Keywords
Danio rerio, Zebrafish, Drug discovery, Toxicology, High-throughput screening, CRISPR/Cas9
This article is included in the Manipal Academy of Higher Education gateway.
The increasing demand for ethically acceptable, economically viable, and translationally relevant animal models in biomedical research positions Danio rerio (zebrafish) as a prominent alternative to traditional rodent systems. This review provides an integrated analysis of zebrafish biology and delineates their expanding applications in pharmacological investigations and toxicological evaluations. Emphasis is placed on genetic homology with humans, optical transparency during embryogenesis, and suitability for high-throughput screening, which collectively support the model’s relevance in contemporary biomedical studies. The historical progression of zebrafish usage is outlined, and critical biological features, such as developmental kinetics, sexual dimorphism, and organogenesis are described to contextualize their utility in disease modeling. Zebrafish are examined for their capacity to assess acute, chronic, and specialized toxicity endpoints, including neurotoxicity, hepatotoxicity, and endocrine disruption. Their roles in investigating inflammation, metabolic disorders, neurodegeneration, cancer, and infectious diseases are also reviewed. Technological advancements, including CRISPR/Cas9-mediated gene editing and the development of transgenic lines, are discussed alongside innovations in imaging and screening methodologies. Regulatory frameworks, as well as compliance with Good Laboratory Practices (GLP), are addressed. The review concludes by evaluating the potential of zebrafish in precision medicine and their capacity to enhance early-phase drug discovery through scalable, cost-effective, and biologically relevant approaches.
Danio rerio, Zebrafish, Drug discovery, Toxicology, High-throughput screening, CRISPR/Cas9
In the areas of drug discovery and toxicology, the need for effective and reliable animal models has long been a cornerstone of biomedical research. Traditionally, mammalian models such as mice, rats, dogs, and monkeys have played a pivotal role in understanding disease pathways and assessing the potency and safety of potential therapeutic agents. However, growing ethical concerns surrounding animal welfare, combined with the high financial and temporal costs associated with transgenic lines of a few of these models, have prompted the exploration of alternative animal systems.1 Non-mammalian organisms, like zebrafish (Danio rerio), fruit fly (Drosophila melanogaster), and nematode (Caenorhabditis elegans),2 are gaining significant attention due to their potential to streamline drug discovery while aligning with the 3Rs - Replacement, Reduction, and Refinement principles.3 As biomedical research grows more complex, zebrafish offer an ethical, predictive alternative for advancing human-relevant studies.
Zebrafish have a history of being used in biomedical research, and since the late 20th century, their use has grown significantly in popularity.4 Initially leveraged for genetic and developmental studies, zebrafish were recognized for their transparent embryos and rapid development, allowing for real-time observation of cellular and developmental processes.5 George Streisinger's work in the early 1980s helped establish zebrafish as a model organism,6 and its utility has since expanded into various research domains, including cancer,7 cardiovascular diseases,8 and neurological disorders.9 With ~70% of human genes having zebrafish orthologs, this genetic similarity makes them valuable for modeling human diseases.10 The development of advanced genetic tools like CRISPR/Cas9 has further enhanced the relevance of zebrafish for studying complex pathologies, providing a powerful platform for gene editing and disease modeling.11
Compared to rodents, zebrafish are cost-effective, highly fecund, and develop rapidly, enabling high-throughput drug and toxicity screening.12 The transparency of zebrafish embryos permits non-invasive, real-time imaging of biological processes, an advantage often limited in opaque mammalian models.13 Zebrafish also face fewer ethical constraints, as they are perceived to have lower sentience than higher vertebrates, further supporting their use as an alternative model.14 Additionally, their genetic homology, ease of manipulation, and suitability for behavioural studies position them as an indispensable tool, complementing traditional mammalian models.15
Recent developments in zebrafish research, namely in toxicity and medication development, are the main topic of this thorough study. Zebrafish are crucial to the future of biomedical research because they have become a potent model organism for comprehending disease processes, assessing toxicity, and screening potential phytochemicals, validating their network pharmacology prediction, and assessing the toxicity and efficacy of novel nano-formulations.16 Despite this expanding literature, no single review has integrated zebrafish biology with mechanistic applications across toxicology, pharmacology, genomics, regenerative medicine, and natural product research. Existing reviews are often narrowly focused on development, toxicology, or neuroscience, but lack a systems-level, translational perspective. We critically examine the strengths and limitations of zebrafish as a translational platform, highlighting where they align with or diverge from mammalian and human systems. Special emphasis is placed on mechanistic insights, the role of emerging technologies, and the translational fidelity of zebrafish findings. By bridging basic biology with applied biomedical sciences, this article positions zebrafish as a next-generation model for integrative and personalized medicine. This comprehensive approach ensured the review encompassed the most current advancements and trends in zebrafish research ( Figure 1).
A common model organism in scientific studies, especially in developmental biology and genetics, is the little freshwater zebrafish. Figure 2 illustrates how the zebrafish development cycle may be broken down into numerous important phases.17 Understanding the growth cycle of zebrafish is crucial for both laboratory studies and ecological assessments.18
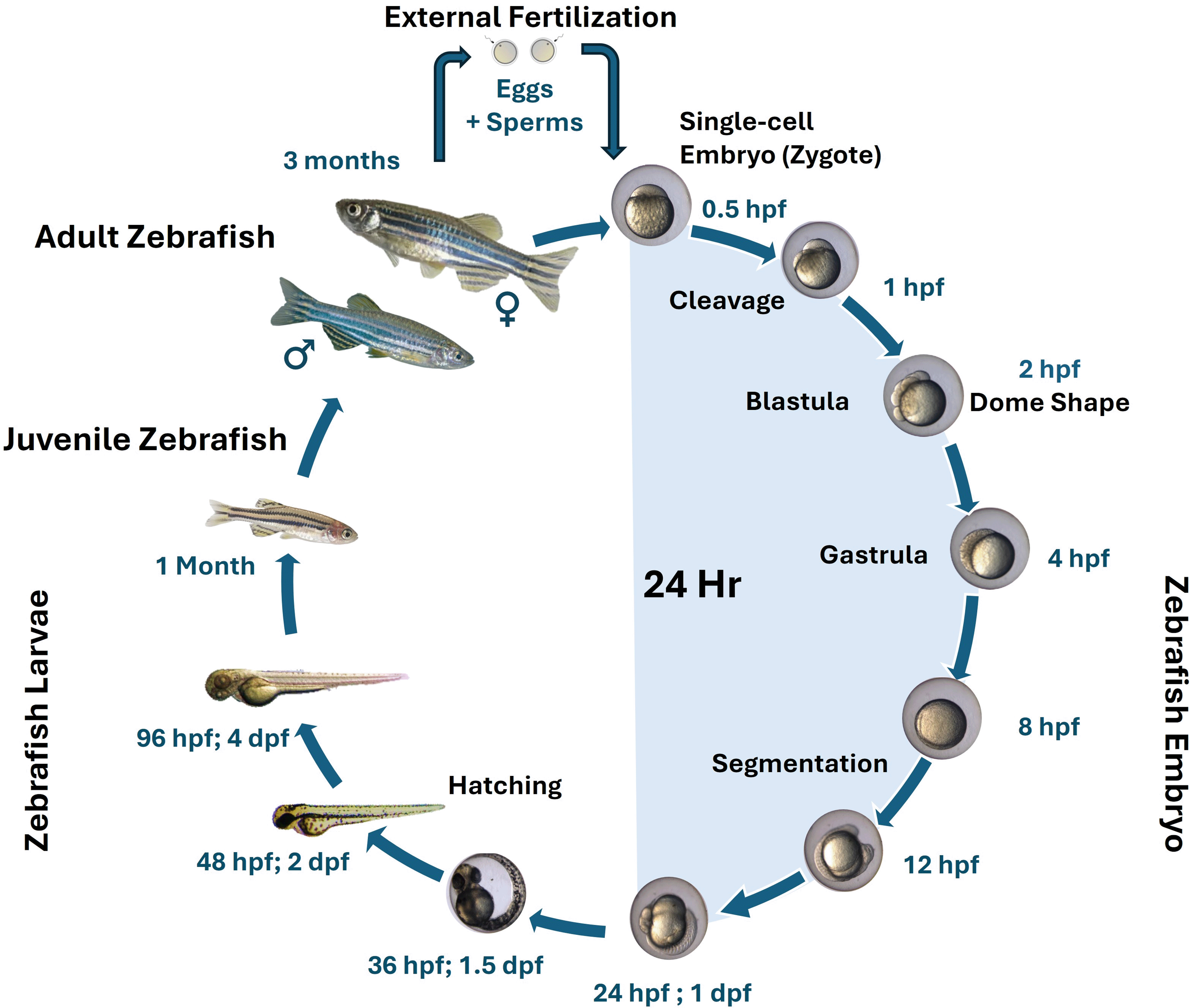
The blue side represents embryonic stages within the first 24 hours, while the other side shows subsequent larval and juvenile growth leading to the adult fish.
Zebrafish exhibit external fertilization, where eggs are laid and fertilized in the water. The fertilization process occurs when a male fish releases sperm over the eggs laid by a female. After fertilization, the eggs begin to develop rapidly.19 Within approximately 36 hours, precursors to all major organs appear, and the embryo becomes transparent, allowing for easy observation of developmental processes.20 Hatching occurs around 48 hours post-fertilization (hpf ), at which point the embryos transition into free-swimming larvae. This stage is characterized by the absorption of the yolk. After hatching, zebrafish enter the larval stage, which lasts until about 14 days post-hatching. They experience major morphological changes at this time, including the development of adult fin structures and the disappearance of the larval fin fold. The larvae are extremely sensitive to their surroundings, and elements like temperature and water quality can have an impact on their growth.18 From about 14 to 60 days, zebrafish are considered juveniles. They continue to grow and develop more distinct adult features. This stage is critical for establishing social hierarchies and behaviours, which can affect growth rates and health.21 Zebrafish mature and reproduce within 3-4 months, enabling rapid multigenerational studies. Males are typically more brightly colored with distinct markings.22 Adult zebrafish can live for up to 5 years under optimal conditions. They are capable of spawning frequently, with females laying hundreds of eggs every few days. This prolific spawning behaviour is advantageous for research purposes, allowing for the collection of large numbers of embryos for experimental use.23
Zebrafish are widely used in genetics, developmental, and behavioral research. Males and females differ in appearance, behavior, and physiology, which can affect outcomes. Table 1 and Figure 3 summarize these key differences.
| Characteristic | Males | Females | References |
|---|---|---|---|
| Body Color | Darker blue with a pinkish-yellow cast | Bluish white with silver stripes | 24 |
| Body Shape | More streamlined and slender | Larger, rounder belly due to egg storage | 25 |
| Activity Level | More active and energetic | Generally, less active | 26 |
| Ventral Fins Coloration | More golden, especially on the ventral fins | Less pronounced coloration | 27 |
| Behaviour | More exploratory, higher velocity in open areas | Less exploratory, more time spent in sheltered areas | 28 |
| Brain Differences | Higher expression of certain genes related to aggression | Different gene expression patterns, especially during differentiation | 28 |
| Metabolite Profile | Higher levels of certain lipids (e.g., ceramide) | Different lipid profiles, indicating metabolic differences | 26 |
Zebrafish are ideal for studying embryogenesis and organogenesis due to their quick development and transparent embryos. External fertilization enables real-time visualization of cleavage, gastrulation, and organ formation (heart, kidney, endocrine tissues). Embryogenesis in zebrafish begins with external fertilization, leading to cleavage and the formation of a blastula and gastrula. Fluorescence microscopy reveals cell migration and differentiation, while key pathways like Wnt, Notch, and Hedgehog regulate these processes.29
In zebrafish, several organs develop sequentially. The heart begins to form around 24-hpf and is fully functional by 48-hpf. It develops from mesodermal precursors through a series of morphogenetic movements.30 Zebrafish develop pronephros, which is the first kidney structure, followed by the mesonephros. The pronephros consists of a simple structure with two nephrons, while the mesonephros develops more complex nephron structures during larval stages, supporting ongoing nephrogenesis.31 The inter-renal gland, the teleostean equivalent of the adrenal gland, forms alongside the embryonic kidney. Recent studies have identified genes involved in the development and steroidogenesis of this organ, revealing similarities between zebrafish and mammalian organogenesis ( Figure 4).32 Zebrafish also provide insights into the effects of genetic mutations and environmental factors on organ development. The use of transgenic lines and gene knockdown techniques has enabled researchers to dissect the molecular mechanisms underlying organogenesis, revealing conserved pathways that are critical for understanding vertebrate development.
Due to its unique advantages over traditional animal models, the zebrafish has emerged as a powerful model organism in toxicology research. As a vertebrate species, zebrafish share significant genetic and physiological similarities with humans, making them relevant for studying the toxicological effects of various substances on human health.4 Zebrafish are transparent during the embryonic and larval phases, which makes it possible to directly see developmental processes and the impacts of toxicants in vivo. This is one of the main benefits of employing zebrafish in toxicology ( Figure 5).33 Additionally, zebrafish have a short life cycle, high fecundity, and are amenable to genetic manipulation, enabling researchers to study acute and chronic toxic effects in a high-throughput manner. Zebrafish models have been extensively used to investigate a wide range of toxicological endpoints, including neurotoxicity, teratotoxicity, genotoxicity, and developmental toxicity induced by various substances such as nanoparticles, pesticides, and pharmaceuticals.34
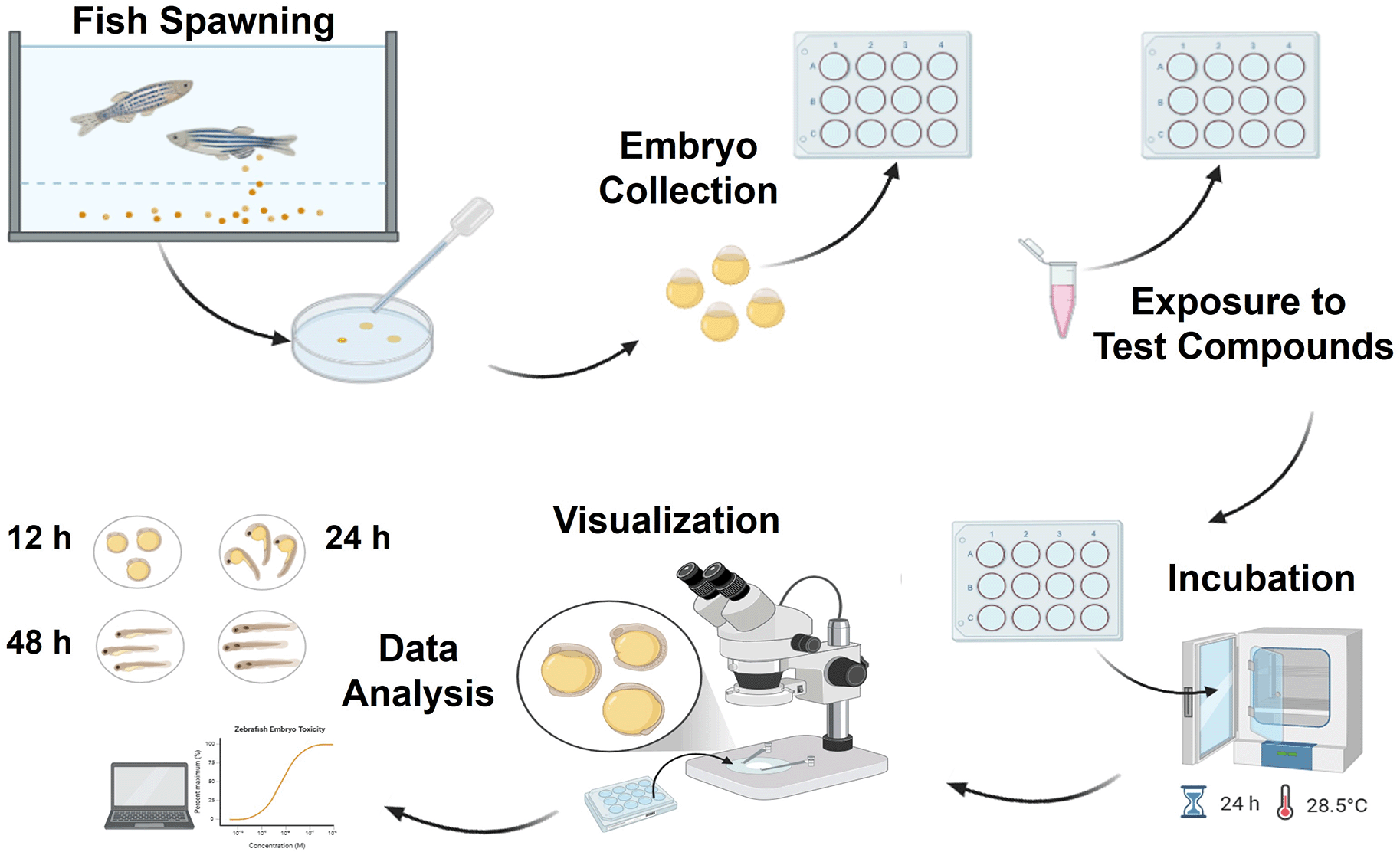
Acute toxicity testing in zebrafish has gained prominence as an efficient and relevant alternative to traditional methods involving adult fish, particularly in assessing chemical safety. The zebrafish model, specifically its embryos and larvae, is utilized in the Zebrafish Embryo Toxicity Test (FET) and the Zebrafish Larvae Acute Toxicity Test (FLT), which evaluate developmental endpoints and acute toxicity, respectively.35 These tests offer advantages such as rapid development, transparency, cost-effectiveness, and high throughput, making them valuable tools in chemical risk assessment, although they may have limitations in detecting neurotoxicants.36 The FLT test exposes larvae to toxicants at a later stage, whereas the FET test concentrates on early embryo development. These two assays provide partially overlapping toxicity profiles and unique information, indicating that their combination can enhance the use of zebrafish embryos and larvae as standard toxicity testing models.37 The use of zebrafish embryos to evaluate acute toxicity is described in the OECD TG-236 guideline. Coagulation, lack of somite development, absence of heartbeat, and non-detachment of the tail are the four main endpoints that the test focuses on. In the extended FET Test, beyond the standard endpoints, additional morphological observations can be included to identify different classes of toxicants, although specificity is higher at sublethal concentrations.38,39 FLT uses zebrafish larvae for four days post-fertilization and is exposed for 48 hours in 6-well plates. It has shown high correlation with traditional acute fish toxicity tests and is simpler to implement than the FET test.40 A modified version of the OECD 236 test uses 4-128 cell stage embryos in a 96-well microtiter plate, improving testing efficiency and throughput. This method has shown good correlation with the standard OECD 236 method and is less time-consuming.41
Chronic toxicity testing in zebrafish is increasingly recognized for its effectiveness in evaluating the long-term effects of chemical exposure, leveraging the species' rapid development and genetic similarities to humans.42 Zebrafish can be subjected to various chronic exposure protocols, often involving prolonged exposure to test substances from early life stages through adulthood. This approach allows researchers to assess a range of toxicological endpoints, including developmental, reproductive, and behavioural impacts.43 The transparency of the zebrafish model throughout early development makes it very useful for observing physiological changes and any harmful consequences in real time. Additionally, research has demonstrated that long-term exposure can yield important insights into toxicity processes, such as neurotoxicity and endocrine disruption. It offers a more thorough comprehension of chemical safety in aquatic ecosystems and its consequences for human health.15
3.2.1 Developmental toxicity
Developmental toxicity testing in zebrafish has emerged as a critical approach for evaluating the effects of chemicals on embryonic development, offering a high-throughput and ethically sound alternative to traditional mammalian models. Recent studies have validated the zebrafish developmental toxicity assay by demonstrating its ability to predict teratogenic effects with high sensitivity and specificity; for instance, a study found that 74.2% of known teratogenic compounds were accurately identified using this model, highlighting its reliability in screening potential developmental toxicants.44
3.2.2 Neurotoxicity
Neurotoxicity testing in zebrafish has become essential in pharmaceutical research for evaluating the neurotoxic effects of drugs and environmental chemicals. Various behavioural assays can be employed to assess neurotoxicity, including locomotor activity tests and assessments of anxiety-like behaviours, which have been shown to correlate well with mammalian responses. Studies indicate that exposure to neurotoxicants, such as heavy metals and certain pharmaceuticals, can lead to significant behavioural changes and alterations in neural morphology in zebrafish.45
3.2.3 Cardiotoxicity
Zebrafish cardiotoxicity testing is useful for evaluating the possible cardiac effects of pharmacological medications and phytomolecules. Using zebrafish models, various endpoints such as heart rate, contractility, and structural abnormalities can be evaluated, providing insights into the mechanisms of drug-induced cardiotoxicity.46 Studies have demonstrated that zebrafish models can effectively detect the cardiotoxic effects of chemotherapeutic agents like doxorubicin, recreational drugs, and environmental pollutants.47 Furthermore, zebrafish have been used to screen for cardioprotective compounds, such as angiotensin receptor blockers and beta-blockers, which can ameliorate drug-induced heart dysfunction.48
3.2.4 Hepatotoxicity
Zebrafish hepatotoxicity testing has emerged as a significant technique for assessing the liver toxicity of medications and phytomolecules, providing a high-throughput and morally sound substitute for conventional animal models. Various studies have demonstrated that exposure to hepatotoxic compounds, such as acetaminophen,49 and certain herbal extracts, like Polygonii multiflori, can induce significant liver damage in zebrafish, characterized by histopathological changes, increased levels of liver enzymes, and alterations in liver morphology.50 For instance, fraxinellone, a compound derived from Cortex Dictamni, has been shown to cause hepatotoxicity through oxidative stress mechanisms, leading to apoptosis and liver degeneration in zebrafish larvae.51
3.2.5 Nephrotoxicity
Due to their transparent bodies and rapid development, zebrafish larvae allow for real-time observation of kidney function and morphology, making them ideal for assessing nephrotoxic effects. Studies have shown that exposure to nephrotoxic agents such as gentamicin and aristolochic acid leads to significant renal damage, characterized by tubular injury, necrosis, and impaired kidney function.52 For example, studies showed that exposure to gentamicin caused renal tubules to widen and elevated the expression of biomarkers for kidney damage, including kidney injury molecule-1 (kim-1) and heme oxygenase 1 (hmox1). Additionally, zebrafish models have been used to screen for different compounds' protective properties, which has helped identify prospective therapeutic agents against drug-induced kidney damage.53
3.2.6 Endocrine toxicity
Endocrine toxicity testing in zebrafish has become a crucial approach for evaluating the effects of pharmaceuticals and phytomolecules on the endocrine system, particularly in the context of endocrine-disrupting compounds (EDCs). Research has shown that exposure to EDCs, such as bisphenol A and certain pesticides, can disrupt sexual differentiation, reproductive functions, and hormone signaling pathways in zebrafish.54 For example, studies have demonstrated that exposure to 17α-ethinylestradiol leads to feminization of male zebrafish and altered reproductive behaviors.55
3.2.7 Environmental and industrial toxicity studies
An important technique for determining how chemicals and contaminants affect aquatic ecosystems and human health is environmental and industrial toxicity testing. To assess the impact of several environmental pollutants, including heavy metals, pesticides, and nanomaterials, on embryonic development and behaviour, the zebrafish embryo toxicity test (ZET) has been used extensively.56 For instance, studies have shown that exposure to polychlorinated biphenyls (PCBs) and heavy metals can lead to significant morphological abnormalities and behavioural changes in zebrafish larvae.57
Zebrafish screens have identified several compounds in early clinical trials for various conditions. Zebrafish embryos are suitable for high-throughput screening in 96- or 48-well plates, allowing for cheaper and efficient screening of compounds. Treatments like EPX-100 (clemizole) for Dravet58 and Trifluoperazine for Diamond-Blackfan59 are also under investigation. Additionally, DB-041, aimed at treating hearing loss after aminoglycoside antibiotic use, is in Phase-I trials. Zebrafish models are also being used to create treatments for lymphatic diseases, melanoma, and graft-versus-host disease (GvHD). Pathogens are delivered into zebrafish embryos either by immersion or microinjection, making them useful as infection models. Five weakly water-soluble nitronaphthofuran compounds were tested for in vivo toxicity and effectiveness using the zebrafish embryo TB model.60
The zebrafish model allows researchers to investigate the pharmacokinetics and pharmacodynamics of drugs at a cellular level. Blood sampling from zebrafish larvae allowed for the measurement of paracetamol and its metabolites. The PK parameters, including clearance and distribution volume, correlated well with those in higher vertebrates. One challenge in using zebrafish, especially larvae, is the difficulty in measuring internal drug exposure. Accurate quantification of drug and metabolite levels is essential for reliable PK and PD studies.61 A case study demonstrated the use of zebrafish larvae to assess the ADME profile of naloxone, an opioid antagonist. The research confirmed that zebrafish metabolize naloxone into the same major metabolites observed in humans. Furthermore, the spatial distribution of naloxone within the larvae mirrored known human pharmacological data, underscoring the model's translational relevance.62 In the context of Traditional Chinese medicine (TCM), researchers have developed an integrated high-throughput strategy using zebrafish to study the ADME characteristics of complex herbal formulations. Utilising techniques like ultra-performance liquid chromatography-high-resolution mass spectrometry (UPLC-HRMS) and desorption electrospray ionisation-mass spectrometry (DESI-MS) imaging, the study successfully identified and characterised multiple metabolites, demonstrating the model's efficacy in complex compound analysis.63 Research comparing immersion and intrayolk microinjection methods in zebrafish embryos revealed insights into compound absorption and distribution. Evidence suggests that not all small molecules are equally absorbed, even if immersion of ZFE is the primary exposure method utilized. This might lead to false-negative readouts and inaccurate findings. Fluorescent dyes administered through these methods allowed for real-time imaging of pharmacokinetics, highlighting the model's utility in studying compound behaviour over time.64
Zebrafish possess a range of nociceptors that respond to noxious stimuli, similar to mammals. Research has identified various molecular pathways involved in pain perception, including the opioid system, transient receptor potential (TRP) channels, and acid-sensing ion channels (ASIC).65–67 Zebrafish models have been used to test various analgesics, including opioids, NSAIDs, and local anaesthetics, demonstrating their effectiveness in reducing pain-induced behaviours. Recent studies have shown that various analgesics acting on COX-1 or COX-2 can effectively alleviate pain-related behaviours in zebrafish following induced injuries like fin amputation.67 Studies have shown that female zebrafish may exhibit more pronounced pain-related behaviours compared to males, indicating the importance of considering sex as a variable in pain research.68 Essential oils from the Piper genus have shown potential antinociceptive and anti-inflammatory effects in zebrafish models.69 Similar to adult zebrafish and other vertebrates, larval zebrafish react to unpleasant stressors, and analgesics can lessen the impact of nociception on activity.70–72
4.3.1 Zebrafish models to screen drugs for neurodevelopmental disorders
Autism: Deficits in social interaction, hyperactivity, anxiety, communication problems, repetitive behaviors, and impaired sensory processing are characteristics of autism. Zebrafish, with their genetic tractability, clear development, preserved brain architecture, and aptitude for high-throughput screening, have lately become a potent supplementary system to rodent models, which have long been at the forefront of ASD research.73 The Simons Foundation Autism Research Initiative (SFARI) has validated 12 ASD risk genes with established zebrafish mutant lines, enabling cross-species comparisons with human and mouse models to identify conserved neurodevelopmental mechanisms.73 For example, oxytocin treatment during the larval stage, particularly at 50μM for 48 hours, significantly ameliorated autism-like behaviors in the zebrafish model. This behavioural improvement was accompanied by the upregulation of key autism-related genes such as Shank3a, Shank3b, and the oxytocin receptor.74 Similarly, tanc2 knockout zebrafish exhibited increased brain size, selective expansion of glutamatergic neurons, and impaired social preference phenotypes consistent with TANC2-associated ASD. These changes reflect an underlying excitatory/inhibitory (E/I) imbalance, a common feature in many neurodevelopmental disorders.75 Other gene models further underscore the utility of zebrafish in elucidating ASD mechanisms. shank2b-/- zebrafish displayed core ASD-like features, including reduced social and kin preference, stereotypic behaviors, and sensory hypersensitivity, which were linked to GABA receptor downregulation and increased seizure susceptibility, implicating GABAergic dysfunction as a major contributor to ASD symptoms.76 The adult brain's downregulation of synaptic structure and function genes was also linked to poor shoaling, decreased social interaction, and motor coordination problems caused by haploinsufficiency of setd5.77 In ctnnd2b heterozygous zebrafish, ectopic Isl1-positive neurons and reduced GABAergic populations disrupted neuronal differentiation in the forebrain, leading to hyperactivity that mirrors CTNND2-related neurodevelopmental disorders.78 Loss of GluN2B, encoded by grin2B, resulted in selective social deficits without broader neurodevelopmental impairments. While grin2B-/- larvae retained normal learning and locomotor function, they exhibited reduced inhibitory neuron marker expression in the subpallium, a region implicated in ASD. This highlights the potential of zebrafish for modeling NMDA receptor-related autism in ways that are not possible in lethal rodent knockouts.79 In a valproic acid (VPA)-induced ASD model, acute treatment with duloxetine (DLX) from 4.5 to 6 dpf reversed hyperactivity, anxiety, and social deficits. DLX also normalized “acetylcholine esterase (AChE)” activity and “Akt-mTOR signaling”, yielding persistent improvements into adulthood.80 Another study developed a comprehensive VPA-induced zebrafish model that integrates behavioural despair assays, molecular alterations, and predictive endpoints into a unified system. Using 7-day-old larvae for early screening and 21-day-old juveniles for assessing social behaviour, this paradigm supports scalable and rapid identification of lead compounds.81
ADHD: Recent zebrafish studies have illuminated novel mechanisms and therapeutic leads for attention deficit hyperactivity disorder (ADHD). CFTR-deficient zebrafish exhibit reduced dopaminergic neuron populations and hyperactivity, both of which are ameliorated by CFTR modulators such as lumacaftor and ivacaftor. Separately, amlodipine, a calcium channel blocker, reduced hyperactivity and impulsivity in both zebrafish and rat ADHD models by targeting L-type calcium channel genes, which have been implicated in ADHD via human GWAS studies.82 Environmental factors also play a significant role: embryonic exposure to bisphenol A (BPA) and perfluorooctanesulfonic acid (PFOS) induced dopamine dysfunction, reduced GABAergic neuron density, and ADHD-like behaviors in zebrafish, highlighting the neurodevelopmental risks of endocrine disruptors during pregnancy.83 CRISPR-generated chmp7+/- zebrafish modeled an ADHD-associated risk variant and displayed significant hyperactivity at 6 dpf, along with reduced brain volume. These early-stage hyperactivity phenotypes correlated with reduced chmp7 transcript levels, mimicking the human risk allele profile. Importantly, methylphenidate treatment successfully reversed the hyperactivity, supporting the model’s pharmacological validity.84 Moderate embryonic exposure to estrogen and the environmental pollutant bisphenol A (BPA) in zebrafish induced distinct neurodevelopmental phenotypes that parallel features of ADHD and ASD, respectively. Estrogen exposure led to hyperactivity and impulsivity, linked to increased Cyp19a1b and reduced tyrosine hydroxylase expression, while BPA exposure caused reduced GABAergic neuron populations, particularly in the midbrain, along with social deficits, repetitive behaviors, and heightened escape responses.85 Using adgrl3.1-/- zebrafish larvae as a genetic model of ADHD, researchers identified robust hyperactivity that was responsive to standard non-stimulant treatments, albeit with sleep-related side effects.86
4.3.2 Zebrafish models to screen drugs for neurodegenerative disorders
Alzheimer’s: Their neurogenic potential, driven by neuron-glia interactions mediated through IL-4, serotonin, and BDNF-NGFR signaling, supports studies of synaptic plasticity and regeneration. Gene manipulations of AD-associated targets such as ABCA7, BACE2, and TNFαIP1 have revealed enhanced synaptic resilience via BDNF and neuropeptide Y pathways.87,88 Behavioural paradigms using agents like aluminium chloride (AlCl3), D-galactose, scopolamine, streptozotocin (STZ), and okadaic acid (OKA) replicate key AD features, including amyloid aggregation, tau hyperphosphorylation, neuroinflammation, and sensorimotor decline. Spatial learning and memory are assessed through T-maze and Y-maze assays, with evidence of cognitive rescue from botanical interventions. For instance, Eulophia ochreata extract improved spontaneous alternation and reduced decision latency in scopolamine-induced zebrafish models, while Carica papaya seed extract reversed OKA-induced deficits and restored Purkinje cell architecture.89 A range of natural compounds, neochlorogenic acid, apigenin, tangeretin, andrographolide, scoparone, and herbal agents like Ocimum sanctum, exhibited neuroprotection through Nrf2 activation, mitophagy enhancement, and GSK-3β or TNF-α inhibition.90,91 Multi-target-directed ligands (MTDLs) such as apigenin-rivastigmine hybrids, donepezil analogs (e.g., DK02, compound 6a), and chromone derivatives showed efficacy through cholinesterase inhibition, Aβ aggregation blockade, and metal chelation with BBB permeability.92–94
Transgenic zebrafish expressing human APP variants replicate hallmark Aβ deposition, cerebrovascular pathology, and behavioural phenotypes. Advanced nanocarriers, including PAMAM dendrimers, carbon dots, exosome-mimetic liposomes, and mesenchymal stem cell-derived vesicles, enable targeted CNS delivery of curcumin, memantine, and RNAi-based therapeutics with reduced systemic toxicity.95 Platforms like LCSF-NR siRNA nanorings effectively silence BACE1 and reduce amyloidogenesis in vivo. Imaging breakthroughs using HBT-PON, PRS, and ESIPT-based fluorescent probes allow real-time tracking of oxidative stress, neuroinflammation, and trace metal accumulation in the zebrafish brain.96 Gene-editing tools such as the PINE-CONE prime editor facilitate SNP-specific modeling of AD risk alleles, while systems-level analyses have revealed early mechanistic events such as serotonin-linked neuroinflammation, oligodendrocyte dysregulation, and ferroptosis.97 Combined AD-comorbidity models incorporating osteoporosis or metal toxicity allow for synergistic drug screening with agents like notopterol and osthole, while gut-brain axis studies highlight microbial shifts induced by Aβ accumulation.98
Parkinson's: Zebrafish possess a dopaminergic system analogous to humans, including tyrosine hydroxylase-positive neurons, enabling modeling of substantia nigra-like degeneration. Zebrafish also exhibit a functional blood-brain barrier, enabling translational screening of CNS-active drugs and nanoparticle delivery systems. Neurotoxin-based models using MPTP, rotenone, 6-OHDA, and manganese reliably induce mitochondrial dysfunction, oxidative stress, and dopaminergic cell loss, mirroring hallmark PD pathology.72,99 Genetic models targeting pink1, parkin, dj-1, or lrrk2 further recapitulate mitophagy deficits and proteostatic imbalance observed in familial PD.100,101 Transparent larvae enable live imaging of neurodegeneration, reactive oxygen species (ROS), and neuronal apoptosis using biosensors and fluorescent dyes. Behaviourally, zebrafish display PD-relevant phenotypes including bradykinesia, postural instability, and anxiety-like responses, assessable through high-throughput locomotor and reflex assays.102 Natural compounds like Naringenin and hesperidin have shown neuroprotective potential in the 6-OHDA zebrafish model by downregulating LRRK2, gsk3β.103,104 Celastrus paniculatus extract reversed motor and memory deficits and restored DJ-1 and LRRK2 expression in rotenone-induced models.105 Synthetic agents such as salicylaldehyde, benzoylhydrazone, and Granulathiazole A effectively counter ferroptosis and ROS-mediated damage via Nrf2/HO-1 pathway activation.106,107 Nanocarrier systems, including DNA tetrahedral nanocages, have facilitated targeted dopamine delivery, enhancing neuronal recovery.108
Epilepsy: Zebrafish central nervous system shares conserved neurotransmitter systems with mammals, including GABAergic, glutamatergic, and dopaminergic circuits. Chemically induced seizure models using agents like pentylenetetrazol (PTZ), kainic acid, or pilocarpine reliably reproduce electrographic and behavioural seizure phenotypes, including hyperlocomotion, head twitching, and convulsive swim bursts. These are quantifiable using automated tracking systems, enabling high-throughput screening of both synthetic and natural anticonvulsants. Mutants like scn1lab, kcna1a, and pcdh19 show spontaneous seizures and hyperexcitability, mimicking syndromes such as Dravet and EA1 syndrome, while CRISPR/Cas9 tools now allow precise modeling of human epilepsy-linked mutations.109–112 High-throughput drug development is made possible using adult fish to test new compounds and investigate pharmaco-resistant seizures. This also encourages the use of zebrafish in personalized medicine.113 Live calcium imaging and optogenetics in transparent zebrafish larvae further permit real-time visualization of seizure onset and neural hyperexcitability.114 Compounds like valproate, cannabidiol, baicalin, and resveratrol have demonstrated efficacy in zebrafish epilepsy models, validating the system for translational drug discovery.112,115–118
Anxiety: They exhibit homologous neurotransmitter systems, including GABAergic, serotonergic, and dopaminergic pathways, quite closely paralleling those involved in human anxiety disorders.119 Using proven paradigms such as the “novel tank diving test (NTD)”, “open field test (OFT)”, and “light/dark box test (LDT)”, zebrafish exhibit measurable anxiety-like responses, such as thigmotaxis, bottom-dwelling, and light/dark zone preference.120,121 These behaviors are modifiable by known anxiolytics such as SSRIs, benzodiazepines, and herbal extracts, demonstrating high predictive validity. Stress can be induced through environmental (e.g., novelty, isolation) or chemical (e.g., neurotoxicants like benzo [a] pyrene, valproic acid, bisphenol),122 while genetic models targeting anxiety-related genes (e.g., nr4a2) offer insight into molecular mechanisms. Agents like caffeine, a central nervous stimulant and adenosine receptor antagonist, exacerbate bottom-dwelling behaviour and startle responses in the NTD, resembling anxiety phenotypes in adult fish.123 Similarly, MK-801, an NMDA receptor antagonist, elicits erratic swimming and avoidance responses indicative of heightened fear circuitry activation.124 The alarm substance assay, using extracts from injured conspecifics, provides an ethologically relevant anxiety model.125 It has been confirmed that exposure causes freezing and an increase in cortisol in both strains and sexes. According to research, female zebrafish were more active and nervous than males; melatonin caused anxiolytic reactions in both sexes, whereas diazepam only caused anxiolytic reactions in males.126 Another robust inducer is corticotropin-releasing factor (CRF), which activates the hypothalamic-pituitary-inter-renal (HPI) axis and mimics endogenous stress hormone release; CRF-treated zebrafish display sustained thigmotaxis and elevated cortisol.127
Depression: A versatile model for depression research, owing to its conserved monoaminergic systems, including serotonin, dopamine, and norepinephrine pathways that mirror key aspects of human neurobiology. Their functional HPI axis enables quantification of cortisol responses to chronic stressors, facilitating the study of neuroendocrine dysregulation commonly observed in depressive disorders.128 Zebrafish display measurable depression-like behaviors, such as reduced exploration, social withdrawal, and increased bottom-dwelling, assessed through validated paradigms like the novel tank diving test, social preference test, open field test, and light/dark box test. Depression can be experimentally induced via pharmacological agents (e.g., reserpine, bisphenol), environmental stressors (e.g., social isolation, novelty exposure), or genetic modifications targeting mood-regulatory genes like nr4a2, akt3, and gnpda2. These models have high translational relevance, with zebrafish demonstrating robust responses to conventional antidepressants like fluoxetine (SSRIs, SNRIs, tricyclics).129
Zebrafish larvae and adults serve as powerful platforms for xenotransplanting human cancer cells, enabling real-time studies of tumor progression and therapy responses. Advances with CRISPR/Cas9 and transgenic lines model key mutations (TP53, KRAS, BRAF) across cancers, while ZTX and zPDX models offer rapid, scalable, and physiologically relevant alternatives to murine models, aided by optical transparency and immunodeficient lines for high-throughput imaging. In solid tumours such as rhabdomyosarcoma (RMS), the use of Tg (flk1:GFP) zebrafish embryos injected with RMS cells enabled rapid screening of both chemotherapeutics and targeted agents, closely mirroring clinical efficacy.130 In colon cancer, the cannabinoid anandamide (AEA) exerted anti-tumour and anti-angiogenic effects not through direct cytotoxicity on HCT116 cells, but by modulating the zebrafish tumour microenvironment, highlighting the model’s value in dissecting host-tumour interactions.131
In non-small cell lung cancer (NSCLC), a combined mouse-zebrafish PDX approach using cryopreserved tissue predicted drug responses and metastatic dissemination within 3 days, outperforming some clinical tests with 91% sensitivity for lymph node involvement.132 For glioblastoma (GBM), a highly invasive and treatment-resistant malignancy, intracranial zebrafish patient-derived orthotopic xenografts (zPDOX) retained both histological and molecular fidelity to original tumours and allowed individualized drug screening within 20 days. Single-cell RNA sequencing confirmed conservation of zebrafish BBB properties, validating their relevance in neuro-oncology. Paediatric cancer studies, including the INFORM trial, integrated zPDX models with organoids, spheroid cultures, and mouse PDXs to identify actionable drug sensitivities and effective therapeutic combinations in relapsed, heterogeneous tumours.133 In hematologic malignancies, SCID zebrafish lines have enabled engraftment and drug screening of chronic myelogenous leukaemia (CML) and acute myeloid leukaemia (AML) cells.134 These models demonstrated short-term survival of human hematopoietic cells and validated the in vivo efficacy of agents such as imatinib, cytarabine, azacitidine, and arsenic trioxide. Zebrafish avatars have shown strong clinical alignment in co-clinical trial settings. For instance, in pancreatic ductal adenocarcinoma (PDAC), zPDX responses to chemotherapy accurately predicted relapse and disease-free survival.135 In rectal cancer, zebrafish xenografts subjected to radiotherapy effectively distinguished radiosensitive from radioresistant tumours based on apoptotic and DNA damage markers.136
Advanced platforms such as automated heartbeat quantification systems (e.g., AISS) and wireless ECG telemetry have enabled real-time, anaesthetic-free cardiac assessment in both larvae and adults. CRISPR/Cas9 mutagenesis has facilitated the creation of cardiovascular disease models for functional genomics and therapeutic testing. Zebrafish have been used to evaluate the cardiotoxicity of tyrosine kinase inhibitors like ponatinib.137 Microfluidic chip-based assays now allow precise anti-thrombotic drug testing, while deep learning models support rapid video-based analysis of cardiac rhythms.138 Notably, the ZebraReg platform has identified cardiac regeneration regulators using transgenic lines.139 These models have proven valuable for investigating metabolic regulators like isosteviol and senkyunolide I for lipid modulation and oxidative stress.140 Heart rate (HR) and heart rate variability (HRV), which are markers of cardiac function, are utilized to evaluate drug-induced cardiotoxicity in zebrafish. To better understand disease causes and medication effects, zebrafish models have been created for a number of cardiovascular disorders, including atherosclerosis, congenital heart abnormalities, and dilated cardiomyopathy. HR and HRV in zebrafish models have been measured using zebrafish heartbeat detecting techniques, signal analysis technologies, and reputable commercial software like “Rvlpulse”, “LabVIEW”, and “ZebraLab”.141 Fully automated in vivo picture capture and analysis to extract pertinent cardiovascular data, such as heart rate, arrhythmia, AV blockage, ejection fraction, and blood flow, are made possible by high-throughput screening systems.142 “pyHeart4Fish” is a new Python-based, platform-independent application designed to automatically quantify cardiac chamber-specific metrics in zebrafish embryos, including heart rate, contractility, arrhythmia score, and conduction score.143
Humans and zebrafish have many genetic and physiological traits in common, especially regarding organs involved in metabolic control, such as the liver, adipose tissue, and the pancreas. Microbiome interactions, β-cell regeneration, and AI-driven analytics are now included in the zebrafish diabetes simulation, going beyond traditional glucose control. Zebrafish-microbiome co-models have illuminated how gut dysbiosis contributes to insulin resistance and inflammation, enabling screening of probiotics and symbiotic with anti-diabetic potential. Transgenic zebrafish lines expressing fluorescent reporters for insulin, Akt, and FoxO1 signaling now permit real-time visualization of pancreatic responses to therapeutic agents.144 Furthermore, AI-based imaging tools and microfluidic platforms are increasingly employed to assess islet morphology, glucose uptake, and metabolic flux in high-throughput settings. Zebrafish models effectively mimic human diabetes, including type 2 diabetes and maturity-onset diabetes of the young (MODY), showing similar pancreatic structure and glucose homeostasis mechanisms.145 Zebrafish models of insulin resistance and liver-specific insulin receptor knockdowns are used to study type-2 diabetes. Overnutrition in zebrafish larvae and juveniles can mimic human obesity, activating pathways like mTOR and increasing β-cell mass. Research using zebrafish has identified new therapeutic targets, such as the centromere protein X (CENPX), which, when inhibited, ameliorates hyperglycaemia and upregulates insulin levels.146 They also replicate human lipid metabolism disorders, such as hyperlipidaemia and atherosclerosis, through genetic and dietary manipulations. Zebrafish have a simpler gut microbiota than humans, sharing some bacterial phyla but lacking the diversity and complexity of the human gut. They produce different digestive enzymes, with higher activity of proteases and lipases, affecting nutrient digestion differently. Zebrafish also have a higher metabolic rate due to their smaller size and ectothermic nature. While their lipid metabolism pathways are similar to humans, zebrafish store more fat in the liver and visceral areas rather than subcutaneously.147
Researchers can investigate the underlying processes and find possible therapeutic molecules by modeling hereditary kidney disorders in zebrafish. Zebrafish larvae have substantial similarities to mammalian models in terms of drug metabolism and kidney injury, making them useful models for identifying drug-induced nephrotoxicity. Zebrafish have become an increasingly powerful model for renal disease research and drug screening owing to their conserved kidney structure, genetic tractability, and suitability for high-throughput assays. The zebrafish pronephros mirrors mammalian kidney function, enabling studies of glomerular filtration, tubular processing, and proteinuria, while genetic tools such as CRISPR/Cas9 and transgenic fluorescent reporters facilitate mechanistic insights and screening pipelines. Applications include modeling drug-induced nephrotoxicity (gentamicin, cisplatin), acute kidney injury via nephrotoxin injection or genetic disruption, and genetic kidney disorders such as polycystic kidney disease, with outputs validated against mammalian data.148,149 Automated imaging and multiparametric pipelines now allow parallel assessment of renal morphology, function, and filtration in live larvae, supporting large-scale chemical library screening.150
Despite structural differences, zebrafish livers share significant functional and genetic similarities with human livers, including comparable cytochrome P450 enzyme activity, which is crucial for drug metabolism.151 Zebrafish models are effective for assessing hepatotoxicity by exposing embryos and larvae to compounds, allowing real-time imaging of liver function. Researchers have identified drugs that cause liver damage, showcasing zebrafish's potential for evaluating drug-induced liver toxicity and discovering safer alternatives. Gender-specific variations in hepatotoxic reactions have been seen in zebrafish used to assess the hepatotoxicity of traditional Chinese medications, including Emodin-8-O-β-D-glucopyranoside (Em8G).152 By using genetic models or nutritional modification to create fatty liver phenotypes in zebrafish, non-alcoholic fatty liver disease (NAFLD) may be studied, and drugs that decrease lipid accumulation can be found. Additionally, zebrafish model alcoholic liver disease by exposing them to ethanol, resulting in inflammation and steatosis. Screening various compounds has revealed protective effects against alcohol-induced liver damage, aiding in the search for potential treatments. In parallel, zebrafish models of fatty liver disease (NAFLD/NASH) have recapitulated hallmark human features: hepatic lipid accumulation, ballooning degeneration, ER stress, ferroptosis, and gut-liver axis dysregulation.153 Tools such as Oil Red O staining, RNA-seq, and fluorescent probes (e.g., ONOO-/viscosity dual-channel sensors) have enabled mechanistic tracking and therapeutic evaluation.154
Zebrafish have been identified as highly regenerative, capable of regrowing amputated fins, lesioned brain, retina, spinal cord, and heart. Studies have shown that these cardiomyocytes can dedifferentiate, re-enter the cell cycle, and proliferate to replace lost tissue. This process allows zebrafish to regenerate up to 20% of their ventricular mass within approximately two months post-injury, without scarring or arrhythmias.155 Effective cardiomyocyte repopulation and scar prevention depend on early responses to cardiac injury, such as coronary revascularization aided by VEGFC signaling. Gene expression programs that support cardiomyocyte dedifferentiation and proliferation are driven by changes in chromatin accessibility, which are controlled by transcription factors like AP-1.156,157 They are also used to model liver injury, providing insights into the cellular and molecular mechanisms of liver regeneration with potential applications for human therapies. Studies indicate that within seven days post-surgery, zebrafish can recover their liver mass to normal levels, often exceeding the original size before fine-tuning back to baseline over the following week.158 The regeneration process in zebrafish involves several key signaling pathways. For instance, the BMP and FGF signaling pathways are crucial during the early stages of liver regeneration. Additionally, hormone activity, ribosome biogenesis, and antioxidant activity also play a part in liver regeneration.159 Zebrafish biliary epithelial cells can transdifferentiate into hepatocytes, a process regulated by signaling pathways such as MAPK, PI3K, and mTOR.160
Zebrafish can regenerate parts of their central nervous system, including the retina and spinal cord, facilitated by Müller glia and radial glial cells. Upon retinal injury, Müller glia dedifferentiate, re-enter the cell cycle, and produce neuronal progenitor cells (NPCs) that proliferate and differentiate into the lost neurons. Key factors such as Midkine-a and transcriptional regulators like Bromodomain (Brd).161–163 Zebrafish can regenerate axonal tracts and entire tissues in the CNS, making them a valuable model for studying CNS injuries and diseases. The absence of small leucine-rich proteoglycans (SLRPs), which are known to hinder axon regeneration in mammals, allows zebrafish to recover functional capabilities more effectively after CNS damage.164 Additionally, their fin regeneration model helps researchers understand the stages of tissue regeneration, including wound healing and blastema formation. Zebrafish fin regeneration begins with the formation of a blastema at the injury site, consisting of mesenchymal progenitor cells derived from dedifferentiated osteoblasts and other cell types. A protective wound epithelium forms over the injury, ensuring the undifferentiated state of blastemal cells.165 The regeneration process balances proliferation and differentiation, with osteoblasts migrating to the injury site to form new bone structures. Several signaling pathways, including Neuregulin-1 (NRG1), Retinoic Acid, NF-κB, and Wnt signaling, regulate this process. Ultimately, the regenerated fin restores its size and morphology, influenced by positional memory within blastemal cells, which guides accurate reconstruction.166,167
The zebrafish genome was first sequenced in 2001, and subsequent efforts have refined and expanded this genomic resource. The zebrafish genome is relatively small compared to the human genome, containing approximately 1.7 billion base pairs and an estimated 26,000 genes. Despite its size, the zebrafish genome shares a remarkable degree of synteny with the human genome, with large conserved chromosomal blocks. This synteny allows for the identification of human orthologs in zebrafish, enabling the study of human genes and diseases in a simpler and more accessible model system.168 Zebrafish research has changed in multiple ways since the zebrafish genome sequence became available. First, it has made it easier to identify genes that are involved in a variety of biological processes, including illness, metabolism, and development. Researchers can find zebrafish genes that are like human genes linked to disorders by comparing the genomes of the two species.10 This makes it possible to investigate these genes in zebrafish to learn more about their functions and possible contributions to the pathophysiology of illness.169 Second, the invention of potent genetic tools for modifying zebrafish gene expression has been made possible by the zebrafish genome sequence.10,169 These instruments include RNA interference (RNAi), which may be used to suppress gene expression, and CRISPR-Cas9, which enables accurate and effective genome editing.170 By using these instruments, zebrafish models of a number of human illnesses have been produced, facilitating the investigation of disease processes and the creation of possible treatment approaches.169
The zebrafish genome has been deconstructed using long-read nanopore sequencing technology, revealing 1,697 novel insertions and deletions over one kilobase in length. This enhances the zebrafish's genetic landscape and provides new insights into its genomic architecture. The assembly also improved the structure and organization of the genome by placing 106 previously unlocalized scaffolds. The research also discovered additional sites of retrotransposon integration, suggesting a dynamic genomic landscape. The new assembly provides a more accurate representation of the zebrafish genome, crucial for future research and interspecies comparisons.171 A study highlights the significant role of zebrafish in genetic and genomic research, particularly in relation to human diseases.171 The study of zebrafish has advanced medical research by deepening our understanding of biological processes and disease causation. Zebrafish are now one of the most potent systems for reverse genetics thanks to the advent of sophisticated genetic tools like CRISPR-Cas9, TALEN, and zinc finger nucleases. Zebrafish models have been utilized to develop new gene knockout models for certain illnesses and have demonstrated therapeutic promise in situations such as retinitis pigmentosa and cystinosis.172 Using a particular area of chromosome 4 (Chr4R) as its focus, the study finds distinct gene expression patterns on the zebrafish sex chromosome. It validates a ZZ/ZW chromosomal sex determination scheme, where Chr4R's right arm contains the primary sex locus. Chr4 has four different areas with different gene expression levels, according to an RNA-seq study. Protein-coding genes found in Region-2, a 41.7 Mb portion, are quiet in the ovaries but expressed in the testes. A Maternal-to-Zygotic Transition gene block, Region-2, is identified in the study as having genes that are expressed in the testes during early embryonic development but are silent in the ovaries. The intricacy of gene regulation is shown by the distinctive features of zebrafish sex chromosomes.173 The study shows that wild zebrafish have higher genetic diversity than lab strains, with GC-biased gene conversion present only in the wild. Distinct populations in Nepal and Bangladesh highlight opportunities for population genetics research. Using RAD-seq, the findings emphasize the need to consider genetic variation in laboratory strains.174
The groundbreaking gene editing technique CRISPR-Cas9 approach makes it possible to target and modify certain genes in the zebrafish genome with accuracy and efficiency ( Figure 6).170 Researchers can produce double-stranded breaks in the DNA at the appropriate place by introducing a Cas9 nuclease and a guide RNA (gRNA) complementary to the target gene sequence.175 Gene knockouts or insertions can result from the healing of these breaks by homologous recombination or non-homologous end joining (NHEJ).176 Zebrafish models of several human illnesses, such as cancer, neurological conditions, and metabolic diseases, have been produced using CRISPR-Cas9. For instance, to examine Alzheimer's, Huntington's, and cystic fibrosis in zebrafish, scientists have been using CRISPR-Cas9 to knock out genes linked to these conditions.169 Gene expression can be decreased when siRNAs attach to the target mRNA and cause its breakdown. The role of genes involved in zebrafish development, cell signaling, and illness has been investigated using RNA interference (RNAi). For example, scientists have been using RNAi to silence genes related to angiogenesis, a process essential to the development and spread of tumours.177
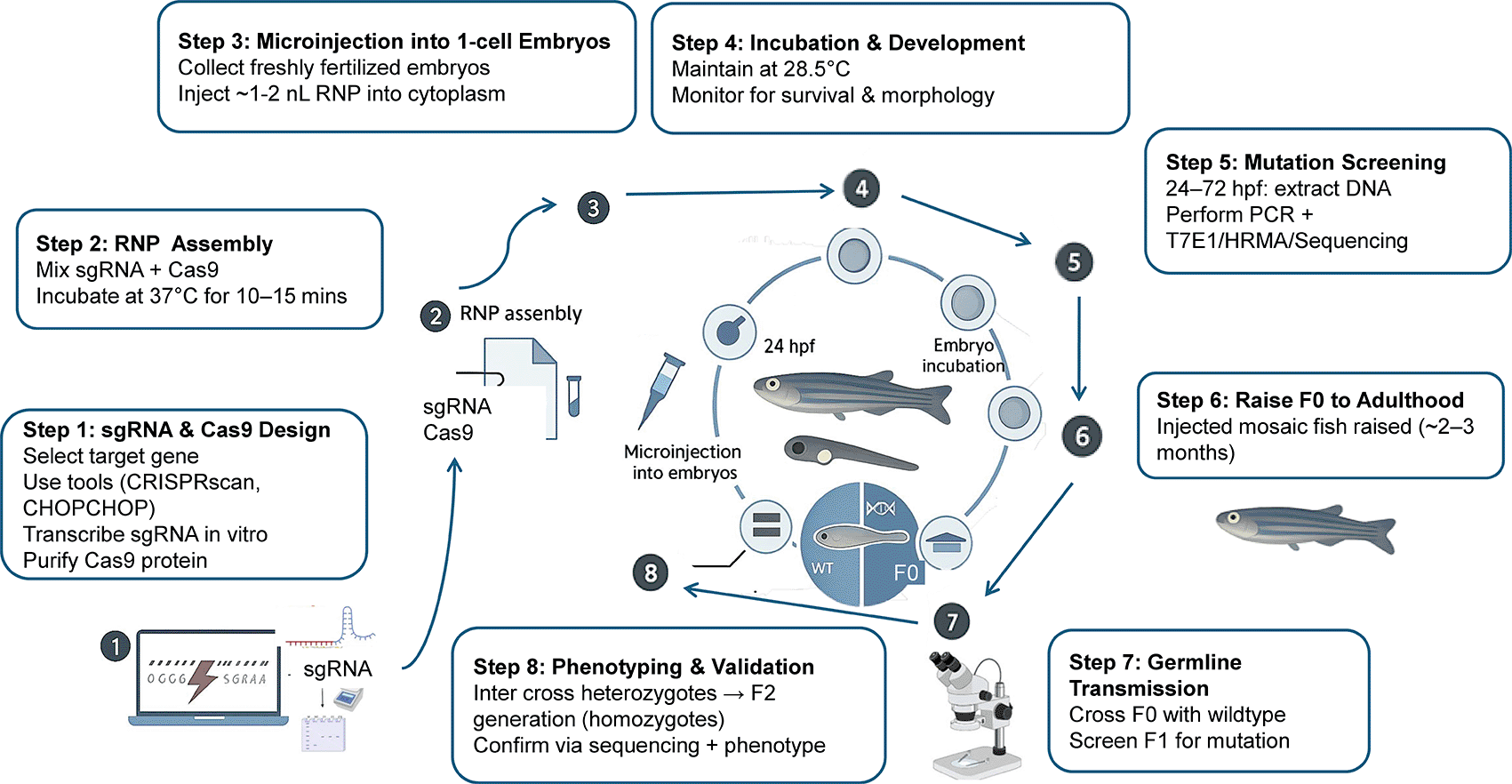
Transgenics zebrafish carry genes that have been artificially introduced into their genome. These genes can be used to study gene function, model human diseases, or produce proteins of interest.178 Transgenic zebrafish can be created using various methods, including microinjection of DNA into fertilized eggs and the use of retroviral vectors.170 Transgenic zebrafish have been used to model various human diseases, including cancer, cystic fibrosis, and Alzheimer's disease.169 For example, researchers have created transgenic zebrafish that overexpress oncogenes or carry mutations in genes implicated in human diseases. These models allow for the study of disease mechanisms and the development of potential therapeutic interventions.179 Table 2 highlights a starting point for researchers interested in using transgenic zebrafish lines. The specific choice of line will depend on the research question being addressed, followed by assessing the advantages and disadvantages of zebrafish, as shown in Figure 7.
Zebrafish are increasingly recognized as a valuable model organism in biomedical research, like traditional mammalian models. As their use expands, regulatory oversight is expected to tighten to ensure animal welfare and scientific integrity across multiple countries ( Table 3). In the United States, existing regulations that apply to mammalian models are likely to be extended to zebrafish. This includes compliance with the Animal Welfare Act and guidelines from Institutional Animal Care and Use Committees (IACUCs).180 A panel discussion at the “World Aquaculture Society's Aquaculture America 2009 Conference” highlighted the need for sensible regulatory guidelines that promote high standards of animal care while facilitating quality research.181 Recommendations were made to develop new guidelines tailored to zebrafish research. The “Organization for Economic Co-operation and Development (OECD)” has established guidelines for the use of zebrafish in toxicity testing. For instance, OECD Test Guideline 236 focuses on fish embryos and aims to provide predictive data similar to that obtained from adult fish, aligning with the “Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH)” regulation in the EU.182 There is a disconnect between zebrafish researchers and regulatory agents, primarily due to the latter's familiarity with mammalian species rather than aquatic organisms. This gap can hinder the development of effective regulations that ensure fish welfare while promoting scientific innovation.
As zebrafish continue to gain traction across biomedical disciplines, emerging innovations such as humanized lines, organoid co-cultures, and gnotobiotic models are expanding their relevance in immunotherapy, infectious diseases, and microbiome research. Integration of advanced tools, including single-cell omics, spatial proteomics, optogenetics, and in vivo imaging, has positioned zebrafish to bridge molecular mechanisms with functional outcomes in cancer, neurodegeneration, and cardiometabolic disease. Collectively, these advances establish zebrafish not merely as model organisms but as versatile, next-generation platforms for high-throughput, integrative, and personalized biomedical research.
The authors express their gratitude to the Manipal Academy of Higher Education, Manipal, India, for awarding the TMA Pai Fellowship to Sandra K.S. and Megh Pravin Vithalkar. They also acknowledge the Anusandhan National Research Foundation (ANRF), New Delhi, India, for granting a Junior Research Fellowship to Beere Vishnusai (CRG/2022/004904).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Partly
Are the conclusions drawn appropriate in the context of the current research literature?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental Toxicology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 01 Dec 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)