Keywords
Acinetobacter baumannii, pgaABCD locus, carbapenem resistance, biofilm
This article is included in the Pathogens gateway.
Carbapenem-resistant Acinetobacter baumannii (CRAB) is an important nosocomial pathogen with high biofilm-forming ability and multidrug resistance. This study aimed to investigate the prevalence of the pgaABCD locus in clinical CRAB isolates and its relationship with biofilm production and antimicrobial resistance.
A total of 150 non-duplicate clinical A. baumannii isolates were collected from healthcare facilities in Shahrekord, Iran. Identification was performed by PCR and biochemical tests. Antimicrobial susceptibility was determined using disk diffusion, and biofilm formation was assessed by the crystal violet method. PCR was used to detect pgaABCD genes. Hemolytic and proteolytic activities were evaluated on blood and skim milk agar. Statistical analyses included chi-square tests and heatmap clustering of resistance profiles.
Results showed that all isolates were multidrug-resistant, showing the highest resistance to ciprofloxacin (100%), ceftazidime (90%), and cefepime (86.66%). Of these, 90 (60%) were CRAB, all forming biofilms, with 55.5% producing strong biofilms. Hemolytic activity occurred in 14% of isolates and was significantly associated with strong biofilm formation (p < 0.0001). Proteolytic activity was observed in all isolates without significant association. The pgaA and pgaD genes were detected in 100% of isolates, while pgaB and pgaC were found in 84.4% and 93.3%, respectively. Strong biofilm formation correlated significantly with pgaB (p < 0.0001) and pgaC (p = 0.0002). Heatmap analysis showed higher antibiotic resistance in strong biofilm producers.
Strong biofilm formation in CRAB is associated with pgaB/pgaC genes and hemolysin production, highlighting the importance of anti-biofilm strategies and molecular monitoring in clinical settings.
Acinetobacter baumannii, pgaABCD locus, carbapenem resistance, biofilm
Carbapenem-resistant Acinetobacter baumannii (CRAB) is a gram-negative opportunistic pathogen and a leading cause of hospital-acquired infections worldwide.1 The growing prevalence of carbapenem-resistant variants of this bacterium has increased its threat to public health, as these strains resist multiple antibiotics, including carbapenems, which are often the last therapeutic option for treating severe resistant infections.2 As a result of the lack of effective treatment alternatives, higher rates of morbidity and mortality are associated with infections caused by CRAB, particularly in intensive care units.3
A. baumannii capacity to create biofilms is a key virulence factor that greatly increases its persistence and antibiotic resistance.4 Biofilms consist of structured bacterial populations surrounded by a self-produced extracellular matrix, allowing them to adhere strongly to both biological tissues and inanimate surfaces. This growth mode differs from that of planktonic (free-living) bacteria, as biofilm-producing bacteria secrete lipoproteins, polysaccharides, fibrous proteins, and various extracellular substances, creating colony-like or microcolony structures. A. baumannii develops biofilms rapidly through a process that includes five different stages.5–7 The resistance conferred by biofilms arises from several mechanisms, including reduced permeability, inactivation of antimicrobial agents by enzymes on the biofilm surface, limited access to essential nutrients and oxygen, specific phenotypic traits of biofilm-associated cells, and evasion of the host immune response.8
Several genetic loci regulate biofilm formation in A. baumannii, which among them pgaABCD locus plays a pivotal role.9 This locus consists of four genes consist of pgaA, pgaB, pgaC, and pgaD which encode enzymes that are integral to the synthesis, modification, and transport of poly-β-1,6-N-acetylglucosamine (PNAG), a principal polysaccharide component of the biofilm matrix.10 PNAG is essential for the initial adhesion of bacterial cells, their aggregation, and the structural integrity of the biofilm.11 Research has shown that removing the pgaABCD locus significantly reduces the organism’s ability to form biofilms, emphasizing the critical importance of these genes in its pathogenicity.9 Furthermore, PNAG not only facilitates biofilm development but also contributes to immune evasion by obscuring bacterial surface antigens and reducing opsonization.12
Investigating the prevalence of the pgaABCD locus in clinical CRAB strains is essential for enhancing our knowledge of their epidemiology and the mechanisms behind their pathogenicity. Although the pgaABCD genes are conserved across various A. baumannii strains, their presence and expression vary among different clones and geographical regions.13,14 This variability in the distribution and expression levels of the pgaABCD locus underscores the necessity of continuous monitoring and molecular analysis of CRAB isolates to assess the spread of virulence factors.15
Despite the increasing research on biofilm formation in A. baumannii, there is still a need to understand the regional variations in the genetic factors that influence this phenotype. Limited molecular data from Iran exist, and no previous study has specifically investigated the distribution of the pgaABCD locus in CRAB isolates or its relationship with biofilm formation and patterns of antimicrobial resistance. This study aimed to address the regional knowledge gap and enhance the overall understanding of the molecular epidemiology of CRAB. Specifically, it tries detect the presence of the pgaABCD locus in clinical CRAB isolates and studying its correlation with biofilm production and antibiotic resistance patterns.
Microbiological media, glucose, agarose, ethidium bromide solution (Cat No. E1385) and crystal violet (Cat No. 101408) were obtained from Merck (Darmstadt, Germany). Antibiotic discs were purchased from MAST (Merseyside, U.K). DNA extraction kit (Cat No. EX6071) and Reddy to use 2x Master Mix (Cat No. MM2062) were obtained from Sinaclon (Tehran, Iran). Primers were synthesized by Pishgam Biotech Co (Tehran, Iran).
In this research, 150 non-duplicate A. baumannii isolates were gathered from healthcare facilities in Shahrekord, Chaharmahal and Bakhtiari Province during 2020–2021. Clinical isolates were obtained from routine diagnostic specimens collected from hospitalized patients. These specimens included blood, urine, respiratory tract secretions (such as tracheal aspirates), wound swabs, and catheter tips. Microbiological and biochemical methods such as gram staining, oxidase and catalase tests, oxidative-fermentative (OF) test under aerobic and anaerobic conditions, triple sugar iron (TSI) test, indole, motility (SIM), Simon citrate, methyl red–Voges Proskauer (MR-VP), and lactose fermentation on MacConkey agar, along with molecular confirmation through 16S rRNA analysis were used to identify the isolates. The primers used in the current study are listed in Table 1.
List of primers with names, target genes, sequences (5’→3’), product sizes, and references.
| Genes | Primers sequences (5' to 3') | amplicon Size (bp) | Reference |
|---|---|---|---|
| 16s rRNA | F: CATTATCACGGTAATTAGTG | 208 | [16] |
| R: AGAGCACTGTGCACTTAAG | |||
| pgaA | F: ATTCAAAAGTCAGTTGATGGGC | 460 | [17] |
| R: TTTTTTGTCCTTGCTCCAGC | |||
| pgaB | F: AAGAAAATGCCTGTGCCGACCA | 490 | [18] |
| R: GCGAGACCTGCAAAGGGCTGAT | |||
| pgaC | F: TGATGGCTTGGACATGGATG | 1189 | [19] |
| R: TGATCTCCACGGAAGCCTCG | |||
| pgaD | F: CCCCTGCTCATCATAATGTAAG | 353 | [17] |
| R: GGTTTTGTTTAATGTGGCTGC |
Hemolysin activity was assessed using streaking and spot assays on blood agar plates, as per established protocols.20 Plates were incubated at 37 °C for 24 h. A lightly cleared zone in the streaking method and a clear halo surrounding grey-green colonies in the spot method indicated red blood cell lysis. The proteolytic activity was measured on skim milk agar following 4 h of incubation at 37 °C, where a clear zone signified a positive result. All procedures were performed in triplicate.
To assess antimicrobial resistance, the Kirby–Bauer disk diffusion assay was employed for all clinical A. baumannii isolates, using standardized procedures outlined in the 2020 CLSI guidelines.21 The antibiotics tested included imipenem (IMP, 10 μg), meropenem (MEM, 10 μg), doripenem (DOR, 10 μg), ampicillin (AMP, 10 μg), doxycycline (DOX, 30 μg), cefepime (CEF, 30 μg), ceftazidime (CAZ, 30 μg), ciprofloxacin (CIP, 5 μg), erythromycin (E, 15 μg), trimethoprim/sulfamethoxazole (STX, 1.25/23.75 μg), gentamicin (CN, 10 μg), amikacin (AK, 30 μg) and colistin (COL, 10 μg). Multidrug resistance (MDR) was defined as resistance to more than three classes of antibiotics.22
The biofilm formation assay for CRAB was conducted in triplicate using the adherent assay method described previously.23 A 200 μl of fresh Trypticase Soy Broth, supplemented with 0.25% glucose (w/v), was inoculated with 10 μl of freshly cultured CRAB strains. A negative control, which lacked glucose, was also included. After incubation, the crystal violet assay was performed following standard protocols, and the optical density was measured at 570 nm using a plate reader. Biofilm formation was classified as weak, moderate, and strong.24
Following the instructions of the manufacturer, a commercial DNA extraction kit (Sinaclon, Iran) was used to extract genomic DNA from the overnight-grown CRAB strains. Molecular identification via 16S rRNA and detection of biofilm-associated genes (pgaA, pgaB, pgaC, and pgaD) were performed using PCR. The primer sequences applied are provided in Table 1. PCR reactions used a 2x Master Mix (Sinaclon, Iran), with each 25 μl reaction comprising 12.5 μl Master Mix, 1 μl each of forward and reverse primers, 1 μl DNA (200 ng/μl), and nuclease-free water to complete the volume. The PCR cycling protocol included initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 59°C for 1 min for all genes, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. PCR products were separated on 1% agarose gel with ethidium bromide and visualized under a gel documentation system.
The percentage of A. baumannii was used to represent its genotypic and phenotypic capabilities for forming biofilms and its antimicrobial resistance profiles. To analyze the association between the presence of hemolysin, proteolytic activity, pga locus genes and the intensity of biofilm formation, the chi-square test was applied. To group the isolates according to their antimicrobial resistance profiles and biofilm-forming ability, the heatmap analysis employed hierarchical clustering with Ward’s linkage and the Euclidean distance metric. Before clustering, data normalization and scaling were conducted using z-score transformation. The heatmap was visualized with GraphPad Prism 9. Additionally, Spearman’s rank correlation coefficient was calculated to assess the relationship between biofilm formation categories and resistance to specific antibiotics, with statistical significance defined as p < 0.05.
The gram-negative bacteria exhibiting lactose (-), Nonmotile, oxidase (-), catalase (+), indole (-), pigment (-), urease (+), citrate (+), H2S (-), and negative for both MR and VP tests were identified as A. baumannii isolates. Subsequently, DNA extraction was performed on all isolates, followed by identification using the PCR method. Based on this analysis, 150 isolates were confirmed as A. baumannii ( Figure 1).
Among all the isolates, 21 (14%) displayed hemolytic activity, with each demonstrating a consistent beta-hemolysis profile. In contrast, proteolytic assays confirmed protease production in all isolates, indicated by distinct hydrolytic zones around the colonies. Statistical analysis showed a significant association between hemolysin production and strong biofilm formation (p < 0.0001). In contrast, no correlation was found for protease activity, likely because it was uniformly present across all isolates.
Of the 150 A. baumannii isolates, the most significant resistance was observed against ciprofloxacin (100%), followed by ceftazidime (90%) and cefepime (86.66%) ( Figure 2).
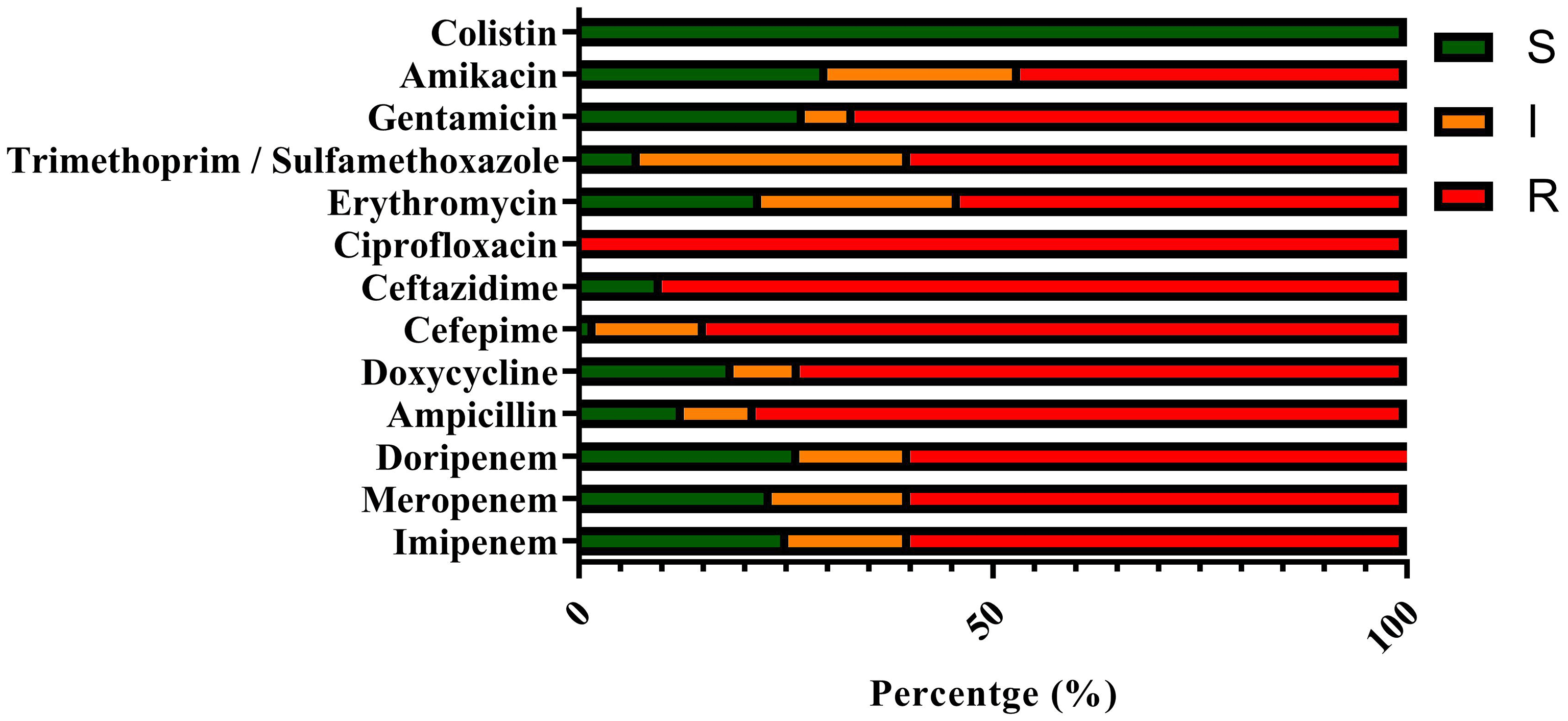
Resistance is expressed in percentages, categorized as S (sensitive), I (intermediate), and R (resistant).
Additionally, the analysis revealed that all isolates (100%) were identified as multidrug-resistant (MDR) ( Table 2), with 60% (90 isolates) classified as carbapenem-resistant A. baumannii (CRAB).
Distribution of isolates categorized as resistant, intermediate, or sensitive to tested antibiotics, based on CLSI guidelines.
All CRAB isolates demonstrated the capacity to form biofilms. Quantitative classification placed 12 isolates (13.33%) in the weak, 28 (31.11%) in the moderate, and 50 (55.55%) in the strong biofilm-producing categories. To improve the transparency and reproducibility in our biofilm categorization, we recorded and analyzed the optical density (OD) values measured at 570 nm for each CRAB isolate. The classification into weak, moderate, and strong biofilm producers was based on established cut-off values. Table 3 shows the distribution of the OD values across these three categories.
Optical density (OD570) values were used to classify isolates as strong, moderate, weak, or non-biofilm formers.
| Biofilm category | OD570 range | Number of isolates | Percentage (%) |
|---|---|---|---|
| Weak | 0.100-0.300 | 12 | 13.33 |
| Moderate | 0.301-0.600 | 28 | 31.11 |
| Strong | > 600 | 50 | 55.55 |
Subsequent heatmap analysis revealed diverse antibiotic resistance patterns among the isolates, correlating with the degree of their biofilm-forming capabilities ( Figure 3). The heatmap visualization highlights the clear patterns of antimicrobial resistance among CRAB isolates based on their biofilm-forming abilities. Significantly, isolates identified as strong biofilm producers demonstrated consistently high resistance rates to nearly all tested antibiotics, especially carbapenems (imipenem, meropenem, and doripenem), fluoroquinolones (ciprofloxacin), and aminoglycosides (gentamicin, amikacin). This extensive resistance in strong biofilm producers indicates a possible interaction between biofilm-mediated protection and genetic resistance mechanisms. In contrast, moderate biofilm producers showed a mixed resistance pattern, displaying moderately high resistance to carbapenems and cephalosporins, but lower resistance levels to colistin and sulfonamides. Weak biofilm producers, on the other hand, typically showed lower resistance and heightened susceptibility to various agents, including colistin, doxycycline, and trimethoprim/sulfamethoxazole.
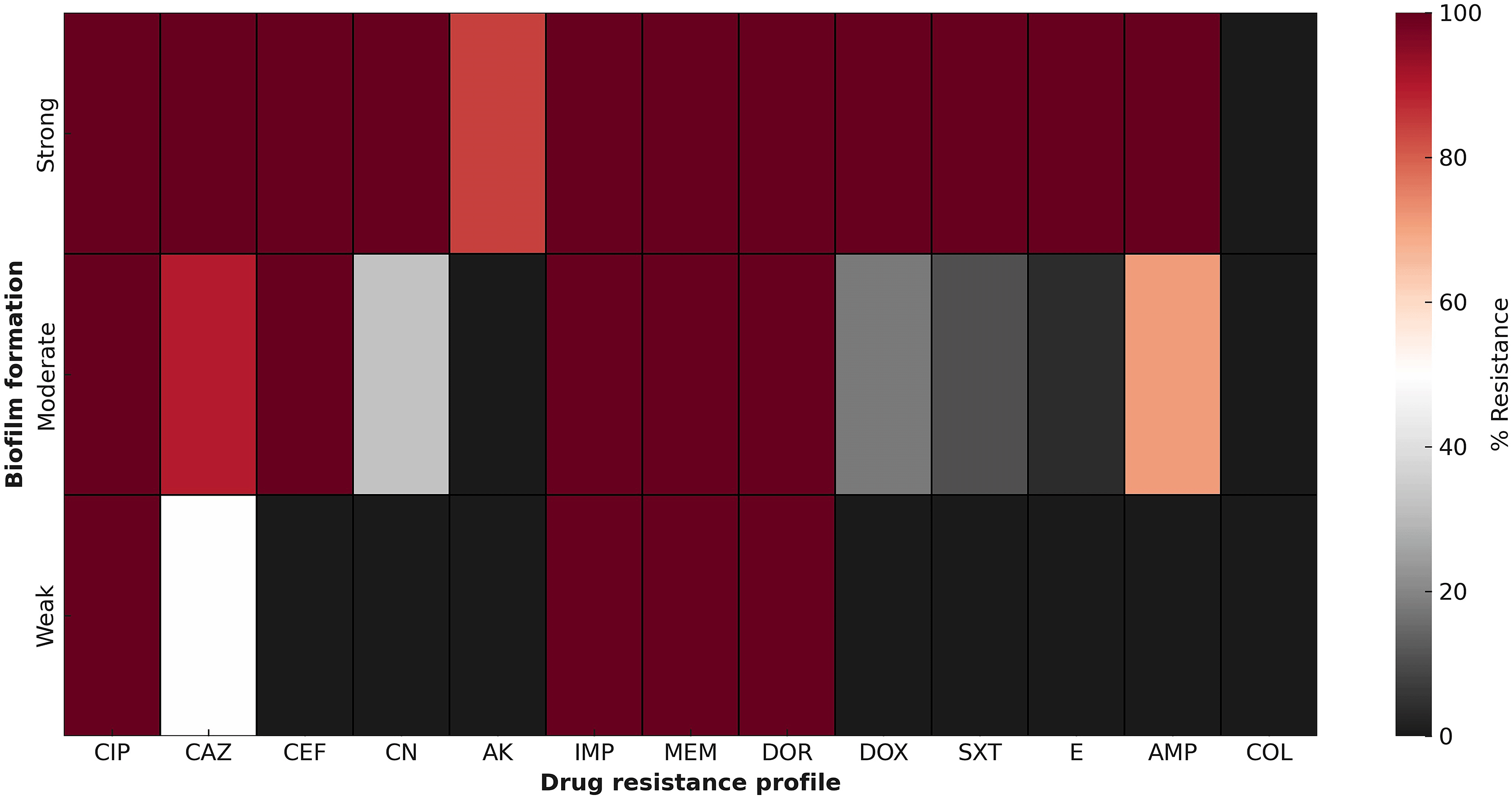
The color scale illustrates the relationship between biofilm production capacity (quality) and specific drug-resistant phenotypes.
In addition, CRAB isolates were tested for the pgaABCD locus genes associated with biofilm formation, including pgaA, pgaB, pgaC, and pgaD. The results indicated that pgaA and pgaD were the genes most commonly associated with biofilm formation, present in 100% of the isolates. In contrast, the frequencies for pgaB and pgaC were 84.4% and 93.3%, respectively. To find out any potential correlation between the amount of biofilm production and the existence of pga genes, a chi-square test was carried out. The analysis demonstrated a statistically significant association between pgaB (p < 0.0001) and pgaC (p = 0.0002) and the strength of biofilm formation. However, no significant correlation was observed for pgaA and pgaD, likely because these genes were present in all isolates. These findings suggest a potential functional distinction for pgaB and pgaC in influencing biofilm phenotype among CRAB strains.
A. baumannii has become a more prevalent pathogen linked to hospital-acquired infections.25 Multiple studies have highlighted its remarkable ability to survive in unfavorable conditions and its significant resistance to various antibiotics, which are believed to be linked to biofilm formation.26–28 Treating these bacteria, especially multidrug-resistant (MDR) strains, is critically important.29 The main therapeutic selection for treating MDR A. baumannii infections that do not respond to third-generation cephalosporins is still carbapenems but, the incidence of CRAB strains is increasing.30 Considering the widespread occurrence of these infections and the diverse patterns of antibiotic resistance in different regions, it is crucial to monitor the prevalence and antimicrobial resistance globally. These findings help us understand the distribution of resistance patterns and support the selection of the most appropriate therapeutic strategies.31
This study provides significant insights into the biofilm-forming capabilities and genetic factors associated with CRAB isolated from clinical samples in Shahrekord, Iran. Our results showed that all CRAB isolates (100%) were able to produce biofilms, with more than half (55.55%) being classified as strong biofilm producers. Additionally, all isolates contained the pgaA and pgaD genes, while pgaC and pgaB were detected in 93.3% and 84.4% of the isolates, respectively. These findings strongly align with previous studies highlighting the crucial role of the pgaABCD operon in the synthesis of poly-β-1,6-N-acetylglucosamine (PNAG), an essential component of the extracellular biofilm matrix.9,19 For example, Choi et al. (2009) found that deleting the pgaABCD locus significantly reduced biofilm formation in A. baumannii.9 Similarly, Li et al. (2021) reported that pgaA and pgaD were highly conserved in CRAB isolates, with frequencies of 97% and 95%, respectively, emphasizing their importance in biofilm-associated virulence.13 Our findings align with those of Kishii et al. (2020), who noted a correlation between the expression of pgaABCD genes and biofilm strength. This suggests that variations in genetics or expression levels could influence biofilm phenotype heterogeneity.14 The slightly lower prevalence of the pgaB and pgaC genes in our study may indicate regional genomic variability, a phenomenon discussed in comparative genomics studies by Lukovic et al. (2025), which documented inter-clonal diversity in virulence gene distribution among CRAB strains across different geographic regions.15
This study highlights the significant connection between biofilm formation and antimicrobial resistance. All CRAB isolates were found to be multidrug-resistant (MDR), with the highest resistance rates against ciprofloxacin (100%), ceftazidime (90%), and cefepime (86.66%). These patterns are consistent with previous research by Sarafan Sadeghi et al. (2019) conducted in Shahrekord32 as well as multicentric surveillance data from Asia-Pacific by Lee et al. (2023), which reported widespread resistance among CRAB strains to carbapenems and fluoroquinolones.31 The biofilm phenotype is closely associated with increased antimicrobial resistance which is attributed to the physical barrier of the biofilm matrix, the reduced cellular metabolism, and the upregulation of resistance genes.8,12 For instance, Yang et al. (2019) found a positive correlation between strong biofilm-forming ability and resistance to multiple antibiotic classes in A. baumannii isolates derived from ICU patients.27
One notable finding of this study is the universal presence of proteolytic activity among the isolates, while only 14% of the isolates demonstrated hemolytic activity, particularly β-hemolysis. Additionally, our research revealed a statistically significant correlation between hemolysin production and strong biofilm formation (p < 0.0001); however, the underlying mechanisms linking these phenotypes remain largely unexplored. Hemolysins in A. baumannii, especially β-hemolysins, have been associated with tissue damage, immune evasion, and enhanced colonization, potentially aiding in the initial adhesion and maturation of biofilms on host surfaces.10,12 The disruption of red blood cells and the subsequent release of host nutrients may create favorable conditions for biofilm development. Conversely, all isolates displayed proteolytic activity, and no significant correlation was observed with biofilm strength, likely due to the uniformity of the proteolytic presence. However, proteases such as OmpA-associated protease and serine proteases have been reported to influence biofilm matrix composition and remodeling.6
Our findings align with global reports, showing a high prevalence of biofilm formation and a strong association between the pgaABCD locus and CRAB virulence. However, unlike studies such as Khanna and Joshi (2024), which reported detection rates of pgaB and pgaD at 70–85% among Indian isolates, we found pgaA and pgaD present in all CRAB isolates in our study. These differences may be due to differences in local strain epidemiology, methodological sensitivity, or varying selective pressures, such as antibiotic use policies.18 Statistical analysis confirmed a significant association between the presence of pgaB and pgaC genes and strong biofilm production, indicating their functional role in enhancing biofilm phenotype heterogeneity. This suggests that pgaB and pgaC could act as variable genetic markers that influence biofilm strength in clinical CRAB isolates. However, the universal presence of pgaA and pgaD across all isolates limited their statistical association with phenotypic variation.
One of the key contributions of this study is the notably high prevalence of the pgaA and pgaD genes, which were identified in 100% of CRAB isolates. This rate surpasses several previous reports from regions such as India and Southeast Asia.18,31 Additionally, the meaningful correlation between the pgaB and pgaC genes and strong biofilm formation (p < 0.0001 and p = 0.0002, respectively) indicates a potentially strain-specific impact on the biofilm phenotype, a detail not consistently emphasized in earlier research. The observed antimicrobial resistance pattern especially the universal resistance to ciprofloxacin and high resistance to ceftazidime and cefepime suggests a regionally unique resistance profile, likely influenced by local antibiotic usage policies. When viewed in the context of global surveillance data, these findings reveal a troubling convergence of biofilm-associated virulence and multidrug resistance in CRAB strains from this area. This underscores the necessity for region-specific molecular monitoring and therapeutic strategies, while also enhancing the global understanding of CRAB epidemiology.
From a clinical perspective, the high prevalence of multidrug-resistant CRAB isolates that produce strong biofilms highlights the urgent need to integrate anti-biofilm strategies into infection control and treatment protocols. The pgaABCD locus, a key factor in biofilm matrix synthesis, presents a promising therapeutic target. Agents that inhibit PNAG synthesis or block pgaABCD gene expression may reduce biofilm formation and improve antibiotic effectiveness. Additionally, the strong link between biofilm phenotype and resistance patterns indicates that biofilm-disrupting agents could enhance the efficacy of conventional antibiotics, particularly against strains resistant to fluoroquinolones and cephalosporins. From an infection control standpoint, hospitals in endemic areas should strengthen disinfection protocols, as biofilm-forming CRAB can survive on non-living surfaces and medical devices. Incorporating biofilm screening into routine microbiological diagnostics could also help in risk stratification and the development of more targeted antimicrobial regimens.
There are a number of limitations in this work that must be addressed. First, the sampling was restricted to a single geographic region (Shahrekord, Iran) and occurred over a relatively short period (2020–2021). Changes in clonal distribution, antibiotic use policies, and hospital environments may influence the results, thereby limiting the applicability of the findings to other time periods or geographic regions. Second, the study lacked detailed metadata, including patient demographics, infection sites, and ward-level environmental factors. These variables could act as confounders affecting both biofilm formation and antimicrobial resistance, and their absence limits our ability to control for stratification or adjust for underlying clinical or environmental influences. Third, gene expression analysis (e.g., qRT-PCR) was not conducted, which restricts our understanding of the functional activity of the pgaABCD genes under biofilm-inducing conditions. Fourth, even though the presence of pgaB and pgaC genes was significantly associated with strong biofilm production, no functional validation experiments (such as gene knockout, knockdown, or complementation assays) were performed to confirm causality, leaving the current findings correlative. Fifth, molecular typing or phylogenetic analysis (e.g., MLST, PFGE, or REP-PCR) was not included, which limits our ability to assess strain-level differences, clonal dissemination, and the spread of biofilm-associated traits. Future investigations should utilize functional genomics tools to validate gene functions in biofilm formation while addressing these limitations by incorporating multi-center sampling, collecting detailed clinical metadata, performing transcriptional profiling, and employing genotyping approaches. This will provide deeper insights into the population structure, transmission dynamics, and evolutionary trajectories of CRAB strains.
This study demonstrates widespread prevalence of biofilm formation ability among CRAB isolates and emphasizes the critical role of the pgaABCD operon especially the pgaB and pgaC genes in determining biofilm strength. All isolates displayed multidrug resistance, with the strongest biofilm producers showing the highest resistance rates. A meaningful relationship between the biofilm phenotype and antimicrobial resistance patterns suggests that biofilm formation enhances bacterial survival under antibiotic pressure. The identification of the key genetic factors participating in biofilm development and their link to resistance highlights the necessity of incorporating anti-biofilm therapeutic strategies and molecular surveillance in the clinical management of CRAB infections. Future research should focus on gene expression analysis and the exploration of biofilm-inhibitory compounds as adjuncts to antibiotic therapy.
This study was reviewed and approved by the Institutional Animal Care and Use Committee of Islamic Azad University, Shahrekord, Iran (Ethics code: IR.IAU.SHK.REC.1401.068). The committee granted a waiver of written informed consent because only anonymized, residual diagnostic specimens were used and no patient-identifiable data were recorded. All procedures involving human-derived materials were conducted in accordance with relevant guidelines and the Declaration of Helsinki. A copy of the ethics approval is available from the corresponding author.
During the preparation of this work, the author(s) used chatGPT 3.5 to proofread and improving clarity and flow. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
The datasets underlying this article are available in Zenodo at https://doi.org/10.5281/zenodo.17222374.33 All data are fully anonymized and openly available under a CC-BY 4.0 license.
Underlying data supporting the results reported in this article include the raw values underlying the means, standard deviations, and other summary statistics presented in the text and tables; the values used to generate graphs and figures; the variables applied in the analyses (e.g., isolate ID, sample type, antibiotic susceptibility and genotypic data); and the data points extracted from images for analysis. These datasets are openly available in Zenodo at https://doi.org/10.5281/zenodo.17222374.33
Extended data supporting this study include supplementary large tables (e.g., complete antibiotic susceptibility profiles). These files are available in Zenodo at https://doi.org/10.5281/zenodo.17222374.33 Extended data are openly available under a CC-BY 4.0 license.
The authors extend their gratitude to the members of the Biotechnology Research Center at the Islamic Azad University of Shahrekord in Iran for their assistance and support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology, antimicrobial resistance, molecular epidemiology, bacterial virulence factors
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology, Immunology, Antimicrobial Peptides
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 24 Nov 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)