) and specificity (79%). Conversely, a cutoff score of 2 provided excellent discriminative performance, yielding 100% sensitivity and 86.3% specificity, which reflects the increased neovascularization commonly observed in malignant tumors. Such results are also consistent with the International Endometrial Tumor Analysis (IETA) consensus,30 which emphasizes abnormal vascular patterns as a key ultrasound feature suggestive of malignancy.
Taken together, our findings reinforce the clinical value of color Doppler vascular assessment in women with abnormal uterine bleeding. The strong association between vascular score ≥2 and malignancy suggests that integrating vascularization into risk prediction models may substantially improve diagnostic accuracy. This parameter could therefore serve as a non-invasive adjunct to guide clinical decision-making and reduce unnecessary invasive procedures.
In our cohort, two clinical characteristics of bleeding were independently protective: having ≤1 hemorrhagic episode (OR 0.20, 95% CI 0.08–0.52, p = 0.001) and lower bleeding abundance (OR 0.30, 95% CI 0.13–0.65, p = 0.002). Clinically, this pattern is plausible: malignant endometrial lesions often generate recurrent and profuse bleeding due to fragile neovascularization and disordered repair, whereas isolated or low-volume events are more typical of benign etiologies (e.g., anovulatory dysfunction, polyps, simple hyperplasia).
Most of the available studies have not addressed the predictive value of bleeding frequency or volume. Specifically, several key works—including Giannella et al.,3 Timmermans et al.,19 Sahu et al.,22 and Getpook et al.23—focused primarily on endometrial thickness or metabolic and clinical factors, without evaluating bleeding characteristics as independent predictors of malignancy. Similarly, Tian et al.21 did not report hemorrhage count or abundance in relation to cancer risk. Bleeding burden was not included as a variable into the nomogram of Ruan et al.20
The closest conceptual overlap is PAD30’s anovulatory pattern,24 which acknowledges that bleeding characteristics carry predictive signal, indirectly supporting our observation that clinical bleeding features can refine risk.
Our findings highlight the potential value of bleeding characteristics as predictive markers in premenopausal AUB. Specifically, the number of hemorrhagic episodes and the abundance of bleeding represent simple, clinically accessible variables that can be systematically collected during patient history. As our data suggest, fewer episodes and lower bleeding abundance are associated with a reduced likelihood of malignancy, underscoring the importance of detailed bleeding history in refining risk stratification.
This study has several strengths. It is, to our knowledge, one of the few to focus exclusively on premenopausal women with AUB, a population for whom risk stratification for endometrial malignancy remains uncertain. The use of a robust reference standard—hysterectomy specimen histology—provides high diagnostic accuracy for outcome classification. Furthermore, the study covered a relatively long period (almost nine years), enhancing the reliability of findings.
By incorporating bleeding patterns—simple, cost-free, and universally available clinical variables—our model complements existing approaches and may enhance decision-making regarding endometrial sampling in premenopausal women with AUB.
However, some limitations should be acknowledged. First, the retrospective single-center design may limit generalizability. Second, external validation was not performed, and the predictive score requires further confirmation in independent cohorts before clinical application. Finally, some variables of potential interest, such as molecular or hormonal markers, were not available in the dataset.
The proposed risk score offers a clinically useful, non-invasive tool to stratify premenopausal women with AUB according to their likelihood of endometrial malignancy:
▪ Low-risk group (score <7):
These women are characterized by protective factors such as a low number of hemorrhagic episodes (≤1) and lower bleeding abundance. The score demonstrated an an AUC of 0.901 and an excellent negative predictive value (98%) in this group, suggesting that unnecessary endometrial biopsies could be safely avoided, thereby reducing patient morbidity and healthcare costs.
▪ High-risk group (score ≥7):
Patients presenting with predictors such as endometrial thickness >9 mm, oral contraceptive use, and the presence of vascularization fall into this category. With a sensitivity of 77% and a specificity of 90%, this group should be prioritized for invasive diagnostic procedures (endometrial sampling or hysteroscopy) to ensure timely detection and management of endometrial malignancy.
This stratification may improve patient-centered care by tailoring diagnostic strategies, optimizing resource allocation, and minimizing unnecessary interventions.
To confirm and expand the clinical applicability of this score, further research should address the following:
▪ External validation: Conduct multicenter studies across diverse populations to confirm reproducibility and generalizability.
▪ Prospective evaluation: Implement prospective trials to assess real-world impact on reducing unnecessary biopsies without delaying cancer detection.
▪ Comparative assessment: Benchmark the score against existing guidelines and other predictive models to establish relative performance.
▪ Model refinement: Explore the addition of serum biomarkers, molecular markers, or advanced imaging parameters to enhance predictive accuracy.
This study developed and internally validated a novel risk prediction score for endometrial malignancy in premenopausal women presenting with AUB. The score based on five key predictors: endometrial thickness greater than 9 mm, oral contraceptive use, and the presence of vascularization as risk factors, while a low number of hemorrhage episodes and lower bleeding abundance were protective.
A cutoff value of 7 or higher identified high risk of endometrial malignancy, with excellent diagnostic accuracy (AUC 0.901). At this threshold, the score achieved a sensitivity of 77% and specificity of 90%, with a particularly high negative predictive value of 98%, making it especially useful for ruling out malignancy among low-risk patients.
These findings suggest that the proposed score could serve as a valuable non-invasive decision-support tool to guide the selective use of endometrial biopsy. While external validation in larger and more diverse populations remains necessary, the model has the potential to improve patient-centered care by reducing unnecessary invasive procedures and ensuring timely diagnosis in women at elevated risk.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The study protocol was approved on 6 Mars 2025 by the institutional ethics committee of Charles Nicolle Hospital, Tunis, Tunisia before conducting the study (approval number: FWA 00032748- IORG0011243).
As this was a retrospective study using anonymized data, informed consent was waived.
All data sets can be assessed and all study findings reported in the article are shared via Harvard Dataverse: “Development and Internal Validation of a Novel Risk Prediction Score for Endometrial Malignancy in Premenopausal Women with Abnormal Uterine Bleeding”, https://doi.org/10.7910/DVN/BTRWYC.31
This project contains the following:
Harvard Dataverse: “Development and Internal Validation of a Novel Risk Prediction Score for Endometrial Malignancy in Premenopausal Women with Abnormal Uterine Bleeding”, https://doi.org/10.7910/DVN/BTRWYC.31
This project contains the following:
This work has been reported in line with the STROBE guidelines.32
Harvard Dataverse: “Development and Internal Validation of a Novel Risk Prediction Score for Endometrial Malignancy in Premenopausal Women with Abnormal Uterine Bleeding”, https://doi.org/10.7910/DVN/BTRWYC.31
This project contains the following:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: statistical genetics, genetic epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Nov 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)