Keywords
Melanoma; FDG PET/CT; neoplasm staging; diagnostic imaging; case management; immunotherapy
This article is included in the Oncology gateway.
Accurate staging guides melanoma treatment. Conventional imaging modalities like contrast-enhanced CT (CECT) is widely used but relies on morphology which may miss early spread. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) combines metabolic and anatomic data and may improve detection.
We conducted a single-centre retrospective diagnostic accuracy study at a tertiary university hospital in Tunisia (December 2019-February 2024). All adults with histologically confirmed melanoma undergoing whole body 18F-FDG PET/CT as well as CECT for initial staging or restaging were included. The reference standard was histopathology, otherwise composite verification with clinical/imaging follow-up ≥6 months. Outcomes were per-patient sensitivity, specificity, predictive values, and accuracy, inter-modality agreement (Cohen’s κ), and management change attributable to 18F-FDG PET/CT.
Of 51 screened, 35 patients were included (23 staging and 12 restaging). Compared with CECT, 18F-FDG PET/CT reclassified stage in 22/35 (62.9%), upstaging 14 (40.0%) and downstaging 8 (22.9%). For nodal disease, 18F-FDG PET/CT showed higher specificity (95.2%, 95% CI [77.3-99.8] versus 66.7%, 95% CI [44.7–84.4]) and accuracy (88.6%, 95% CI [73.3-96.8], versus 65.7%, 95% CI [47.8-80.9]) with similar sensitivity (78.6%, 95% CI [49.2-95.3] versus 64.3%, 95% CI [35.1-87.2]). For distant metastases, 18F-FDG PET/CT achieved markedly higher sensitivity (92.9%, 95% CI [66.1–99.8] versus 50.0%, 95% CI [23.0-77.0]) and accuracy (91.4%, 95% CI [76.9-98.2] versus 68.6%, CI [50.7-83.1]), with high specificity for both (90.5%, 95% CI [69.6-98.8] versus 81.0%, 95% CI [58.1–94.6]). Agreement with CECT was fair for nodes (κ=0.27) and poor for distant sites (κ=0.16). Management decisions were available in 32/35. 18F-FDG PET/CT changed treatment in 15/32 (46.9%). No adverse events occurred.
In this first Tunisian series, 18F-FDG PET/CT improved diagnostic performance over CECT, especially for distant metastases, and frequently redirects management. Findings support integrating its integration into melanoma care pathways when results may influence therapy.
Melanoma; FDG PET/CT; neoplasm staging; diagnostic imaging; case management; immunotherapy
Cutaneous melanoma currently shows the fastest progression rate among all cancer types worldwide. According to the International Agency for Research on Cancer (IARC), the number of new melanoma cases rose from 132,600 in 2000 to approximately 330,000 in 2022.1 The lifetime risk estimated at 1 in 500 in 1935 is now escalating as melanoma affects approximately 1 in 50 individuals in Western populations.2 Although melanoma accounts for a minority of skin cancers, it is responsible for the majority of skin cancer-related deaths due to its high metastatic potential.3 With 93 new cases recorded in 2022, malignant melanoma ranks 24th out of all malignancies in Tunisia (age-standardised incidence rate [ASR-W]: 0.45 per 100,000), and with 39 deaths (ASR-W mortality rate: 0.31 per 100,000), it ranks as the 24th most common cause of cancer-related deaths.4 Despite these rates in comparison to high-incidence countries, melanoma is viewed as a disproportionately significant public health concern because of its poor prognosis at advanced stages and its high potential for metastasis.
The first-line treatment for the patients with resectable primary tumours is surgery, followed by therapeutic lymphadenopathy or sentinel node biopsy if required. While surgery remains central for early stages, the introduction of effective and immune-based treatments has significantly improved outcomes for patients with advanced disease.5,6 In this regard, precise patient classification and staging are essential for guiding therapeutic choices and predicting prognosis. Conventional imaging modalities, including contrast-enhanced computed tomography (CT), are widely used for staging. But because CT is based on morphological criteria, it may fail to detect early metastatic disease. In contrast, 18F-fluorodeoxyglucose positron emission tomography coupled with computed tomography (18F-FDG PET/CT) integrates metabolic and anatomic data in a single acquisition, enabling whole-body assessment for metabolically active lesions.7 Reviews have shown that 18F-FDG PET/CT achieves higher diagnostic capabilities than CT alone for detecting both nodal and distant metastases, particularly in advanced disease stages. Despite strong evidence in the American Joint Committee on Cancer (AJCC) stages III-IV, the role of 18F-FDG PET/CT remains less well established in early stages.8 The usefulness of this hybrid modality both in staging and restaging has been shown by observational studies conducted in real-world settings, and it has a significant influence on clinical decision-making at various phases of disease.
In Tunisia, 18F-FDG PET/CT has only been available since late 2019, and its recent integration represents a significant advance in oncology, including melanoma. The lack of published national data prompted this first Tunisian study to assess its utility in routine clinical practice. Therefore, we aimed to determine its optimal use across different disease stages in light of current treatment options. The objectives of this observational retrospective study were as follows:
• To compare the diagnostic performance of 18F-FDG PET/CT versus contrast-enhanced CT in the initial staging and restaging of melanoma.
• To assess the added clinical value of 18F-FDG PET/CT in guiding therapeutic management.
• To discuss the implications of incorporating 18F-FDG PET/CT into the routine melanoma work-up considering current international evidence.
This is a single-centre diagnostic accuracy study that was conducted using the retrospective cohort design. It encompassed the collection of data retrieved from all eligible patients’ records from the hospital information system in the Nuclear Medicine Department of Sahloul University Hospital, Sousse, Tunisia, from December 2019 to February 2024.
Tunisia has a 5-year melanoma prevalence of 0.58 per 100,000 inhabitants,4 among the lowest globally. This study represents the first Tunisian evaluation of 18F-FDG PET/CT compared to conventional CT for melanoma staging and restaging. Ethical committee approval was obtained to access, collect, and analyse the data for the study.
A total of 35 were eligible adult patients (≥18 years), who were referred for either initial staging of melanoma after primary diagnosis or restaging for suspected recurrence. Inclusion criteria included a histologically confirmed cutaneous or mucosal melanoma and completion of both contrast-enhanced thoraco-abdomino-pelvic CT (TAP CT) and 18F-FDG PET/CT within the study period. Patients were excluded if the 18F-FDG PET/CT had been performed for reasons other than initial staging or recurrence evaluation, such as response assessment or routine surveillance. Patients were also excluded if their imaging datasets were incomplete or if histopathological or clinical follow-up data were unavailable or insufficient. In addition, individuals with a follow-up period less than six months after the 18F-FDG PET/CT examination were excluded from the study.
Because melanoma incidence in Tunisia is low and 18F-FDG PET/CT was only recently introduced, we included all eligible patients during the study period. This exhaustive consecutive sampling over four years yielded 35 cases. Although this number is below the ideal target, which corresponds to total sample sizes of 36-64 at local disease prevalences and is reduced to 44 with a more permissive precision of ±0.12 is applied as suggested by published precision-based approaches to diagnostic accuracy studies.9,10 The analyses were conducted with exact 95% confidence intervals and the results are interpreted with appropriate caution.
* Contrast enhanced Computed Tomography (CECT)
Contrast-enhanced TAP CT scans were acquired using a multidetector scanner, with intravenous iodinated contrast (1.5-2 mL/kg). Axial, coronal, and sagittal reconstructions were obtained with slice thickness of 1-5 mm. CECT pathological lymph nodes were defined as those with a short-axis diameter is greater than 15 mm, a round shape, loss of the fatty hilum, central necrosis, irregular margins and heterogenous or rim enhancement. CECT was interpreted independently by radiologists blinded to 18F-FDG PET/CT results but with access to the same clinical information.
* 18F-FDG PET/CT
PET/CT examinations were performed on a hybrid Biograph mCT64 scanner (Siemens®) installed in 2019, compliant with the European Association of Nuclear Medicine (EANM) guidelines. Patients fasted ≥6 h. Capillary blood glucose levels were checked prior to the radiotracer injection and had to be below 2 g/L. After an intravenous injection of 3 MBq/kg of FDG, patients rested for 60 minutes. The PET/CT scan included a topogram and CT. All patients underwent extended whole-body PET, which was acquired from vertex to toes. The acquisition was divided into overlapping beds (8 to 10 beds) with acquisition times of 2 to 3 minutes per bed. CT imaging was performed within 1 minute before PET imaging with the patient in precisely the same position. The acquisition parameters for 64-detector helical CT were of low dose for attenuation correction. Additional CT focused on a particular body region with intravenous iodinated contrast was acquired when indicated. For PET/CT, two reconstructions were performed: one with attenuation correction and the other without. Native slices with a thickness of 5 mm were generated, followed by reconstructions with two specific settings: one standard reconstruction with a slice thickness of 2 mm and another focused on parenchymal structures with a slice thickness of 1 mm. PET/CT readers were blinded to CT findings but had access to clinical information. No adverse events, such as tracer reactions or scan-related issues, occurred during our study for either CT or PET/CT examinations.
Image interpretation
18F-FDG PET/CT was interpreted as positive when there was focal and intense FDG uptake above physiological background, matched to anatomic structures, and not explained by benign processes. Interpretations were performed independently by two blinded nuclear medicine physicians: one resident and a specialist with more than 5 years of experience and an interest in oncology imaging.
The same clinical information were provided for all patients and the interpretation of each imaging modality (18F-FDG PET/CT and CECT) was interpreted independently.
To minimize inter-reader variability, discordant 18F-FDG PET/CT cases were reviewed jointly for consensus. All imaging data were acquired using the same scanner and standardized acquisition parameters throughout the study period.
The composite reference standard was histological analysis (biopsy or surgical specimen) when available. Otherwise, findings were evaluated based on composite follow-up over ≥6 months, combining clinical examination, laboratory markers, and confirmatory imaging (ultrasound (US), Magnetic Resonance Imaging (MRI), bone scan, repeat CT, or 18F-FDG PET/CT). This approach is backed by Dinnes et al.’s11 review of melanoma imaging studies published in the Cochrane Database of Systemic reviews. In the case of absence of the gold standard confirmation, and if both modalities were positive for the same lesion and remained positive within a period of 3 months, they were classified as true positive (TP). Conversely, a lesion was classified as a false positive (FP) when it was positive on imaging but subsequently refuted by the reference standard within a 3- to 6-month period without any treatment, either through histopathological analysis or by stability/regression on clinical and imaging follow-up inconsistent with malignant disease. On the other hand, if they were initially negative and remained negative after a 6-month period with no associated treatment, they were classified as true negative (TN), or false negative (FN) if proven otherwise with the gold standard. Equivocal 18F-FDG PET/CT or CECT findings were prespecified as positive only if corroborated by correlative imaging or histology, otherwise classified as indeterminate and excluded from primary accuracy analyses. For each accuracy analysis (nodes and distant), we only included patients who had full verification data. If verification was missing, those patients were excluded from that specific analysis. Histopathological reviewers and clinicians interpreting follow-up imaging had access to PET/CT and CECT findings as part of routine care, and their assessments therefore reflected real-world clinical practice rather than blinded review conditions.
For the staging analysis, 18F-FDG PET/CT, and contrast-enhanced thoraco-abdomino-pelvic CT (TAP CT) findings were evaluated on a per-patient basis for nodal status (N stage) and distant metastatic status (M stage) according to the AJCC 8th edition TNM classification. Analyses were performed separately for regional nodal metastases (N status) and distant metastases (M status). Subgroup analyses (initial staging vs. restaging, and AJCC stage I–II vs. III–IV) were pre-specified in the study design.
Agreement between CT and 18F-FDG PET/CT was assessed using Cohen’s kappa coefficient with 95% confidence intervals and interpreted according to Landis and Koch’s scale. Concordance was calculated for both binary classification (disease present vs. absent).
Diagnostic performance was expressed as follows: Sensitivity was defined as the proportion of true positives among all patients with the target condition; specificity as the proportion of true negatives among all patients without the target condition; positive predictive value (PPV) as the proportion of true positives among all positive test results, negative predictive value (NPV) as the proportion of true negatives among all negative test results, and diagnostic accuracy as the proportion of correctly classified patients (true positives plus true negatives) among all patients evaluated.
Therapeutic strategies were determined by the institutional multidisciplinary oncology board based on all available clinical, imaging, and histopathological information. For each patient, the initial management plan was established after conventional imaging (CECT, US and MRI), prior to the availability of 18F-FDG PET/CT results.
The impact of 18F-FDG PET/CT on therapeutic management was evaluated by comparing the pre 18F-FDG PET/CT treatment plan with the final plan decided after 18F-FDG PET/CT interpretation. Management status was classified as “no change” when 18F-FDG PET/CT did not alter the planned therapeutic approach, which could include active surveillance, surgical treatment (lymph node dissection or metastasectomy), systemic medical therapy (immunotherapy, chemotherapy, or targeted therapy), radiotherapy, or a combination of these. The status “modified” was applied when 18F-FDG PET/CT findings led to a documented change in the treatment plan. The changes were further categorised as:
*Inter-modality changes are defined as a shift from one therapeutic modality to another (e.g., from surgery to systemic medical therapy or from systemic therapy to surveillance).
*Intra-modality changes are defined as adjustments within the same therapeutic modality (e.g., modifying the type of systemic therapy or changing the intent or target volume of radiotherapy).
All systemic therapies in this study consisted of PD-1 inhibitor-based immunotherapy (nivolumab alone or in combination with ipilimumab). Targeted therapy was not administered, as molecular testing for BRAF mutation status was not performed due to limitations in resources and diagnostic availability in our setting.
Data were analysed using SPSS software, version 25 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to summarize patient demographics, tumour characteristics, and imaging findings. Categorical variables were presented as counts and percentages, while continuous variables were expressed as means ± standard deviations (SD) for normally distributed data or medians with interquartile ranges (IQR) for non-normally distributed data, as determined by skewness, kurtosis, and normality tests.
Agreement between contrast-enhanced TAP CT and 18F-FDG PET/CT was assessed using Cohen’s kappa coefficient with 95% confidence intervals (CI), interpreted according to Landis and Koch’s scale (very good ≥0.80, good 0.60-0.79, moderate 0.40-0.59, weak 0.20-0.39, very weak <0.20). Kappa analysis was performed for nodal status (N stage) and metastatic status (M stage). This was applied to sensitivity, specificity, PPV, NPV and accuracy to assess differences in diagnostic performance between the two modalities.
From 51 consecutive patients screened, 23 were included for initial staging and 12 for recurrence work-up. 6 were excluded (6 not included upfront with 2 synchronous neoplasia, 1 ocular melanoma, 3 incomplete records, and 10 excluded after screening) ( Figure 1).
Demographic, clinical and histopathologic data on the included patients are presented in Table 1.
Thirty-five patients were included (20 men and 15 women). Median age at melanoma diagnosis was 60 years (range, 32-83). No treatment occurred between the imaging tests and the reference standard verification. The indication for 18F-FDG PET/CT was initial staging in 23/35 (65.7%) and restaging in 12/35 (34.3%). A primary cutaneous melanoma was documented in 33/35 (94.3%), while 2/35 (5.7%) had an unknown primary. The lower limb 13/33 (39.4%, 4/9), and the head and neck 11/33 (33.3%, 10/1) were the most affected anatomic sites.
Nodular melanoma accounted for more than half of the cases 19/33 (57.6%, 12/7) and ulceration was present in 22/33 of the patients (66.7%).
According to AJCC, 11/35 (31.4%) were stage I/II (9 staging and 2 restaging), 13/35 (37.1%) stage III (9 staging and 4 restaging), and 11/35 (31.4%) stage IV (5 staging and 6 restaging), indicating a broad disease spectrum across both the staging and restaging cohorts.
Among patients classified as free of nodal or distant metastases by the reference standard, the most frequent alternative findings were benign lymphadenopathy (reactive or inflammatory), postoperative changes, and non-specific pulmonary or hepatic nodules that remained stable on follow-up.
Across the entire cohort, 18F-FDG PET/CT changed the disease classification in 22/35 patients (62.9%), compared with CT alone. Overall, 14/35 (40.0%) were upstaged, 8/35 (22.9%) were downstaged, and 13/35 (37.1%) showed concordant staging with CT. Collectively, these findings indicate a substantial incremental yield of 18F-FDG PET/CT over CT for both nodal and distant disease assessment, resulting in frequent stage migration at both initial staging and during evaluation for recurrence.
In the whole cohort of the 35 melanoma patients (including both initial staging and restaging cases), 18F-FDG PET/CT clearly outperformed contrast-enhanced CT (CECT) in detecting metastatic disease. Overall, 18F-FDG PET/CT identified more true-positive metastases and missed fewer lesions than CECT, leading to higher sensitivity and accuracy for both regional nodal and distant metastasis detection ( Figures 2 and 3).
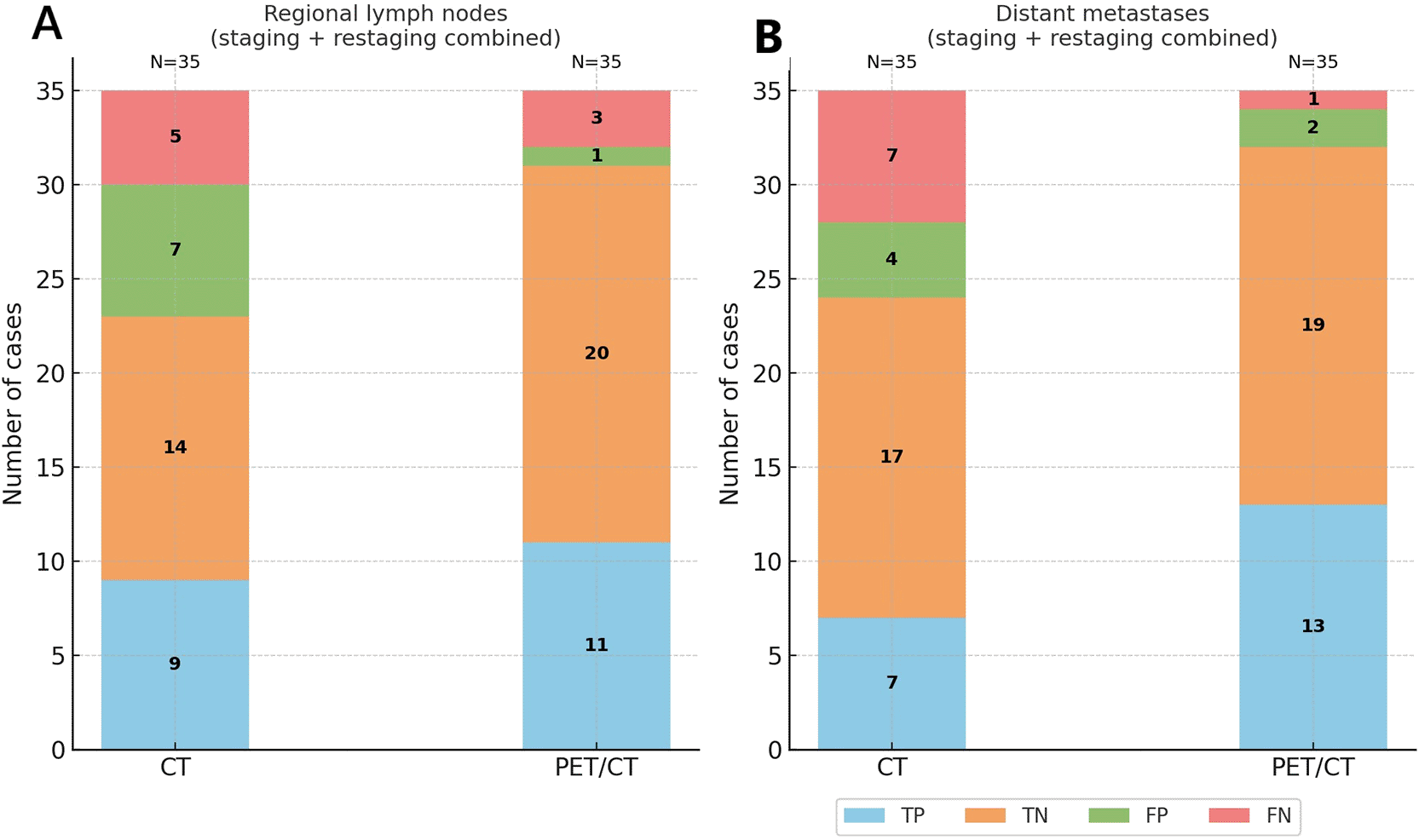
Stacked bar charts illustrate the distribution of TP, TN, FP, and FN for CT and 18F-FDG PET/CT across (A) regional lymph node involvement, (B) distant metastases, and (C) overall staging (combined nodal and distant assessment). 18F-FDG PET/CT consistently outperformed CT by reducing false negatives and increasing true positive detection, particularly for distant metastases.
Abbreviations: CT = computed tomography; 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography; TP = true positive; TN = true negative; FP = false positive; FN = false negative.
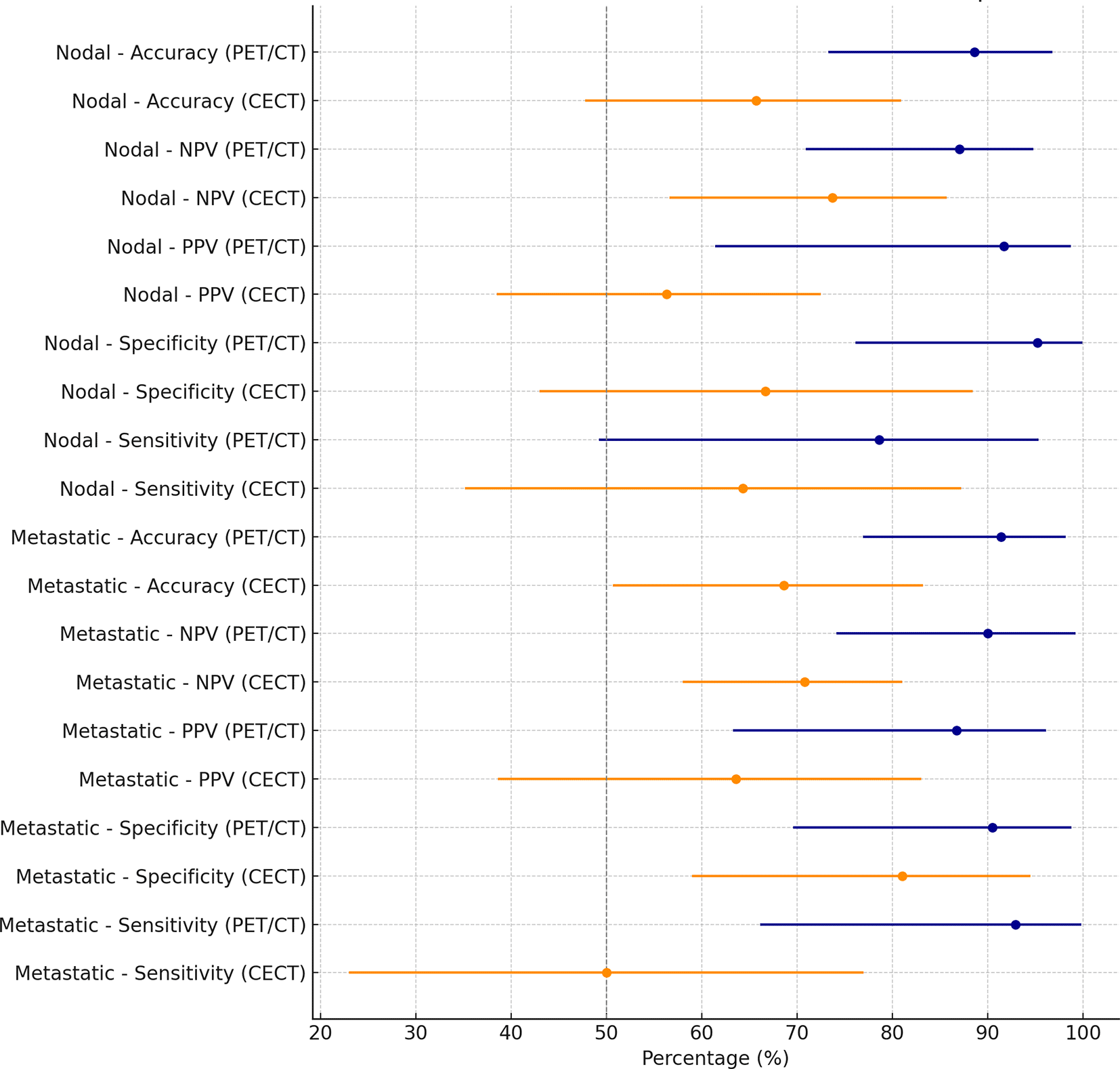
This forest plot compares the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy of CECT and 18F-FDG PET/CT for detecting regional lymph node and distant metastatic disease in the combined staging and restaging cohort.
Abbreviations: PPV = positive predictive value; NPV = negative predictive value; 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography; CECT = contrast enhanced computed tomography.
For the initial staging cohort, compared with CECT, 18F-FDG PET/CT showed higher sensitivity (91.7% vs 58.3%) and accuracy (91.3% vs 69.7%) with similar specificity (90.9% vs 81.8%) for detecting nodal involvement. At recurrence, 18F-FDG PET/CT achieved perfect sensitivity (100% vs 50% for CECT) and superior accuracy (91.7% vs 58.8%) with comparable specificity (75.0% for both).
Taking the whole cohort into account, 18F-FDG PET/CT correctly identified 11 of the 14 patients with nodal involvement (78.6% sensitivity), slightly higher than CECT which detected 9 of 14 (64.3% sensitivity).
18F-FDG PET/CT had far fewer false positives than CECT (only 1 vs. 7). Consequently, 18F-FDG PET/CT achieved a higher specificity for nodal disease (95.2% vs. 66.7%) and a much better positive predictive value (91.7% vs. 56.3%) ( Table 2). The NPV also favoured 18F-FDG PET/CT (87.0% vs. 73.7%), reflecting fewer missed nodal metastases.
The overall accuracy in detecting regional nodal metastasis was 88.6%, notably higher than the 65.7% with CECT ( Table 2). Figure 2A illustrates these findings: 18F-FDG PET/CT confirmed more true-positive nodal metastases and avoided most of the false positive detected in CECT (which often misclassified benign enlarged nodes as malignant). In summary, 18F-FDG PET/CT provided a more reliable assessment of regional lymph node status in the combined cohort, largely by reducing false positives and modestly lowering false negatives ( Figure 2A, Figure 3).
The advantage of 18F-FDG PET/CT was even more pronounced for distant metastasis detection.
In the initial staging group, 18F-FDG PET/CT markedly outperformed CECT with higher sensitivity (87.5% vs 37.5%), higher specificity (93.3% vs 86.0%), and greater overall accuracy (91.3% vs 69.6%). For the restaging group, 18F-FDG PET/CT achieved perfect sensitivity (100% vs 50% for CECT) and superior accuracy (91.7% vs 58.8%) with comparable specificity (75.0% for both).
In the whole cohort, 18F-FDG PET/CT detected 13 of 14 patients with distant metastatic disease, corresponding to a sensitivity of 92.9% which is two-fold higher than CECT since the latter detected only 7 of 14 (50.0% sensitivity). This means that 18F-FDG PET/CT missed just one patient with distant metastases (1 false negative) compared to seven missed by CECT (7 false negatives) ( Table 3, Figure 4). In other words, 18F-FDG PET/CT correctly identified nearly all patients with distant spread, dramatically lowering the false-negative rate for metastasis staging (as visualized in Figure 2B). Specificity for distant metastases was high for both modalities (90.5% with 18F-FDG PET/CT vs. 81.0% with CECT), but 18F-FDG PET/CT still produced fewer false positives (2 vs. 4). The combination of much higher sensitivity and slightly improved specificity gave 18F-FDG PET/CT a markedly superior positive predictive value for distant metastases (86.7% vs. 63.6%) and a significantly better overall accuracy (91.4% vs. 68.6%) compared to CECT ( Table 3). Notably, the negative predictive value of 18F-FDG PET/CT was about 90% for distant disease, substantially higher than CECT’s NPV of 70.8%, indicating that a negative 18F-FDG PET/CT scan virtually ruled out unseen metastases in this cohort, whereas a negative CT was less reassuring. Figure 2B underscores 18F-FDG PET/CT’s benefit in distant staging by showing far more true positives and far fewer missed metastases than CECT. The forest plot in Figure 3 further summarizes these performance metrics, highlighting the consistently superior sensitivity, specificity, and predictive values of 18F-FDG PET/CT for detecting distant metastatic melanoma in the combined analysis.
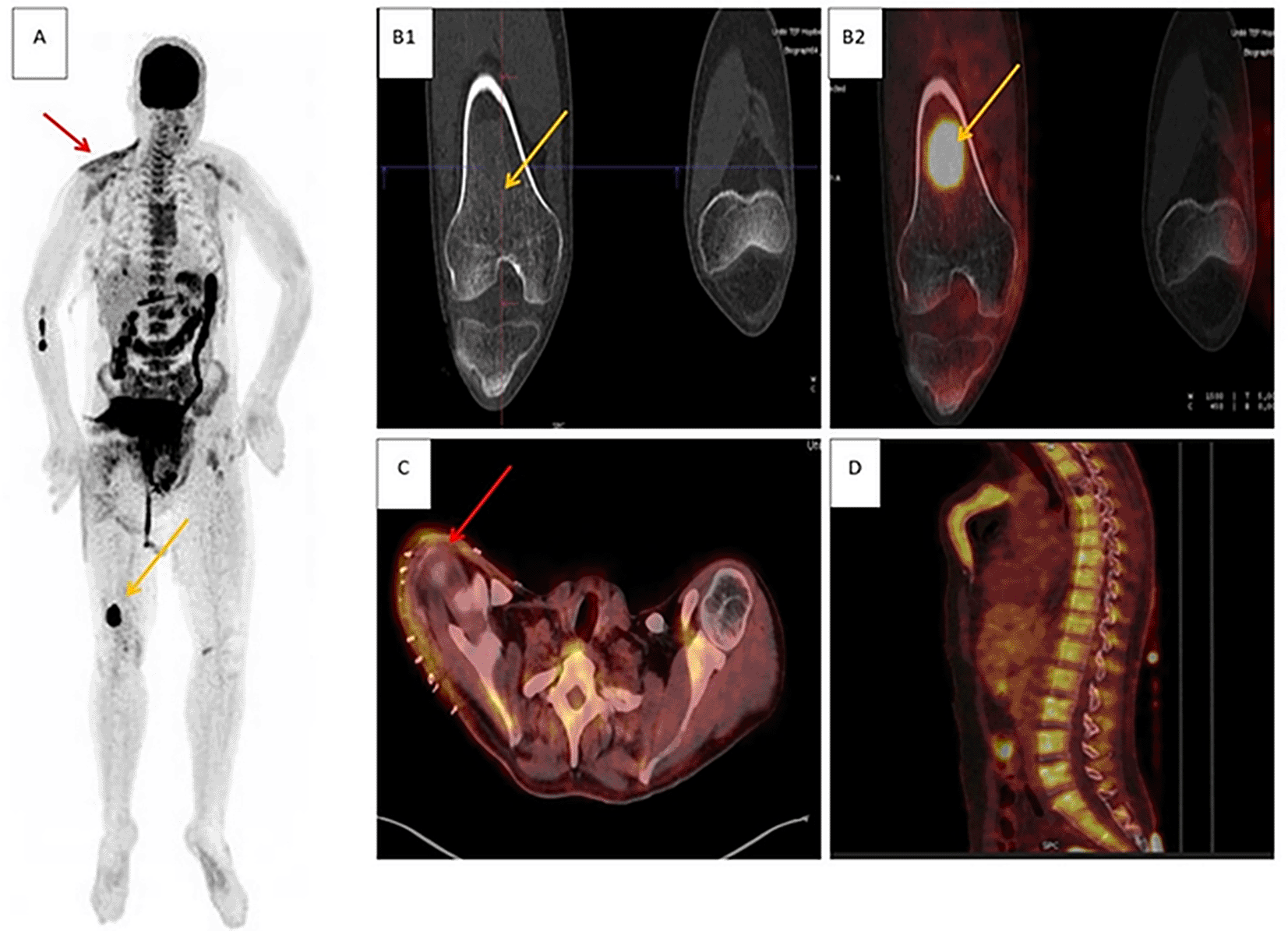
A 35-year-old man with rhabdoid melanoma of the right shoulder underwent wide excision without nodal dissection. Clinical exam, cervical ultrasound, and TAP CT were unremarkable, and disease was classified T4b N0 M0 (stage IIC). 18F-FDG PET/CT demonstrated an intensely hypermetabolic, well-circumscribed ground-glass diaphyseal lesion at the distal right femur, consistent with bone metastasis; diffuse homogeneous uptake in the right scapular region was compatible with post-operative change, and diffuse osteomedullary uptake was reactive. Metastatic origin of the femoral lesion was confirmed on femoral MRI, and the patient was reclassified T4b N0 M1c (stage IV), receiving radiotherapy plus nivolumab.
Panels: (A) maximum-intensity projection (MIP); (B1) coronal CT and (B2) coronal 18F-FDG PET/CT fusion showing the right femoral lesion (yellow arrows); (C) axial 18F-FDG PET/CT fusion showing post-operative changes in the right shoulder (red arrows); (D) sagittal 18F-FDG PET/CT fusion demonstrating diffuse reactive osteomedullary uptake.
Abbreviations: 18F-FDG PET/CT = fluorodeoxyglucose positron emission tomography/computed tomography; CT = computed tomography; TAP CT = thoraco-abdomino-pelvic CT; MRI = magnetic resonance imaging; MIP = maximum-intensity projection.
The anatomical distribution of distant disease differed by modality. CT predominantly identified visceral metastases (central nervous system, lung, liver), whereas 18F-FDG PET/CT detected a broader range of sites, including extraregional nodes, muscle, small bowel, bone marrow and adrenal (n = 10) in addition to viscera. These distributions are summarized in Table 4 for CT and Table 5 for 18F-FDG PET/CT respectively and are consistent with 18F-FDG PET/CT’s higher sensitivity and lower false-negative rate for distant spread in the combined cohort.
| Metastatic site | (n) | % |
|---|---|---|
| Staging (n = 23) | ||
| Central nervous system | 2 | 40 |
| Lung | 1 | 20 |
| Bone | 1 | 20 |
| Liver | 1 | 20 |
| Total | 5 | 100 |
| Restaging (n = 12) | ||
| Central nervous system | 2 | 33.4 |
| Lung | 3 | 50 |
| Liver | 1 | 16.6 |
| Total | 6 | 100 |
At the patient level, sensitivity and specificity were consistently high, with excellent negative predictive values across AJCC stage groups ( Table 6). Notably, in stage I-II disease, 18F-FDG PET/CT achieved 100% sensitivity (95% CI 15.8-100.0) and 100% NPV (95% CI 59.0-100.0) for nodal status. In advanced stages (III-IV), specificity reached 100% (95% CI 39.8-100.0) for nodal involvement, while sensitivity for distant metastases remained high at 90.9% (95% CI 58.7-99.8). These findings highlight 18F-FDG PET/CT’s strong rule-out value for both nodal and metastatic spread, even in early-stage patients.
In the combined cohort of 35 patients, the agreement between CECT and 18F-FDG PET/CT was only moderate to fair which shows the complementary nature of the two modalities rather than redundancy.
For the detection of regional lymph node involvement, the observed agreement between CECT and 18F-FDG PET/CT was 65.7%. The corresponding Cohen’s kappa was 0.27 (95% CI, 0.00-0.57, p = 0.096), indicating only fair concordance ( Figure 2A). This modest agreement largely reflected the higher false-positive rate of CECT and the higher true-positive yield of 18F-FDG PET/CT, as shown in the diagnostic performance analysis. In practice, this means that while the two modalities sometimes aligned in identifying nodal disease, 18F-FDG PET/CT more often reclassified nodal status, thus detecting occult metastases not visible on CECT or refuting suspicious but benign enlarged nodes.
For the detection of distant metastatic disease, the observed agreement was even lower at 60.0%. Cohen’s kappa was 0.16 (95% CI, 0.00-0.48; p = 0.344) ( Table 7), reflecting poor concordance between the two imaging techniques ( Figure 2B). The low kappa was driven by the substantial number of distant metastases detected by 18F-FDG PET/CT but missed by CECT, as well as several false-positive findings on CT.
Management impact was assessed in 32/35 patients (91.4%) and three patients (8.6%) lacked post-imaging decision documentation and were excluded from denominators. 18F-FDG PET/CT prompted management changes nearly half of evaluable patients (15/32,46.9%). Among these patients, inter-modality change like the shift from surgery or surveillance to systemic therapy or radiotherapy was found in 12 cases (37.5%). On the other hand, intra-modality shift like the change in the treatment regimen or in the radiotherapy field was found in the remaining 3 patients (9.4%). No change occurred in 17/32 (53.1%) ( Figure 5).

Flows represent the number of patients whose management remained unchanged or was modified following 18F-FDG PET/CT findings. Management modifications were classified as inter-modality changes (shift between different treatment modalities, e.g., from surgery to systemic therapy) or intra-modality changes (adjustments within the same modality, e.g., modification of systemic regimen or radiotherapy field).
Abbreviations: 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
Subgroup patterns mirrored the pooled effect. During initial staging (evaluable n = 20), 18F-FDG PET/CT altered management in 8/20 (40.0%) with 60% unchanged. During restaging (evaluable n = 12), 18F-FDG PET/CT changed management in 7/12 (58.3%), and 41.7% remained unchanged ( Figure 5). Most escalations were triggered by 18F-FDG PET/CT detection of previously occult nodal or distant disease or clarification of equivocal CT findings, aligning with the higher sensitivity and accuracy reported for 18F-FDG PET/CT in this study ( Figure 6).
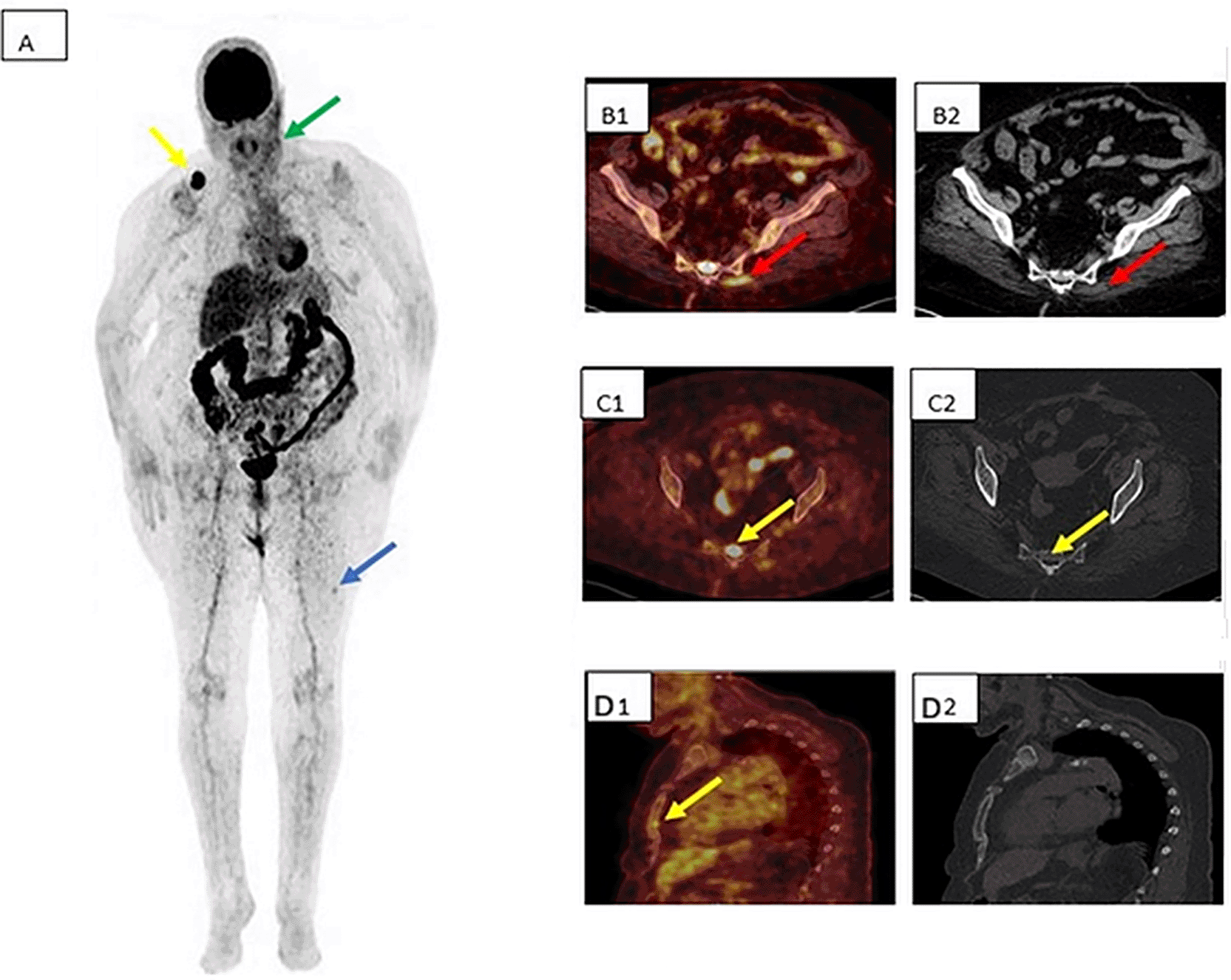
A 70-year-old woman with nodular melanoma of the left cheek presented with left cervical nodal recurrence. Distant work-up with TAP CT was negative.18F-FDG PET/CT revealed, in addition to the cervical nodal disease (green arrow), secondary lesions involving bone, skeletal muscle, and skin, resulting in upstaging to stage IV and initiation of immunotherapy without lymph-node dissection.
Panels: (A) MIP; (B1) axial 18F-FDG PET/CT fusion and (B2) axial CT showing left gluteal muscle involvement; (C1) axial 18F-FDG PET/CT fusion and (C2) axial CT showing sacral bone metastasis (yellow arrow); (D1) axial 18F-FDG PET/CT fusion and (D2) axial CT showing a sternal bone lesion occult on CT; blue arrow indicates cutaneous involvement of the left thigh.
Abbreviations: 18F-FDG PET/CT = fluorodeoxyglucose positron emission tomography/computed tomography; CT = computed tomography; TAP CT = thoraco-abdomino-pelvic CT; MIP = maximum-intensity projection.
This retrospective study evaluated the value of 18F-FDG PET/CT in cutaneous and mucocutaneous melanoma for initial staging and restaging, quantified its diagnostic yield relative to CECT, and assessed its usefulness in treatment planning. The analysis was patient-based and focused on accuracy for nodal and distant disease and on inter-modality concordance in the combined cohort. Because 18F-FDG PET/CT depicts metabolically active disease rather than relying solely on morphologic change, it was hypothesized to uncover clinically occult metastases and drive stage migration more often than conventional imaging. The study therefore tried to translate the findings into a pragmatic, context-adapted imaging pathway for routine practice.
18F-FDG PET/CT has a high diagnostic yield in nodal staging and generally outperforms conventional imaging. Dancheva et al.12 reported sensitivity and specificity of 84.5% and 98.9% for regional nodal staging with 18F-FDG PET/CT versus 22.5% and 97.7% for conventional CT and US. In a 250-patient series, Reinhardt et al. showed 18F-FDG PET/CT to be more accurate than PET or CT alone across clinical contexts, with a smaller advantage in initial staging.13
Gellén et al. observed high diagnostic accuracy across stages (accuracy 91.3% in stages I/II, up to 96.2% in unresectable stage III) and noted that 18F-FDG PET/CT detected small nodal metastases (8-20 mm) in half of true-positive cases.14
These findings align with our cohort: For lymph nodes, 18F-FDG PET/CT showed higher sensitivity, specificity, and accuracy than CECT: 78.6% vs 64.3% sensitivity, 95.2% vs 66.7% specificity, and 88.6% vs 65.7% accuracy, all at the patient level. Fewer PET/CT false positives than CECT (1 vs 7) and modest reduction in false negatives likely reflect metabolic detection and fewer size-based misclassifications.
Our study’s performance for nodal disease was best in stages III-IV, where specificity reached 100%. While in stage I-II, it showed a strong rule-out performance for occult nodal spread with a 100 sensitivity that should be interpreted cautiously across the small early-stage strata.
Lower sensitivity pooled estimates for nodal metastases were reported in a recent meta-analysis by Zamani-Siahkali et al.15 with 10,403 patients (sensitivity of 56% and a specificity of 97%). This limitation is partly explained by the restricted spatial resolution of PET for sub 5 mm lesions. For example, Crippa et al.16 reported sensitivity of 83% for nodal lesions more than 5 mm, but only 23% for those less than 5 mm.
Additional corroboration from a Cochrane review11 shows that in a direct comparison after primary resection, 18F-FDG PET/CT remains favoured and more sensitive than CT for nodal disease (38% versus 23%) with 100% specificity for both.17
Patients with Breslow thickness <1 mm have a very low metastasis risk, making imaging low-yield.23–25 Sentinel Lymph Node Biopsy (SLNB) remains the most sensitive method for micrometastases and the key prognostic factor when Breslow >1 mm.18–20 18F-FDG PET/CT may, however, be useful in stage I/II patients with high-risk features (e.g., T4 or ulceration).18,19,21
The 2024 National Comprehensive Cancer Network (NCCN) guidelines8 are concordant in this context and suggest no additional imaging or SLNB for pT1a without clinical suspicion; from stage IB upward, regional nodal ultrasound is recommended, and if nodes are clinically/radiologically negative, SLNB is preferred for micrometastases and prognostication.8 In our cohort, 18F-FDG PET/CT correctly identified nodal disease in three patients initially classified N0.
Building on this nodal performance, the clinical question shifts to occult distant spread, where 18F-FDG PET/CT’s role is particularly relevant. A 2012 meta-analysis (74 studies including 10,528 patients) found that 18F-FDG PET/CT achieved the highest performance for distant disease (sensitivity 80%, specificity 87%, odds ratio 25), outperforming CT (51%, 69%; OR 2.29) and PET alone (74%, 75%; OR 8.14).22 Similarly, Dancheva et al.12 reported a sensitivity of 100% and a specificity of 98.3% for 18F-FDG PET/CT, compared with 12.3% and 94.5% respectively, for conventional imaging. In line with these findings, Zamani-Siahkali et al.15 reported pooled estimates for distant-metastasis detection of 88% sensitivity and 94% specificity for 18F-FDG PET/CT.
Our results are consistent with the published literature. For distant disease, the advantage was even greater, with markedly fewer missed metastases and better overall accuracy (92.9% vs 50.0% for sensitivity, 90.5% vs 81.0% for specificity, and 91.4% vs 68.6% for accuracy). These results explain why inter-modality agreement was only fair for nodes (κ = 0.27) and poor for distant sites (κ = 0.16). This poor concordance was largely explained by the broader organ capture of 18F-FDG PET/CT, which identified numerous extra-visceral metastases missed by CECT and simultaneously reduced false positives in our cohort. Such incremental yield has been repeatedly confirmed in large series, including Reinhardt et al.,13 where 18F-FDG PET/CT correctly staged 97.2% of 250 patients and detected significantly more visceral and non-visceral metastases than CT alone. Similarly, Weber et al.23 found 18F-FDG PET/CT to be significantly more sensitive than ultrasound across per-patient (sensitivity of 71% for 18F-FDG PET/CT and 48% for US, and specificity of 96% versus 97% respectively), per-examination, and per-lesion analyses, particularly for distant and abdominal metastases. This broader organ detection strengthens the rationale for whole-body 18F-FDG PET/CT when unsuspected extra-visceral spread would alter management.
Data summarised by Dinnes et al.11 further support the advantage of 18F-FDG PET/CT in initial staging: in Veit-Haibach et al. study,17 18F-FDG PET/CT detected two additional distant metastases over CT (sensitivity 42% vs CT 25% and identical specificity 93%), and in Arrangoiz et al. study24 (thick, clinically node-negative primaries), 18F-FDG PET/CT detected all five distant metastases (sensitivity 100% and specificity 94%). Bronstein et al.25 found that 18F-FDG PET/CT detects unexpected metastases in a small but significant percentage (12%), and in 6% of scans, unexpected metastases were found in the extremities, areas not typically included in conventional imaging and detected in the whole-body scan of the PET/CT. For intracranial disease, and when suspected, liver, MRI remains the preferred for brain and hepatic metastases respectively.16
In stage-stratified analyses of our cohort, diagnostic performance for distant remained robust and stronger than in nodal disease across AJCC groups. In stage I-II, 18F-FDG PET/CT favoured rule-out with 100% specificity, while in stage III-IV, it provided both rule in and rule out confidence (sensitivity of 90.9% and a specificity of 100%). This suggests that 18F-FDG PET/CT can confidently exclude metastatic disease even in early-stage patients, and reliably confirm disease in advanced stages.
The 2024 NCCN and the European consensus-based interdisciplinary guidelines recommends whole-body imaging with CT or 18F-FDG PET/CT for stage III macrometastatic disease and stage IV. It may be considered for poor-prognosis stage IIC and discussed in stage IIIA with micrometastases.8,26
Melanoma recurrence is common after resection and reaches up to 30%, particularly in advanced disease, with 50 to 80% relapsing in locoregional disease and up to 95% in the presence of distant metastases.27 Reported accuracies for 18F-FDG PET/CT in restaging are high reaching 96% in the prospective diagnostic study by Mijnhout et al.,28 while Agrawal et al.29 reported accuracies of 92% for regional nodes and 86% for distant disease, confirming 18F-FDG reliability.
These diagnostic gains translated into meaningful therapeutic impact. In almost half of evaluable patients, management changed after 18F-FDG PET/CT, most commonly due to the discovery of occult nodal or distant foci that shifted care from local procedures to systemic therapy or clarified equivocal CT findings and prevented unnecessary interventions. This aligns with prior prospective and retrospective work showing that PET-based staging alters treatment plans in a substantial share of melanoma patients, with variability reflecting differences in stage mix and surgical candidacy.25,30–33
The signal was strongest in advanced or restaging settings, consistent with the literature: in higher stages the pre-test probability of spread is greater, the incremental detection of 18F-FDG PET/CT is larger, and downstream decisions are more likely to change.13 By contrast, in truly early stages without high-risk features, 18F-FDG PET/CT rarely altered management in this study, which is concordant with published recommendations.12,13 Taken together, these observations indicate that 18F-FDG PET/CT acts as an add-on to conventional imaging when results are likely to influence therapeutic planning, and as a replacement whole-body modality in the restaging context where a comprehensive disease map is required for systemic therapy decisions.
The therapeutic context has evolved with the adoption of immunotherapy and BRAF/MEK-targeted therapy as standards. 18F-FDG PET/CT is most useful before systemic treatment to establish a baseline, document true disease extent, and aid prognostic stratification.34–37 About 40% of metastatic melanomas harbour BRAF V600; early metabolic response on PET can separate prognostic groups under BRAF inhibition, with more homogeneous early decline in uptake associated with longer time to progression.38,39 When BRAF V600 is absent, adjuvant pembrolizumab improves distant metastasis-free and relapse-free survival in resected stage III melanoma, and pre-therapeutic 18F-FDG PET/CT helps exclude occult stage IV disease before adjuvant therapy is started.40,41 In our cohort, 18F-FDG PET/CT supported initiation, continuation, addition, or avoidance of immunotherapy according to the disease map it provided. Under immunotherapy, 18F-FDG PET/CT may display atypical patterns like pseudoprogression, hyperprogression, or dissociated responses. These particular aspects can confound conventional criteria. Immune-adapted frameworks (imRECIST for CT and iPERCIST for PET) help distinguish true progression from inflammatory flare or mixed response and guide more appropriate decisions.42–44
18F-FDG PET/CT also depicts immune-related adverse events, prompting timely holds or targeted work-up when indicated.45 These imaging nuances strengthen the link between accurate disease characterization and tailored therapy observed in this study.
Finally, emerging tools point to future refinements. Pre-treatment metabolic tumour volume on FDG PET carries independent prognostic value in patients receiving immune checkpoint inhibitors, opening paths for risk-adapted imaging and therapy.46,47 New tracers, such as the melanin-avid 18F-N-(2-(Diethylamino)ethyl)-5-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy) picolinamide (18F-PFPN), may improve lesion conspicuity and help differentiate inflammatory uptake from melanocytic disease during immunotherapy, though performance may be lower in amelanotic subtypes; these advances were outside the scope of this study but indicate where next-generation staging and response assessment may head.48,49
In this study’s setting, and while considering the international recommendations and local care patterns, we favour a context-adapted approach that integrates 18F-FDG PET/CT selectively and escalating its use when the pre-test probability of metastasis or the likelihood of therapeutic consequence is high and deferring it when expected yield is limited. For non-advanced stages (I-II), no routine 18F-FDG PET/CT is indicated and SLNB should be considered for stage IB (ulceration or high mitotic index). For stages IIA-IIB, locoregional ultrasound is recommended; if negative, sentinel lymph node biopsy may be undertaken. For advanced stages (IIC-IIIC), locoregional ultrasound and TAP CT are generally recommended, with 18F-FDG PET/CT considered as an alternative to consolidate staging and inform therapy. For stage IV, 18F-FDG PET/CT or TAP CT plus brain MRI are generally advised to achieve comprehensive staging prior to definitive systemic or surgical management.
This study has several limitations, notably its retrospective and single-centre nature. Another constraint is the small sample size which is below the idealized target derived from literature-based planning. As a result, confidence intervals were wide in early-stage strata, so estimates, particularly the 100% values, should be interpreted cautiously and validated prospectively. It nonetheless provides interpretable diagnostic accuracy estimates. This could be explained largely by the low incidence of melanoma as well as the recent implementation of 18F-FDG PET/CT in Tunisia. Another explanation could be the lack of regulatory rule that requires 18F-FDG PET/CT to prescribe immunotherapy or targeted therapy. In addition, because histopathologists and clinicians assessing the reference standard had access to imaging results, the possibility of review bias cannot be excluded, which may have slightly inflated accuracy estimates. To mitigate these limitations and enhance generalizability, future investigations should adopt a prospective, multi-centre design.
This study results show that 18F-FDG PET/CT improves detection over CT, explains the observed low inter-modality concordance by identifying additional true disease in an unselected population at advanced and early stages of the disease. Pooled 18F-FDG PET/CT sensitivity and specificity surpass CT for distant metastases and are higher for nodal disease. These findings align with contemporary evidence and support the judicious integration of 18F-FDG PET/CT into melanoma care pathways. It enables clinicians to tailor treatment more appropriately, whether that means intensifying therapy for those with unseen metastases or safely de-escalating interventions for those without evidence of spread. This synergy between diagnostic precision and therapeutic decision-making ultimately optimizes patient care, potentially contributing to improved outcomes as suggested in emerging real-world analyses.
This study was conducted in accordance with the ethical standards of the institutional and national research committees. The protocol was approved by the Ethics Committee of Sahloul University Hospital, Faculty of Medicine of Sousse, University of Sousse, Tunisia (Approval No. [HS52-2025]).
Written informed consent was obtained from all participants to participate in the study.
The authors used a generative AI assistant (GPT-5 Thinking, OpenAI) to help translate sections, improve grammar and clarity, and format figures.
The anonymized data underlying this study are available in the Zenodo: Impact and diagnostic accuracy of 18F-FDG PET/CT in Malignant melanoma staging and restaging: Tunisian Cohort Dataset. https://doi.org/10.5281/zenodo.17507763.50
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Zenodo: Standards for Reporting of Diagnostic Accuracy Studies (STARD 2015) checklist for Impact and diagnostic accuracy of 18F-FDG PET/CT in Malignant melanoma staging and restaging: Tunisian Cohort Dataset. https://doi.org/10.5281/zenodo.17507763.50
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)