Keywords
Enterobacter cloacae, risk factors, antimicrobial resistance, multidrug resistance, hospitalized patients.
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
Enterobacter cloacae is considered an important hospital-acquired pathogen associated with bloodstream, respiratory, and wound infections. Its increasing ability to acquire multidrug-resistant (MDR) traits poses a serious challenge for both infection control and therapeutic management. This study aimed to determine the antimicrobial resistance profiles, prevalence of MDR, and associated clinical risk factors of E. cloacae isolated from hospitalized patients.
Four hundred clinical specimens such as blood, pus, and sputum were collected between July 2024 and July 2025 from hospitalized patients suspected to have a bacterial infection. Risk-factor data were obtained through medical record review. Manual and automated identification with antimicrobial susceptibility testing were performed using the BD Phoenix™ automated microbiology system.
Most infected patients with E. cloacae were male (64%) and aged 60 years or older (56%). Significant risk factors associated with MDR E. cloacae infections included prior antibiotic exposure (p = 0.008), hospitalization longer than 10 days (p = 0.021), and ICU admission (p = 0.034). All isolates (100%) were resistant to ampicillin, amoxicillin–clavulanate, cefazolin, and cefuroxime. Third- and fourth-generation cephalosporins (ceftazidime 70%, cefepime 70%, ceftriaxone 64%), along with ciprofloxacin (44%), levofloxacin (44%), and tigecycline (50%), showed high resistance rates, indicating reduced activity of major antibiotic classes. Imipenem resistance was detected in 34% of isolates, while amikacin (10%), meropenem (10%), and ertapenem (10%) retained good activity. Ceftolozane–tazobactam remained fully effective (100% susceptibility). The overall prevalence of MDR was 86%.
A high prevalence of MDR E. cloacae was identified among hospitalized patients, with strong associations to ICU stay, prolonged hospitalization, and prior antibiotic use. Despite widespread resistance to β-lactams and fluoroquinolones, ceftolozane–tazobactam, amikacin, meropenem, and ertapenem remain effective therapeutic options. The findings emphasize the importance of strengthening local antimicrobial stewardship programs and establishing region-specific surveillance data to guide empirical treatment and support infection-prevention strategies.
Enterobacter cloacae, risk factors, antimicrobial resistance, multidrug resistance, hospitalized patients.
Enterobacter cloacae is a Gram negative opportunistic bacterium that is part of the Enterobacter cloacae complex (ECC) and has developed as being one of the most significant causes of hospital acquired infections worldwide. It is commonly involved in bloodstream, respiratory infections, urinary tract infections, and wound infections, especially in immunocompromised and critically ill patients. The clinical impact of this micoorganism is that it has both intrinsic and acquired antimicrobial resistance and this complicates its treatment and leads to high morbidity and mortality in hospitalized patients.1,2
In recent years, E. cloacae has exhibited a highly serious evolution in resistance to various classes of antibiotics. This is mostly caused by over-expression of chromosomal AmpC β-lactamase, formation of extended-spectrum β-lactamases (ESBLs), and most recently, production of carbapenemases such as KPC and NDM.3,4 These mechanisms confer multidrug resistance (MDR) in Gram-negative pathogens, resulting in broad-spectrum β-lactam resistance such as cephalosporins with carbapenems often being the last treatment option.
It has become a serious international health concern because MDR E. cloacae strains have emerged and spread widely. Various studies have also attributed multidrug resistance to certain clinical and hospital based risk factors such as length of hospital stay, intensive care unit (ICU) admission, prior antibiotic exposure, and the use of invasive medical devices.5,6 These factors acting in synergy and create selective pressure that contributes to the persistence and dissemination of antimicrobial resistant strains in healthcare settings.
Although increasing attention is being paid to E. cloacae as an important nosocomial pathogen, information about its resistance patterns, risk factors, and trends of multidrug resistance is still scarce, especially in developing countries. The emergence of β-lactamase producing strains, such as AmpC or ESBL producers, represents a serious challenge for therapy because early or inaccurate detection often results in inappropriate antibiotic use. Moreover, the impact of clinical factors on MDR E. cloacae such as ICU hospitalization, invasive devices, prolonged hospitalization, and previous antimicrobial exposure infections has not been well defined in local hospital settings.
The absence of region specific surveillance data has prevented the formulation of effective policies, antimicrobial stewardship protocols, and infection prevention measures. Against these gaps, the current study was developed with three main objectives: (i) to assess the prevalence of E. cloacae in major clinical specimens from a tertiary care hospital in Kirkuk, Iraq; (ii) to evaluate the antimicrobial resistance patterns and MDR profiles of the isolates; and (iii) to outline the key patient- and hospital-related risk factors associated with MDR E. cloacae infection. Therefore, the current research aimed to explore the antibiotic resistance characteristics, prevalence of multidrug resistance and clinically significant risk factors of E. cloacae isolates obtained from hospitalized patients.
Four hundred clinical specimens such as blood, pus, and sputum were collected between July 2024 to July 2025 from hospitalized patients who were suspected to have a bacterial infection. The samples were taken under strict aseptic conditions and immediately carried to the microbiology laboratory to be processed. Patients admitted to the hospital with a clinical suspicion of infection and a culture-confirmed E. cloacae isolate were eligible for inclusion. To prevent bias from repeated sampling, only one isolate per patient was used in the analysis. The final dataset excluded cases with incomplete demographic or clinical information, outpatient samples, and surveillance or colonization specimens. Risk factors data of patients were obtained through medical record review. Evaluated risk factors included: patient age, sex, comorbidities (diabetes mellitus, chronic kidney disease, malignancy), recent hospitalization, length of stay, ICU admission, use of invasive medical devices (urinary catheters, central venous catheters, ventilators), prior antibiotic exposure, and immunosuppressive conditions.
Clinical samples (blood, sputum, and pus) were inoculated on MacConkey agar and blood agar plates and incubated aerobically at 37°C for 18–24 hours. The suspected Enterobacter colonies were observed as large, moist, lactose fermenting colonies on MacConkey agar. Gram-negative bacilli morphology was confirmed through Gram staining.
Biochemical tests were done such as oxidase, catalase, citrate utilization, indole production, urease activity, triple sugar iron (TSI) agar reaction, and motility tests. The isolated that showed oxidase-negative, indole-negative, citrate-positive, urease-variable, motile, and producing an acid butt and slant without H2S on TSI agar were identified as E. cloacae.1,2
Final identification of E. cloacae was conducted by using the BD Phoenix™ automated microbiology system (Becton, Dickinson and Company, USA), this medical device incorporates biochemical panels and advanced interpretive algorithms to rapidly and accurately identify Gram-negative bacteria. The system was calibrated and quality-controlled according to the manufacturer’s guidelines to ensure the results’ accuracy and reproducibility. The BD Phoenix™ platform was chosen because it integrates automated biochemical identification with MIC based susceptibility testing and uses standard CLSI breakpoint interpretations. It also demonstrates good agreement with reference methods in routine clinical work. In our laboratory, it is the regular system for Gram negative identification and antimicrobial susceptibility testing, supported by ongoing internal and external quality control checks.
Antimicrobial susceptibility testing (AST) of E. cloacae isolates was conducted by using the BD Phoenix™ automated system. The minimum inhibitory concentrations (MICs) of a wide range of antibiotics from major antimicrobial classes were determined such as β-lactams, carbapenems, aminoglycosides, folate pathway inhibitors, fluoroquinolones and tetracyclines.
Interpretation of susceptibility and resistance was done according to Clinical and Laboratory Standards Institute guidelines (CLSI, 2023). Multidrug resistance (MDR) was defined as resistance to at least one agent in three or more antimicrobial categories based on international consensus.
Data were entered and analyzed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The Chi-square (χ2) test or Fisher’s exact test was used to evaluate associations between multidrug resistance (MDR) and potential risk factors, including age, gender, comorbidities, hospitalization history, ICU admission, use of invasive devices, and prior antibiotic exposure, when applicable.
The Chi-square test was also used to compare antimicrobial resistance rates among different antibiotic classes. A p-value < 0.05 was considered statistically significant. Resistance trends were visualized using graphical summaries created in Microsoft Excel 2021, categorized by antibiotic class.
Enterobacter cloacae (50 clinical isolates; 12.5%) were obtained as mentioned in Figure 1 and were represented by a variety of clinical samples, i.e., blood (20 isolates; 40%), sputum (18 isolates; 36%), and pus (12 isolates; 24%). The variation in distribution between the types of specimens was not statistically significant (p > 0.05). The majority of patients were male (32; 64%), and over half of them were of the ≥60 years age group (28; 56%); nevertheless, there was no significant correlation between gender and age group and type of infection (p > 0.05).
In terms of comorbid conditions, diabetes mellitus (18; 36%) was most common, followed by chronic kidney disease (10; 20%), and malignancy (8; 16%) as mentioned in Table 1. There was a moderate yet insignificant relationship between multidrug resistance and the presence of one or more comorbidities (p = 0.09).
Hospital-related factors were significant risk factors for infection. Recent hospitalization was recorded in 34 patients (68%) and a hospital stay longer than 10 days was noted in 30 patients (60%). Both factors were found to be significantly related to the isolation of MDR E. cloacae (p = 0.021 and p = 0.034, respectively). The percentage of patients admitted to the ICU was 22 (44%), and multidrug resistance also showed a significant association with ICU admission (p = 0.031).
Invasive medical devices such as urinary catheters (26; 52%), central venous catheters (20; 40%) and mechanical ventilation (15; 30%). There was a significant association of MDR infection with urinary catheterization (p = 0.028), whereas central venous catheters and ventilators were not significantly associated (p > 0.05). Previous antibiotic use within the last three months was reported in 33 patients (66%), and it was significantly correlated with MDR E. cloacae (p = 0.008). Nine patients (18%) had immunosuppressive conditions, but these did not correlate significantly with resistance patterns (p > 0.05).
Table 2 summarizes the antimicrobial susceptibility results of the E. cloacae isolates. Ampicillin, amoxicillin-clavulanate, cefazolin, and cefuroxime were resistant in all isolates (100%). The prevalence of ceftazidime (35; 70%), cefepime (35; 70%), and ceftriaxone (32; 64%) among third-generation cephalosporins was also high (p < 0.001) with significant resistance rates. Ceftolozane-tazobactam retained full activity (100% susceptibility) in all isolates. When resistance was assessed by antibiotic class, β-lactams and cephalosporins showed the highest resistance levels. This was followed by moderate resistance to fluoroquinolones (ciprofloxacin and levofloxacin, 44% each) and tigecycline (50%). In contrast, aminoglycosides (Amikacin) and carbapenems generally retained better antimicrobial activity.
Among carbapenems, 17 isolates (34%) were resistant to imipenem, while 5 isolates (10%) were resistant to ertapenem and meropenem. Resistance to amikacin was observed in 5 isolates (10%), whereas gentamicin resistance was much higher (37; 74%), and the difference between the two aminoglycosides was statistically significant (p < 0.001). Moderate resistance was observed with trimethoprim-sulfamethoxazole (30; 60%), ciprofloxacin (22; 44%), levofloxacin (22; 44%), and tigecycline (25; 50%), with no significant differences between them (p > 0.05).
Ceftolozane-tazobactam, amikacin, ertapenem and meropenem were found to be the most effective antibiotics with resistance rates ≤10% each. These agents were significantly more active compared to other classes of antibiotics (p < 0.001) and represent the preferred therapeutic options in this study.
Figure 2 shows the analysis of multidrug resistance (MDR) patterns, indicating that most E. cloacae isolates were resistant to multiple antibiotic classes. The highest resistant pattern was observed in type 1 and 2; five isolates (10%) were resistant to seven antibiotic classes encompassing 15 individual antibiotics, and seven isolates were resistant to seven classes with 13 antibiotics. While the lowest resistant pattern was observed in type 8; five isolates (10%) were resistant to three antibiotic classes only encompassing six individual antibiotics. The MDR prevalence was statistically significant (p < 0.001). Overall, resistance was high, particularly to β-lactams and cephalosporins, with (42; 84%) of isolates classified as multidrug-resistant as mentioned in Figure 3.
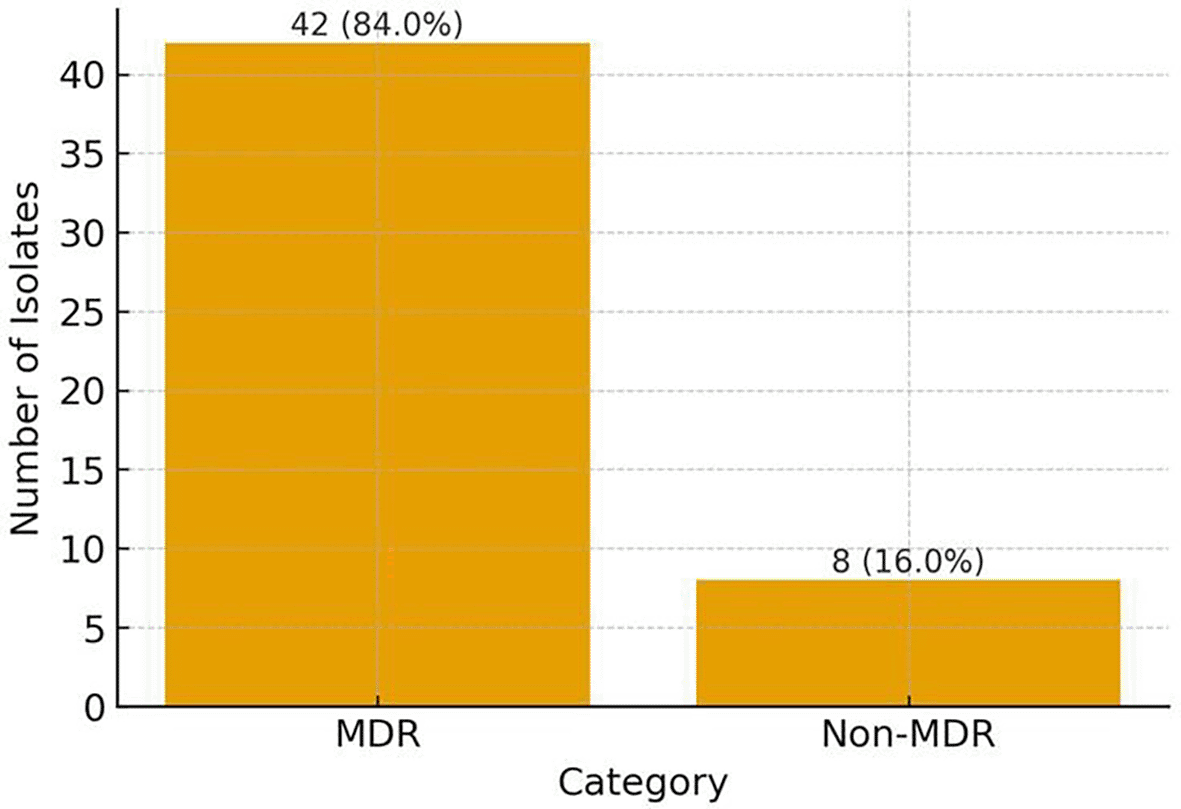
*MDR = Multi-drug Resistance.
The present study presents a recent overview of the clinical and microbiological features of Enterobacter cloacae infections, patient-related risk factors, common antimicrobial resistance phenotypes, and multidrug resistance (MDR) patterns. The current findings indicate approximately high prevalence of E. cloacae among bloodstream, respiratory, and wound infections. The difference in the E. clocae rates between the different studies may be associated with the type of clinical specimens, the population under study and the disparity in the hygienic practices in hospitals.7–9
E. cloacae isolates were in close correlation with hospital associated risk factors; this agreed with Cai et al. and Ibrahim et al. studies in 2024 and 2025 respectively.10,11 The high proportion of E. cloacae was isolated from elderly and male patients due to immunosenescence, frequent hospitalizations, and increased exposure to invasive procedures, this agreed with previous reports.12,13 Comorbid conditions, such as diabetes mellitus, chronic kidney disease, and malignancy were prevalent in the infected patients, consistent with host immunocompromised status, this supported by previous studies.14,15
Isolation of E. cloacae was significantly associated with prolonged hospitalization, ICU stay, and prior antibacterial exposure. These results are in line with previous investigations showing that nosocomial transmission and selection pressure from extensive antibiotic use are serious contributors to colonization and infection by resistant Enterobacter species.16–18 Invasive medical devices such as urinary and central venous catheters increased the risk of infection by facilitating entry of the microrganism and biofilm formation.19
Antimicrobial susceptibility analysis indicated high resistance to most β-lactam antibiotics such as ampicillin, amoxicillin-clavulanate and first to third generation cephalosporins due to overproduction of chromosomal AmpC β-lactamases and extended-spectrum β-lactamases (ESBLs) in E. cloacae.3,20 The resistance rate to gentamicin (74%), trimethoprim-sulfamethoxazole (60%), and moderate resistance to fluoroquinolones (42%) also demonstrates the widespread distribution of multidrug-resistant strains and these findings are in line with the study of Mosaffa et al. in 2024.21
Notably, ceftolozane-tazobactam, amikacin, ertapenem and meropenem were the most effective antibiotics, with 100% susceptibility for ceftolozane-tazobactam and 10% resistance rates for the mentioned three antibiotics. This indicates that carbapenems and aminoglycosides remain effective treatment options but requires close monitoring. These results agreed with recent global surveillance reports emphasizing the continued efficacy of carbapenems and β-lactam/β-lactamase inhibitor combinations antibiotics in treating AmpC and ESBL producing E. cloacae.22,23 However, the 34% resistance rate to imipenem is concerning because it may indicate the emergence of carbapenemase-producing strains, which are increasing worldwide.24–26
Multidrug resistance in this study was 84% similar to previous reports reported MDR prevalence between 70% and 94% in clinical E. cloacae isolates.27 High MDR levels complicate clinical care by limiting treatment options, ceftolozane-tazobactam offers a promising alternative but adherence to antibiotic stewardship is essential to maintain its effectiveness.28 Clinically, the resistance rates to third and fourth generation cephalosporins and fluoroquinolones in this region are markedly high, which makes them unsuitable for routine empirical use in our hospital when treating suspected severe E. cloacae infections. Instead, ceftolozane–tazobactam and carbapenem sparing regimens are used selectively, guided by local susceptibility patterns and stewardship practices, as reflected in our data. Integrating microbiology surveillance findings into the regional antimicrobial stewardship program in Northern Iraq could support more accurate empirical therapy recommendations, reduce unnecessary broad spectrum antibiotic use, and help slow the further emergence of MDR E. cloacae.
This research has several constraints. First, although the isolates were obtained from two tertiary care hospitals, the total number of samples was still limited, which may influence how well the findings apply to broader patient populations. Second, only phenotypic susceptibility testing was performed, without molecular confirmation of resistance genes; therefore, the exact mechanisms responsible for carbapenem and cephalosporin resistance were not fully identified. Third, the statistical analysis relied on bivariate tests without multivariable logistic regression or confidence intervals, reducing the ability to account for potential confounding factors in the associations between risk factors and MDR. Finally, clinical outcomes such as mortality, ICU stay duration, and treatment response were not systematically assessed, which limits the direct evaluation of how MDR patterns may influence patient prognosis.
Overall, these findings highlighted the importance of continuous antimicrobial surveillance, strict infection control and responsible use of broad spectrum antibiotics to prevent and limit the transmission of MDR E. cloacae in healthcare facilities.
Multidrug resistant Enterobacter cloacae infections were in strong association with hospital related risk factors such as prolonged hospital stay, ICU admission, antibiotic exposure, and invasive device use. The overall MDR rate (84%) reveals that antibiotic resistance is a growing concern in healthcare settings, requiring enhanced infection control measures and antibiotic stewardship programs.
Ceftolozane-tazobactam, amikacin, ertapenem and meropenem were the most active antibiotics against multidrug resistant Enterobacter cloacae in this study, but careful antibiotic use, routine susceptibility testing, and rapid diagnostic implementation are necessary to guide targeted therapy, support the development of region specific empirical treatment protocols, and reduce the transmission of MDR E. cloacae in healthcare settings.
This study was approved by the Ethics Committee of Kirkuk Teaching Hospital and the Ethics Review Board of the Ibrahim Scientific Consulting Office (Approval No. 2). Ethical approval was granted on June 15, 2024, prior to the start of the study. All procedures involving human participants were conducted in accordance with institutional and national ethical standards and in line with the principles of the Declaration of Helsinki.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0). The dataset is available at: https://doi.org/10.6084/m9.figshare.30642515. Hasan SA, Mohamed AH, Karim GF. Clinical Dataset on Antimicrobial Resistance and MDR Risk Factors of Enterobacter cloacae isolates from hospitalized patients in Iraq. Figshare; 2025.29
The authors thank the patients and microbiology lab staff for their participation and support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Davin-Regli A, Lavigne J, Pagès J: Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clinical Microbiology Reviews. 2019; 32 (4). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Antimicrobial resistance, phages, food microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 22 Dec 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)