The effective management of chronic diseases necessitates innovative approaches that go above traditional healthcare models, supporting sustained patient engagement and adherence. Gamification, as discussed in previous sections, provides an effective approach for achieving these goals by incorporating motivational design concepts into health interventions. This section presents a comprehensive VHC Gamification Framework, an interactive design conceptualised to prepare for system implementation, though not yet functional. This framework specifically designed for integration within a Virtual Health Coach (VHC) system. The proposed framework builds upon the theoretical foundations discussed in Section 2, particularly SDT, BE, and HBM, to create a robust and adaptable system capable of addressing the multifaceted challenges inherent in chronic disease management.
The design prioritises patient-centred care, aiming to transform routine health tasks into engaging and rewarding experiences, thereby enhancing intrinsic motivation and facilitating positive behavioural change.
The VHC framework is designed to provide a structured yet adaptable blueprint for VHC systems, ensuring that gamified elements are not merely superficial additions but are deeply integrated to drive significant health outcomes. An overview of the VHC framework’s interactive user interface design is depicted in
Figure 1.
5.1 Core elements of the VHC gamification framework
The proposed VHC gamification framework comprises several interconnected core elements, each designed to utilise psychological principles to enhance patient engagement and adherence. These elements do not operate independently; instead, they constitute a cohesive system that collectively supports a dynamic and adaptive user experience. The framework adopts a modular structure, enabling it to be tailored and adjusted to accommodate different chronic conditions and the specific needs of individual patients. These core elements were derived through a synthesis of the motivational theories discussed in section 2 and the evidence reviewed in section 3 and are presented as provisional design constructs. Their refinement will be guided by co-design activities with clinicians and patients—such as workshops, surveys, and focus groups—to ensure condition-specific relevance and contextual validity, as elaborated in section 5.2 and revisited in the evaluation plan in section 6.
5.1.1 Adaptive challenges
Adaptive challenges form a cornerstone of the VHC gamification framework, ensuring that goals and tasks remain optimally challenging and motivating for each patient. This element directly addresses the SDT principle of competence, where individuals are more engaged when tasks are neither too easy nor too difficult. The system dynamically adjusts goal-setting based on real-time patient data, including biometric readings, activity levels, and adherence metrics. For instance, a patient consistently meeting their daily step count might receive a slightly increased target, while a patient struggling with medication adherence could be presented with smaller, more achievable interim goals. This continuous recalibration prevents disengagement due to either boredom or frustration, maintaining a reasonable state of ‘flow’ where patients are immersed, engaged and motivated with the app.
The framework’s ability to personalise challenges ensures that each patient’s journey is tailored to their evolving capabilities and health status, fostering a sustained sense of accomplishment and progress. This adaptive mechanism also aligns with the Health Belief Model by reducing perceived barriers to action, as tasks are always within the patient’s achievable range, thereby boosting self-efficacy.
Figure 2 illustrates an example of the adaptive challenge interface designed for the VHC framework. The interface presents three concurrent challenges (daily steps, medication adherence, and heart-rate–zone training), each showing the current difficulty band, progress, time remaining, streak, and an Adaptive Insight that explains any automatic adjustment. Beneath, the Adaptive Intelligence layer summarises how goals are tuned: real-time adjustment from behavioural telemetry; smart difficulty scaling to avoid plateaus or burnout; biometric integration that ingests psychophysiological readings—heart rate and heart-rate variability, resting heart rate, sleep duration/quality, and activity load—to enforce safety and calibrate effort; and temporal adaptation to align tasks with individual routines and energy profiles. Finally, the Data Collection → AI Analysis → Dynamic Adjustment pipeline indicates how continuous data streams inform personalised targets (e.g., step goals rise when recovery markers such as HRV and sleep are favourable, and ease when elevated resting HR or poor sleep signals fatigue; cardio zones are updated from recent heart-rate profiles; medication prompts respect circadian and adherence patterns).
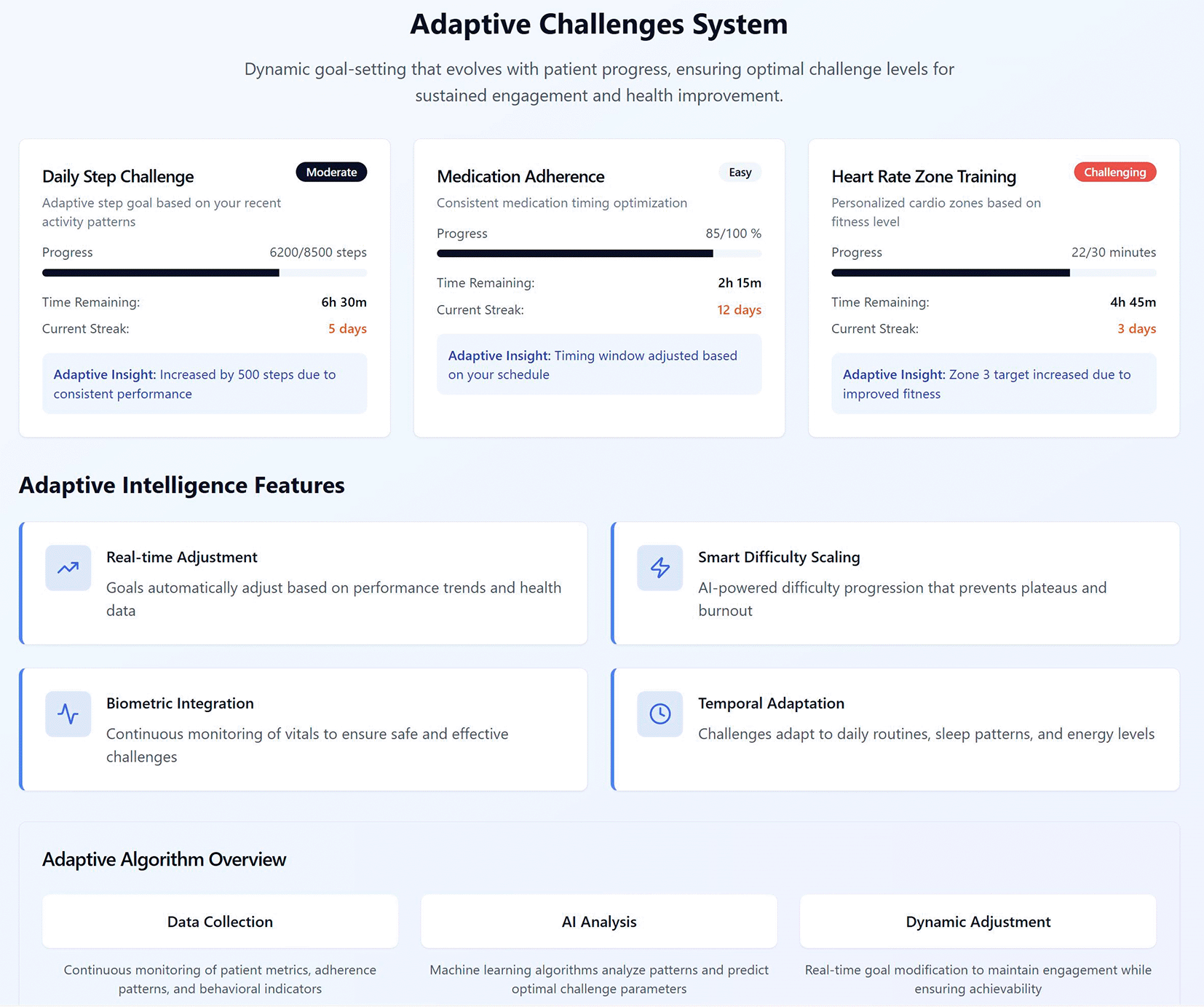
Figure 2. Adaptive challenge interface design within the VHC framework.
5.1.2 Rewards and incentives
Rewards and incentives are integral to the framework, primarily drawing upon principles from BE to reinforce positive health behaviours. The system incorporates a diverse range of incentives, including points, badges, and milestones, awarded for actions such as consistent medication adherence, achievement of exercise routines, or successful completion of educational modules. Points can serve as a virtual currency, redeemable for in-app benefits or recognition, while badges provide symbolic representations of achievement, fostering a sense of mastery and progress.
Milestones, marking significant achievements or sustained behavioural changes, offer larger, more impactful rewards, reinforcing long-term engagement. The design of these incentives considers the BE concept of present bias, where immediate rewards are often more motivating than delayed, larger benefits. By providing instant feedback and tangible (albeit virtual) rewards, the framework leverages this bias to encourage consistent engagement. Furthermore, the strategic implementation of loss aversion, as discussed in Section 2, can be employed by framing certain rewards as potentially lost if adherence falters, thereby increasing motivation to maintain positive behaviours.
Figure 3 illustrates how the VHC operationalises rewards and recognition to reinforce adherence and activity while personalising incentives to patient profiles. The header band summarises a user’s progression—total points, current level, badges earned, day streak, and progress to next level—with tabs to switch between Points, Badges, and Milestones. The Points view shows a timestamped Recent Points Activity ledger (e.g., exercise completed, medication taken, quiz finished), while Incentive Programmes outline configurable schemes such as Weekly Challenges, Adherence Rewards, and Social Impact. The lower panel displays the Badges gallery, with condition-relevant titles, brief criteria, tiering (Bronze/Silver/Gold/Platinum), and associated point values. Badge rules and point weightings are personalised from patient personas and profiles captured at onboarding (e.g., adherence-focused, activity-builder, community-oriented) and linked to the clinical profile (condition, regimen, goals), so the system emphasises badges most salient to each user (e.g., “Medicine Pro” for adherence, “Heart Hero” for cardio targets, “Social Butterfly” for community support). Awarded badges are written to the user profile (with privacy controls) and can surface across social spaces and clinician dashboards, enabling recognition, role eligibility (e.g., mentor/champion), and condition-specific progression without over-reliance on generic rewards.
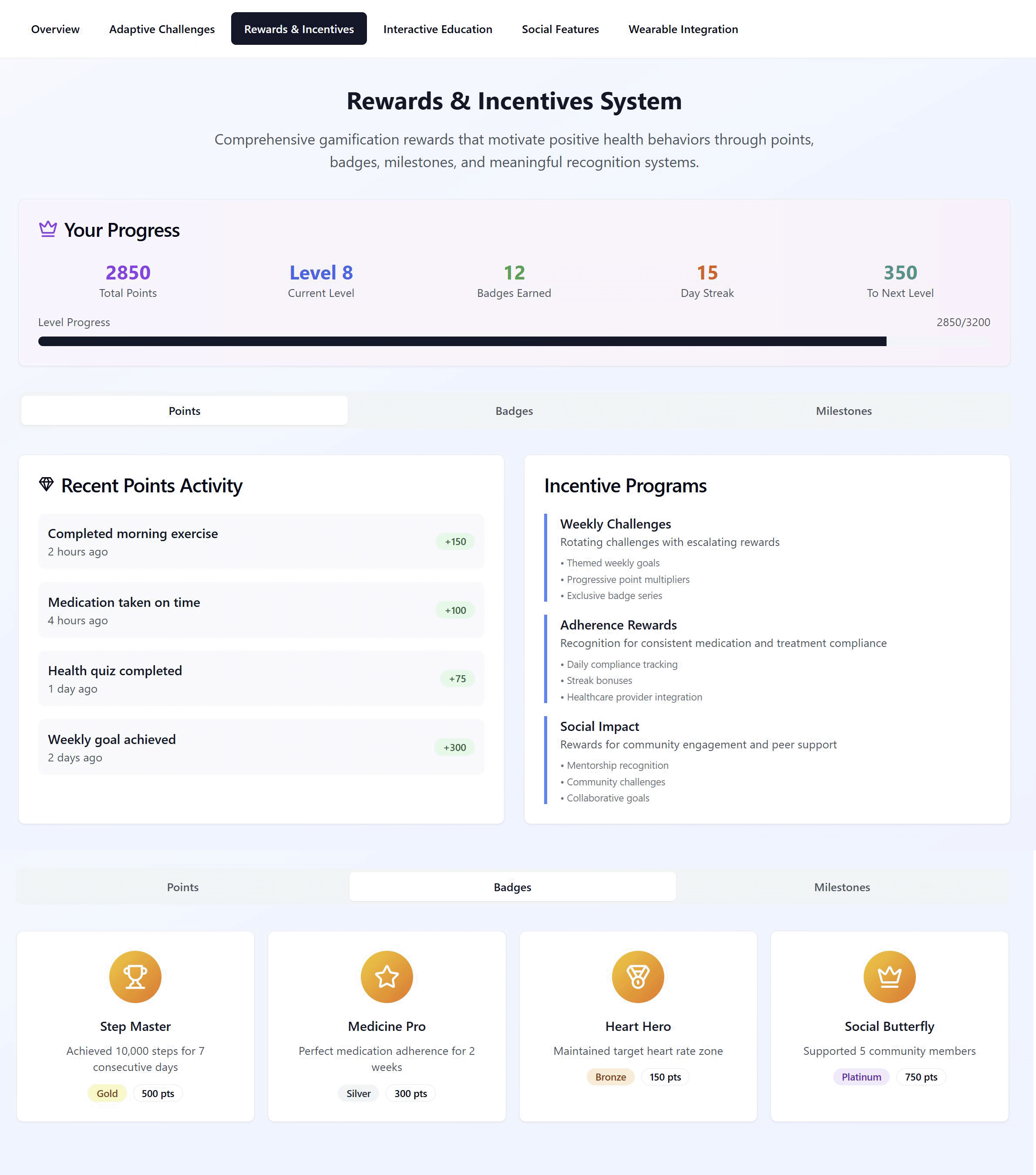
Figure 3. VHC framework’s rewards system design.
5.1.3 Interactive education
Interactive education within the VHC gamification framework transforms passive information consumption into an engaging learning experience. This element directly supports the HBM by enhancing perceived benefits of action and reducing perceived barriers through increased health literacy. Instead of static text or generic videos, the framework employs engaging tools such as quizzes, interactive simulations, and scenario-based learning modules to explain treatment options, disease progression, and lifestyle modifications. For example, a patient with diabetes might navigate a virtual environment to understand the impact of different food choices on blood glucose levels, receiving immediate feedback on dietary choices. Comparable modules can be tailored to other clinical contexts. For neurological conditions such as multiple sclerosis, a fatigue-pacing exercise can ask users to plan a day’s activities under fluctuating energy and heat sensitivity, providing immediate feedback on symptom-flare risk and the effectiveness of pacing strategies. For endocrine disorders, a medication-timing simulation (e.g., for levothyroxine) can demonstrate how dosing in relation to meals or supplements affects hormone control and symptoms, prompting users to establish and rehearse practical routines approved by clinicians. This active learning approach not only improves knowledge retention but also fosters a deeper understanding of their condition and the rationale behind recommended behaviours. By making complex health information accessible and enjoyable, interactive education enables patients to make informed decisions and actively participate in their self-management, thereby boosting their self-efficacy and confidence in managing their chronic condition.
Figure 4 provides a view of an interactive educational module designed for the VHC framework. The module presents a personalised Learning Journey summary (courses enrolled, average completion, modules completed, learning points), followed by a catalogue of Available Learning Modules with type labels (interactive course, video series, interactive guide, adaptive learning), duration, number of modules, difficulty level, and current progress. A live interactive question panel demonstrates immediate correctness feedback with a short rationale, while the Advanced Learning Tools section highlights three engines: AI-powered assessments that adapt item difficulty and sequencing, provide real-time scoring, and identify knowledge gaps; interactive simulations for practising management decisions; and gamified learning paths that unlock content and track progress. Outputs from the AI assessments update the learner model and user profile, which in turn personalise subsequent modules, set appropriate difficulty, recommend next topics, and allocate learning points—ensuring that educational content is tuned to the individual’s needs and can inform tailoring elsewhere in the VHC.
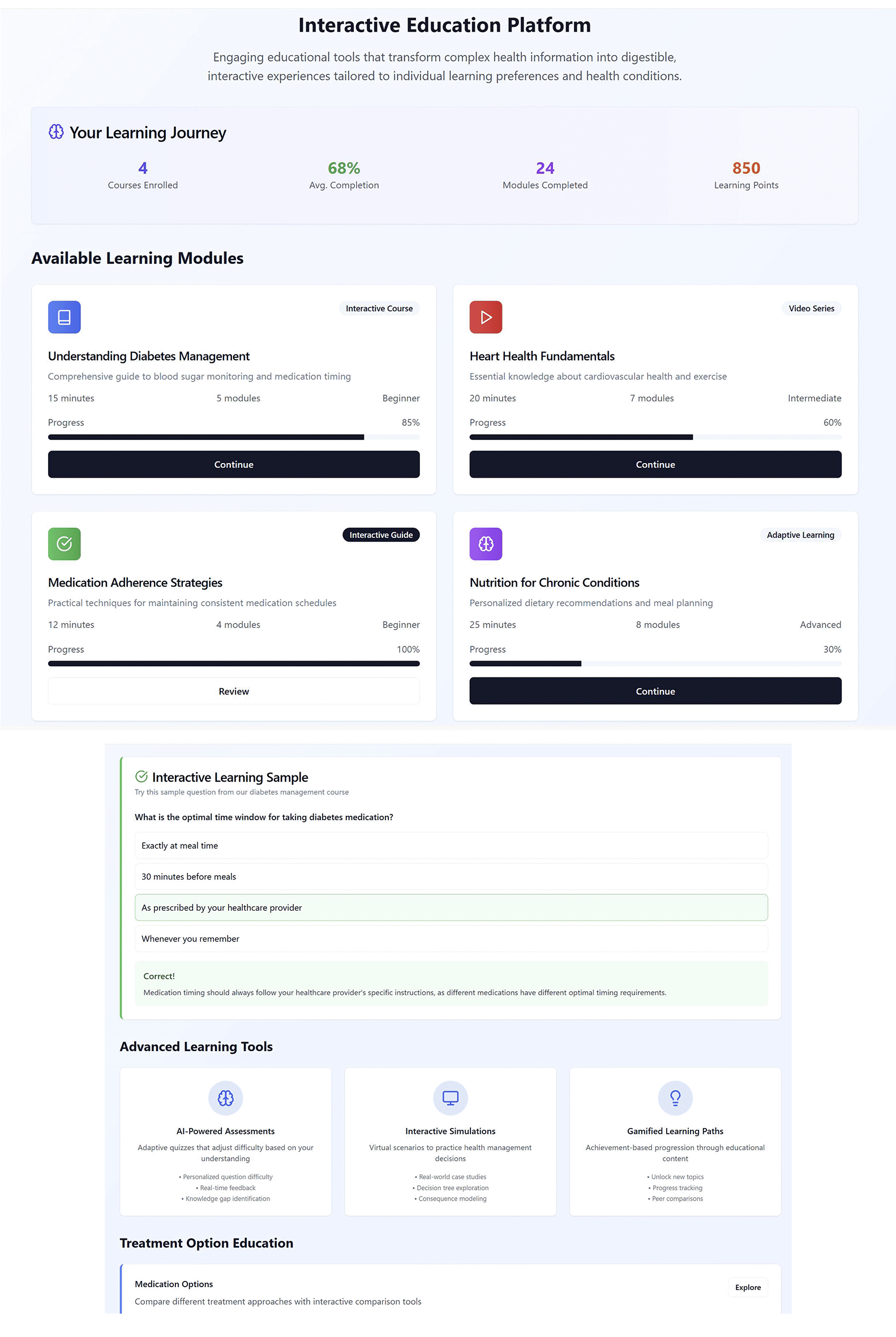
Figure 4. View of the interactive educational module designed for the VHC framework.
5.1.4 Social features
Social features are integrated into the VHC gamification framework to employ peer-based motivation and support, aligning with the SDT principle of relatedness and aspects of Behavioural Economics. The framework facilitates social interaction through shared challenges, leaderboards, and community forums, allowing patients to connect with peers facing similar health challenges. Shared challenges encourage collaborative goal attainment, fostering a sense of camaraderie and mutual support. Leaderboards, while potentially competitive, can also serve as a source of inspiration and recognition, motivating individuals to improve their performance. Community forums—including closed or private patient groups on external platforms—provide a safe space for patients to share experiences, offer advice, and celebrate successes, creating a supportive ecosystem that reinforces positive behaviours. The social comparison inherent in these features can also leverage BE principles, such as social norms and peer influence, to encourage adherence. However, careful design is necessary to mitigate potential negative effects of competition, ensuring that the focus remains on collective well-being and individual progress rather than solely on comparative performance. These considerations are particularly salient for younger, digitally literate cohorts whose disease stability is pharmacologically maintained yet symptoms persist. Many already draw on closed online peer groups for practical advice and emotional support. Within the VHC, such communities are treated as optional, patient-controlled extensions: users may link participation from approved closed groups (e.g., sharing milestones or receiving prompts) while protecting identifiable data. Moderation, signposting to evidence-based resources, and lightweight misinformation safeguards (e.g., clinician-curated FAQs and content flags) are incorporated to preserve psychological safety.
Figure 5 illustrates the VHC Social Features & Community module. The module opens with a Community Overview that gives a pulse of activity—active members, support groups, active challenges, and weekly interactions—and a tab bar to switch between Community, Challenges, and Leaderboard views. The main pane lists Community Members with role labels (e.g., Mentor, Champion), condition tags, level, last activity, cumulative points, streaks, and helpful-vote counts, making peer expertise and engagement visible at a glance. To the right, three feature cards summarise the social tools: Peer Support Groups (condition-specific, moderated discussions with optional expert Q&As), Collaborative Challenges (team goal-setting, shared progress tracking, and team rewards), and a Mentorship Programme (mentor matching, experience sharing, recognition). A Community Feed at the bottom surfaces achievements and tips with timestamps and simple reactions, supporting lightweight participation and recognition alongside deeper group or challenge activity.
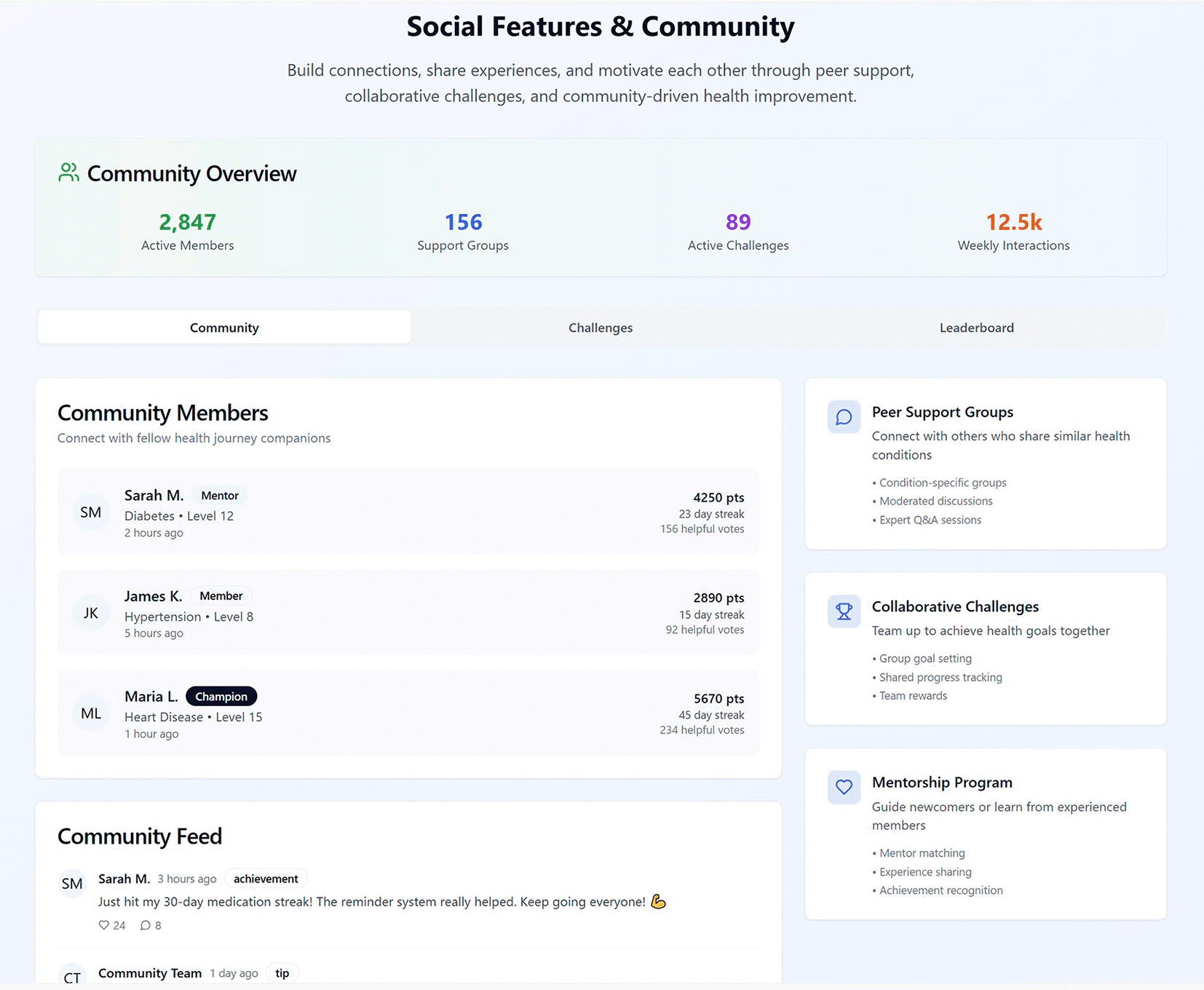
Figure 5. Social feature designed for the VHC framework.
5.1.5 Wearable integration
Wearable integration is a critical component of the VHC gamification framework, enabling the collection of real-time patient data to provide context-aware feedback and personalise the gamified experience. This element underpins the adaptive nature of the challenges and the relevance of rewards, drawing on the immediate feedback mechanisms highlighted in Section 2. By seamlessly integrating with wearable devices (e.g., smartwatches, fitness trackers), the VHC system can automatically track metrics such as heart rate, sleep patterns, activity levels, and even medication adherence (where applicable).
This continuous data stream allows the system to provide immediate, personalised feedback, such as congratulating a patient on reaching their daily step goal or prompting them to take their medication. The real-time data also informs the dynamic adjustment of challenges, ensuring that the gamified experience remains relevant and responsive to the patient’s current health status and progress. This integration not only automates data collection, reducing patient burden, but also enhances the perceived accuracy and relevance of the gamified interventions, thereby strengthening patient trust and engagement.
Figure 6 illustrates how wearable data is presented in the VHC framework design. The Wearable Integration System shows that the VHC can connect to multiple possible sources, not that a patient would wear two watches at once. The Connected Devices panel lists whichever integrations a user could link—e.g., a Fitbit Sense 2, an Apple Watch, or the iPhone Health app—so patients can use what they already own and switch devices over time. In practice, the VHC designates one primary source per signal and de-duplicates overlaps (e.g., heart-rate/ECG from Apple Watch, steps from Fitbit, medications from the Health app), while displaying connection status, last sync and battery.
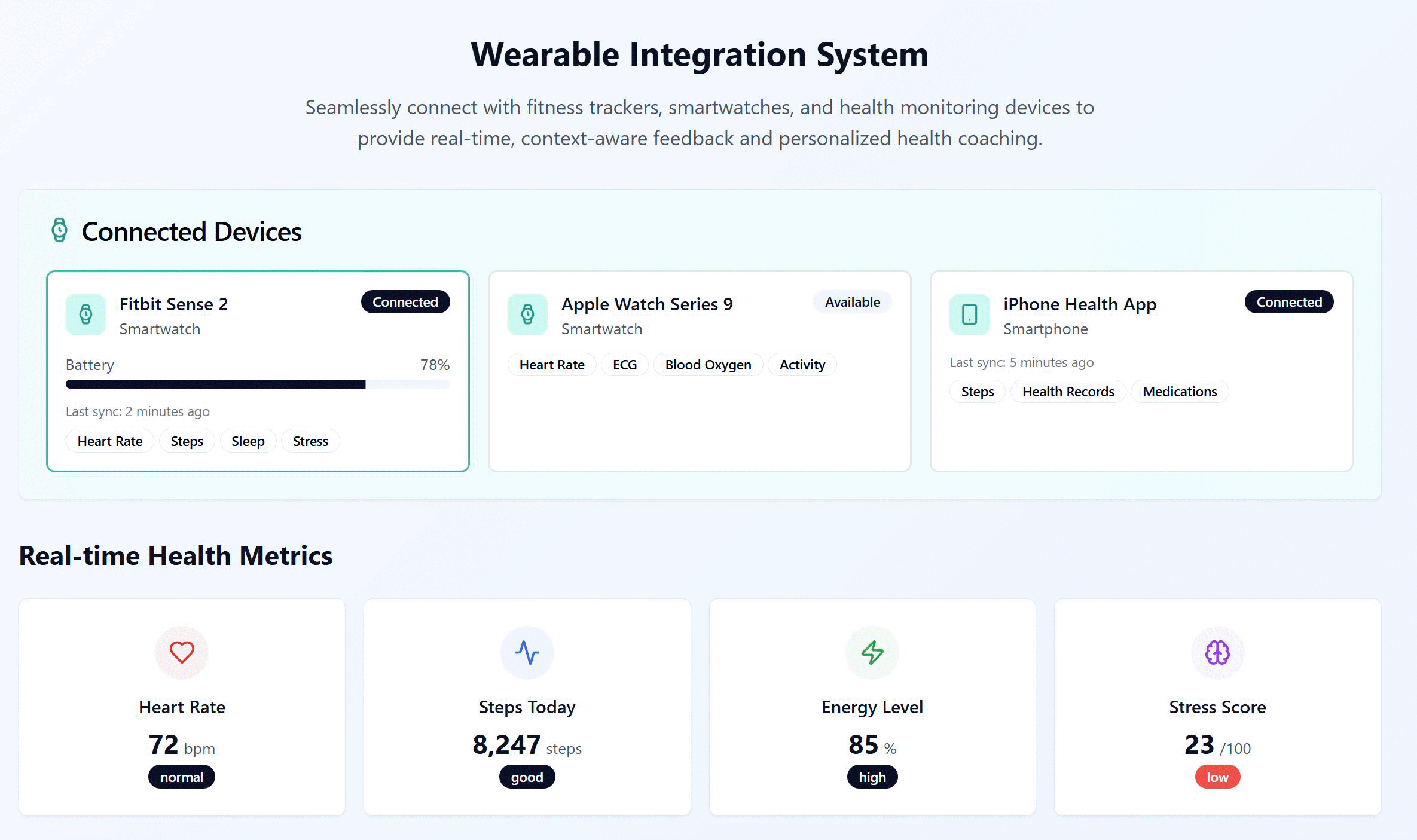
Figure 6. Wearable data presentation in the VHC framework design.
Beneath, Real-time Health Metrics tiles translate incoming data into interpretable summaries: Heart Rate with a status band, Steps Today, an Energy Level estimate (informed by sleep quality, HRV and resting HR), and a Stress Score (largely HRV-derived). Together these views make interoperability explicit and convert psychophysiological streams into actionable, safety-aware feedback for adaptive coaching.
The aforementioned core elements collectively form a cohesive VHC Gamification Framework, designed to provide a holistic and engaging experience for patients managing chronic conditions. This framework is not simply a collection of different features but an integrated system where each component reinforces the others, creating a powerful motivational loop. For instance, data from wearable devices would update adaptive challenges; successful completion would lead to rewards; and progress could be contextualised through interactive education and, if desired, shared via social features. Our approach addresses multiple psychological needs and behavioural drivers simultaneously in preparation for system implementation.
Table 5 maps these VHC framework features to the core theoretical constructs that informed our design.
Table 5. Mapping VHC framework features (Trackwise-Designed) to core gamification theories.
| VHC Framework Feature (Trackwise-Designed) | Primary Theoretical Alignment (SDT, BE, HBM) | Specific Theoretical Construct(s) Addressed | Intended Impact on User Motivation/Behaviour | Example User-Facing Manifestation in VHC Framework Design |
|---|
| Adaptive Challenges | SDT, HBM, BE | Competence, Autonomy, Optimal Challenge (Flow), Reduced Perceived Barriers, Self-Efficacy, Sustained Motivation | Builds confidence through mastery; ensures tasks are engaging; makes goals feel achievable; maintains interest by avoiding stagnation or frustration. | User receives an adjusted daily step goal based on the previous week’s performance; the difficulty of a dietary quiz escalates as the user demonstrates enhanced knowledge. |
| Rewards and Incentives | BE, SDT, HBM | Immediate Reinforcement, Present Bias, Competence Feedback, Status Incentives, Cues to Action, Perceived Benefits | Provides immediate positive feedback; makes progress tangible; reinforces desired behaviours; signals mastery of tasks. | User earns points for logging medication punctually; unlocks a badge for a 7-day activity streak; achieves a milestone for consistent blood glucose monitoring. |
| Interactive Education | HBM, SDT | Perceived Severity, Perceived Benefits, Perceived Barriers, Cues to Action, Self-Efficacy, Knowledge, Autonomy | Increases understanding of condition and treatment; clarifies benefits of adherence; reduces fear/misconceptions; empowers informed choices. | User completes a short, animated module explaining medication mechanisms; engages in a quiz about managing symptoms; accesses tailored tips for healthy eating. |
| Social Features | SDT, BE, HBM | Relatedness, Social Comparison, Peer Competition/Collaboration, Social Norms, Social Cues | Fosters a sense of community and support; motivates through friendly competition or shared goals; normalises healthy behaviours. | User participates in an optional team-based step challenge; (anonymously) observes how their activity compares to similar users; shares an achievement badge with a designated support group. |
| Wearable Integration | Enabling feature for all theories | Data for Personalisation, Real-Time Feedback, Objective Progress Tracking, Context-Aware Cues | Provides objective data for adapting challenges; enables timely and relevant feedback; makes progress visible; triggers context-specific reminders. | VHC framework utilises smartwatch data to suggest a brief walk during a prolonged sedentary period; displays a graph illustrating improved sleep quality following adherence to a bedtime routine. |
The synergistic interplay ensures that the VHC system can continuously adapt to individual patient needs, preferences, and progress, thereby maximising its efficacy in supporting long-term adherence and improved health outcomes. The framework’s iterative nature, based on continuous data feedback and adaptive adjustments, positions it as a dynamic tool for chronic disease management, moving beyond static interventions to personalised care. This integrated approach aligns with the comprehensive nature of CCM, as introduced in Section 1, by providing a structured yet flexible system for patient support and engagement.
5.3 System architecture for TrackWise
The successful deployment of the VHC Gamification Framework within a real-world clinical setting necessitates a robust and scalable system architecture. For TrackWise, the architecture is envisioned as a multi-layered system, ensuring secure data handling, seamless integration with existing healthcare infrastructure, and a responsive user experience. At the foundational layer, secure data acquisition from wearable devices and patient input forms the basis for all subsequent operations. This data is then processed and analysed by an intelligent backend, which incorporates algorithms for adaptive challenge generation, reward allocation, and educational content delivery. This backend also facilitates the social features, managing peer interactions and community functionalities. A critical component of the architecture is its interoperability with Electronic Health Records (EHRs) and other clinical systems, enabling the seamless exchange of patient data and ensuring that the VHC system complements, rather than complicates, existing clinical workflows. The user-facing application, accessible via mobile devices or web browsers, provides an intuitive and engaging interface for patients to interact with the gamified elements. Security and privacy protocols are paramount, adhering to stringent healthcare data regulations to protect sensitive patient information. The modular design of the architecture allows for future expansion and integration of new technologies, ensuring the long-term viability and effectiveness of the VHC Gamification Framework within the Trackwise ecosystem. This architectural design directly supports the practical implementation of the theoretical principles outlined in Section 2, translating abstract concepts into a functional and impactful digital health solution.
To support the dynamic and personalised nature of the VHC framework’s interactive design, we planned a robust and scalable system architecture within the Trackwise project, in preparation for implementation. A conceptual overview of this planned architecture is presented in
Figure 7.
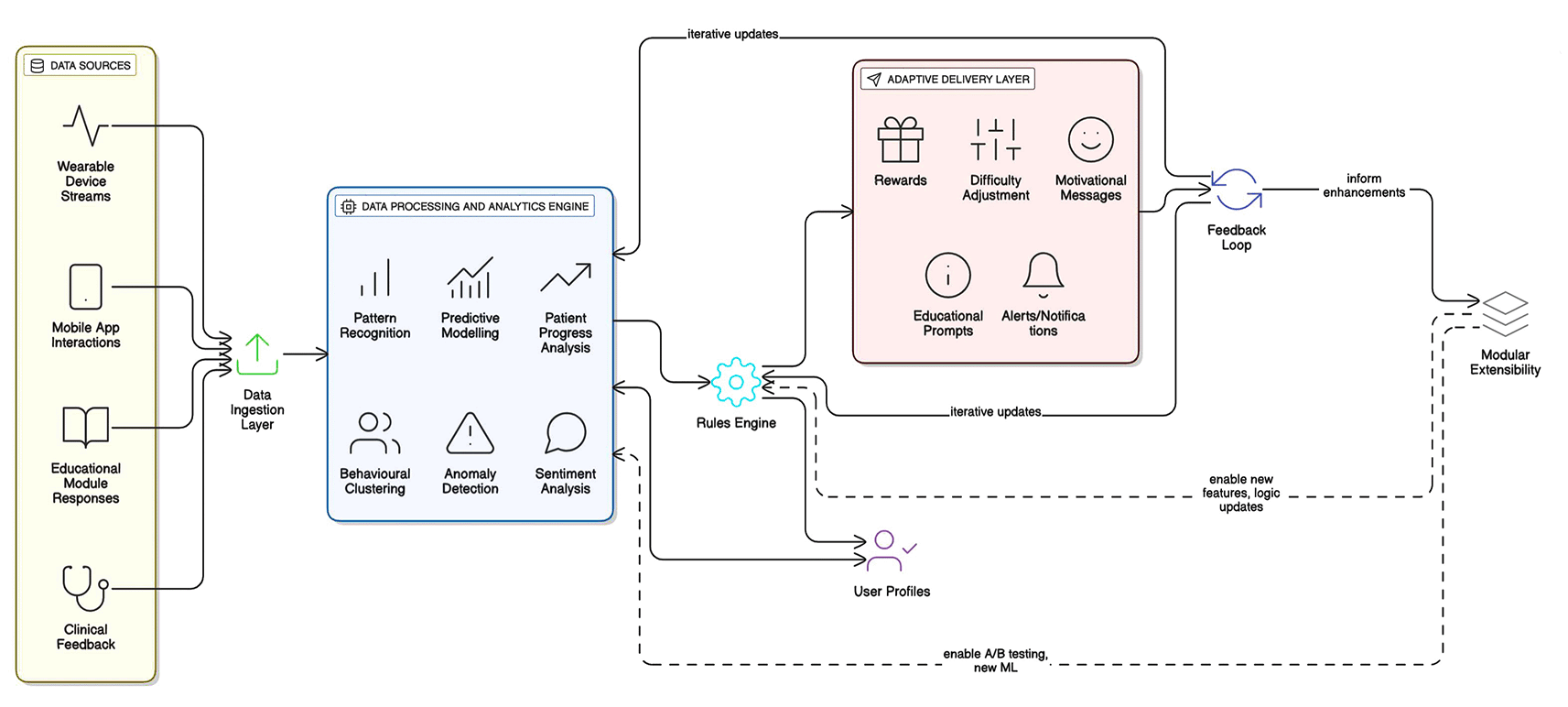
Figure 7. Conceptual Architecture of the VHC framework.
The proposed architecture comprises several key components. A data ingestion layer would be responsible for collecting real-time information from multiple sources, including user interactions with the eventual mobile application, responses to educational modules, and continuous data streams from integrated wearable devices. This raw data would feed into a data processing and analytics engine. Here, algorithms would analyse patient progress, identify patterns, and trigger adaptations to challenges and educational content. This engine is crucial for implementing the ‘Adaptive Challenges’ feature effectively and for personalising the user experience.
A core component of the planned system is the rules engine, which would embody the logic derived from SDT, BE, and HBM. This engine would determine how and when to deliver rewards, adjust difficulty levels, and provide specific educational prompts or motivational messages based on the user’s profile, progress, and engagement patterns. User profiles would be maintained, storing not only health data and progress but also inferred preferences and motivational states, allowing for increasingly refined personalisation over time. The system is designed to support rapid feedback loops, ensuring that rewards, alerts, and educational content are delivered in a timely manner to maximise their impact, a principle highlighted in Section 2. The architecture is conceived as modular, facilitating future updates, A/B testing of new features, and the integration of more advanced machine learning capabilities for predictive analytics and enhanced personalisation. This iterative design approach, incorporating potential feedback from clinicians, is considered vital for the long-term efficacy and relevance of the VHC once implemented.






Comments on this article Comments (0)