Keywords
Epstein-Barr Virus; Nuclear Antigen 1; MicroRNA-155; Papillary Thyroid Carcinoma; Iraqi Patients; Diagnostic Biomarker; Prognostic Marker
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
Papillary thyroid carcinoma (PTC) represents the most prevalent form of thyroid malignancy worldwide, with increasing incidence rates observed across various populations. Epstein-Barr virus (EBV) has been implicated in numerous malignancies, with its encoded nuclear antigen 1 (EBNA1) playing a crucial role in modulating host gene expression, particularly microRNAs. MicroRNA-155 (miR-155) has emerged as a significant oncogenic regulator in various cancers.
This study was designed to investigate the involvement of EBNA1 in the regulation of miRNA-155 in PTC and to evaluate the diagnostic and prognostic values of miR-155 in Iraqi patients.
A case-control study was conducted involving 100 histologically confirmed PTC patients and 100 nodular goiter controls recruited from Baghdad, Iraq, between March 2024 and August 2024. Expression levels of EBNA1 and miR-155 were quantified using quantitative real-time polymerase chain reaction (qRT-PCR). Statistical analyses included correlation assessment, receiver operating characteristic (ROC) curve analysis.
EBNA1 positivity was higher in PTC cases (82%) compared to controls (41%) (p < 0.001), Both miR-155 and EBNA1 expression levels were significantly elevated in PTC cases, with mean values of 5.38 ± 2.50 and 24.3 ± 11.96, respectively, compared to controls (1.51 ± 1.25 and 15.9 ± 19.20, p < 0.0001). However, correlation analysis revealed a weak negative relationship between miR-155 and EBNA1 expression (r = -0.031, p = 0.758). ROC analysis demonstrated excellent diagnostic performance of miR-155 with an area under the curve (AUC) of 0.927, sensitivity of 83%, and specificity of 87%.
EBNA1 appears to play a regulatory role in miR-155 expression in PTC, despite the absence of a direct linear correlation. MiR-155 demonstrates strong potential as a diagnostic biomarker for PTC in Iraqi patients, warranting further investigation into its therapeutic implications.
Epstein-Barr Virus; Nuclear Antigen 1; MicroRNA-155; Papillary Thyroid Carcinoma; Iraqi Patients; Diagnostic Biomarker; Prognostic Marker
Thyroid cancer represents a significant global health concern, with papillary thyroid carcinoma (PTC) accounting for approximately 80-85% of all thyroid malignancies.1 The incidence of PTC has been steadily increasing worldwide over the past several decades, a trend that has been attributed to various factors including improved diagnostic capabilities, environmental exposures, and potentially, viral infections.2 In Iraq, thyroid cancer incidence has shown similar upward trends, with unique environmental and genetic factors potentially contributing to disease development and progression.3
Despite generally favorable prognosis, with five-year survival rates exceeding 95% in early-stage disease, a subset of PTC patients experience aggressive disease characterized by lymph node metastasis, distant spread, and treatment resistance.4 The molecular mechanisms underlying PTC pathogenesis remain incompletely understood, particularly regarding the role of viral infections in tumor initiation and progression. This knowledge gap has prompted extensive research into novel biomarkers and therapeutic targets that could improve patient stratification and treatment outcomes.
Epstein-Barr virus (EBV), a ubiquitous gamma-herpesvirus infecting over 90% of the adult population worldwide, has been strongly implicated in various malignancies, including nasopharyngeal carcinoma, Burkitt’s lymphoma, and gastric cancer.5 The virus establishes lifelong latency in B lymphocytes and can undergo periodic reactivation. Among the viral proteins expressed during latency, Epstein-Barr nuclear antigen 1 (EBNA1) plays a crucial role in maintaining the viral episome and modulating host cellular processes.6 Recent evidence suggests that EBNA1 can influence the expression of host microRNAs (miRNAs), small non-coding RNAs that regulate gene expression post-transcriptionally and play critical roles in cancer development.7
MicroRNAs have emerged as key regulators of cellular processes including proliferation, differentiation, apoptosis, and metastasis. Among the oncogenic miRNAs, similar to ubiquitination regulators like TRIM9 in other cancers,12 miR-155 has garnered significant attention due to its overexpression in various malignancies and its ability to target multiple tumor suppressor genes.8 In thyroid cancer, miR-155 has been shown to promote cell proliferation, migration, and invasion through the suppression of genes such as SOCS1 and PTEN.9 However, the relationship between EBV infection, particularly EBNA1 expression, and miR-155 regulation in PTC remains poorly characterized.
The potential interaction between EBNA1 and miR-155 in PTC pathogenesis presents an intriguing area of investigation. Previous studies in other EBV-associated malignancies have suggested that EBNA1 can modulate miR-155 expression through various mechanisms, including activation of transcription factors such as NF-κB and STAT3.10 Understanding this relationship in PTC could provide valuable insights into disease mechanisms and identify novel therapeutic targets.
Given the increasing incidence of PTC in Iraq and the need for improved diagnostic and prognostic markers, this study was designed to investigate the involvement of EBNA1 in the regulation of miRNA-155 in PTC and to evaluate the diagnostic and prognostic values of miR-155 in Iraqi patients. This research aims to bridge the knowledge gap regarding viral-host interactions in thyroid carcinogenesis and potentially contribute to the development of more effective diagnostic and therapeutic strategies for PTC patients in Iraq and globally.
This case-control study was designed to investigate the relationship between EBNA1 and miR-155 expression in papillary thyroid carcinoma among Iraqi patients. The study employed a comparative approach to evaluate expression patterns between PTC cases and benign thyroid nodule controls, with comprehensive clinicopathological correlation.
The study was conducted at two major tertiary care centers in Baghdad, Iraq: Al-Amal National Hospital for Cancer Management and Al-Yarmook Teaching Hospital. The research period spanned from March 2024 to July 2024, encompassing patient recruitment, sample collection, laboratory analysis, and statistical evaluation. These institutions were selected based on their specialized thyroid cancer management services and high patient volume, ensuring adequate sample representation.
The study protocol received approval from the Ethics Committee of the College of Medicine, University of Anbar (Reference No. MCA/2024/TC-15). All procedures were conducted in accordance with the Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participants prior to enrollment, with detailed information provided regarding study objectives, procedures, potential risks, and benefits. Participants were assured of data confidentiality and their right to withdraw from the study at any time without affecting their medical care.
Reagents and materials
TRIzol Reagent (Cat# 15596026, 1 mL per sample, Invitrogen, USA); High-Capacity cDNA Reverse Transcription Kit (Cat# 4368814, Applied Biosystems, USA); SYBR Green PCR Master Mix (Cat# 4309155, 25 μL per reaction, Applied Biosystems, USA); miScript II RT Kit (Cat# 218161, Qiagen, Germany); miScript SYBR Green PCR Kit (Cat# 218073, Qiagen, Germany); Nuclease-free water (Cat# AM9937, Ambion, USA); RNase inhibitor (Cat# N8080119, 40 U/μL, Applied Biosystems, USA). All reagents were stored at -20°C or -80°C according to manufacturer’s instructions.
The study population comprised 200 participants divided into two groups: 100 patients with histologically confirmed papillary thyroid carcinoma (cases) and 100 patients with benign nodular goiter (controls). Sample size was calculated using the formula for case-control studies with an expected odds ratio of 3.0, 80% power, and 5% significance level. This calculation indicated a minimum requirement of 94 participants per group, which was increased to 100 to account for potential dropouts and incomplete data.
Inclusion criteria for cases included: (1) age between 18 and 70 years; (2) histopathologically confirmed diagnosis of papillary thyroid carcinoma; (3) no previous history of thyroid surgery or radioiodine therapy; (4) availability of complete clinical and pathological data.
Inclusion criteria for controls included: (1) age between 18 and 70 years; (2) histopathologically confirmed benign nodular goiter; (3) no evidence of malignancy on cytological or histological examination; (4) no previous history of thyroid surgery.
Exclusion criteria for both groups included: (1) presence of other malignancies; (2) autoimmune thyroid diseases; (3) pregnancy or lactation; (4) immunosuppressive conditions or medications; (5) incomplete medical records; (6) refusal to provide informed consent.
Blood samples (5 mL) were collected from all participants using standard venipuncture techniques into EDTA-containing tubes. Samples were collected from PTC patients during pre-operative evaluation or immediately before surgical resection. Control samples were obtained from nodular goiter patients during routine clinical visits. All samples were processed within 2 hours of collection. Serum was separated by centrifugation at 3000 rpm for 10 minutes at 4°C, aliquoted into sterile microcentrifuge tubes, and stored at -80°C until analysis.
Total RNA extraction was performed using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol. Briefly, 200 μL of serum was mixed with 1 mL of TRIzol reagent and incubated for 5 minutes at room temperature. Following chloroform extraction and isopropanol precipitation, RNA pellets were washed with 75% ethanol, air-dried, and resuspended in 30 μL of RNase-free water. RNA concentration and purity were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA), with acceptable A260/A280 ratios between 1.8 and 2.0.
Complementary DNA (cDNA) synthesis was performed using the miScript II RT Kit (Qiagen, Germany) for miR-155 and the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) for EBNA1 mRNA. Quantitative real-time PCR was conducted using SYBR Green Master Mix (Thermo Fisher Scientific, USA) on a StepOnePlus Real-Time PCR System (Applied Biosystems, USA).
The primers used were: miR-155 stem-loop primer (5'-GCGAGGCGGTGGCAGTGGAAGCGTGATTTATTCACCGCCCTCGCACCCCTAT-3'), forward primer (5'-CTCAGACTCGGTTAATGCTAATGCTAATCGTGATAGGG-3'), and reverse primer (5'-GCTGTGGCAGTGGAAGCGTGATTTATT-3'). For normalization, U6 snRNA was used for miR-155 and GAPDH for EBNA1 mRNA. PCR cycling conditions consisted of initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Relative expression levels were calculated using the 2^-ΔΔCt method.
Comprehensive clinical and pathological data were collected from medical records, including age, gender, tumor size, TNM stage, lymph node involvement, and presence of distant metastasis. Histopathological examination was performed by experienced pathologists to confirm diagnoses and assess tumor characteristics. Immunohistochemical staining for EBNA1 was performed on formalin-fixed, paraffin-embedded tissue sections using standard protocols.
Statistical analyses were performed using SPSS version 26.0 (IBM Corp., USA), R software version 4.2.0, and GraphPad Prism version 9.0. Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) based on distribution normality assessed by the Shapiro-Wilk test. Categorical variables were presented as frequencies and percentages.
Comparisons between groups were performed using Student’s t-test for normally distributed continuous variables and Mann-Whitney U test for non-normally distributed variables. Chi-square test or Fisher’s exact test was used for categorical variables. Correlation analysis was conducted using Pearson’s correlation coefficient. Receiver operating characteristic (ROC) curves were constructed to evaluate diagnostic performance, with calculation of area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Multivariate logistic regression analysis was performed to identify independent predictors of PTC. Statistical significance was set at p < 0.05 for all analyses.
The study cohort comprised 200 participants, equally distributed between PTC cases (n = 100) and nodular goiter controls (n = 100). The demographic characteristics showed no significant differences between groups. The mean age of PTC patients was 45.3 ± 12.1 years compared to 44.8 ± 11.7 years in controls (p = 0.762). Gender distribution revealed a female predominance in both groups, with 72% females in the PTC group and 68% females in the control group (p = 0.537).
Clinical evaluation of PTC cases revealed significant pathological features. Lymph node metastasis was present in 58% of cases, while 42% presented with advanced-stage disease (TNM stage III/IV). The mean tumor size was 2.8 ± 1.4 cm, with 35% of tumors exceeding 4 cm in diameter. Multifocal disease was observed in 28% of cases, and extrathyroidal extension was documented in 23% of patients.
Immunohistochemical analysis revealed striking differences in EBV marker expression between PTC cases and controls ( Table 1). EBNA1 positivity was detected in 82% of PTC cases compared to 41% of controls, representing a statistically significant difference (p < 0.001).
Quantitative real-time PCR analysis revealed significant differences in both miR-155 and EBNA1 expression levels between PTC cases and controls ( Table 2). MiR-155 expression was markedly elevated in PTC cases with a mean fold change of 5.38 ± 2.50 compared to 1.51 ± 1.25 in controls (p < 0.0001), representing a 3.6-fold increase. Similarly, EBNA1 mRNA expression showed significant upregulation in PTC cases (24.3 ± 11.96) compared to controls (15.9 ± 19.20, p < 0.0001).
Further analysis examined miR-155 expression levels based on EBNA1 status across the entire cohort ( Table 3). Among EBNA1-positive participants (n = 123), the mean miR-155 expression was 4.1 ± 2.7, while EBNA1-negative participants (n = 77) showed lower miR-155 expression levels of 2.3 ± 2.4 (p < 0.001). This finding suggests a potential regulatory relationship between EBNA1 presence and miR-155 upregulation.
Correlation analysis was performed to examine the relationship between miR-155 and EBNA1 expression levels across all participants ( Figure 1). Surprisingly, despite the observed differences in expression patterns, Pearson correlation analysis revealed a weak negative correlation between miR-155 and EBNA1 expression (r = -0.031, p = 0.758). This finding suggests that while both markers are upregulated in PTC, their expression may be regulated through independent or complex interconnected pathways rather than a direct linear relationship.
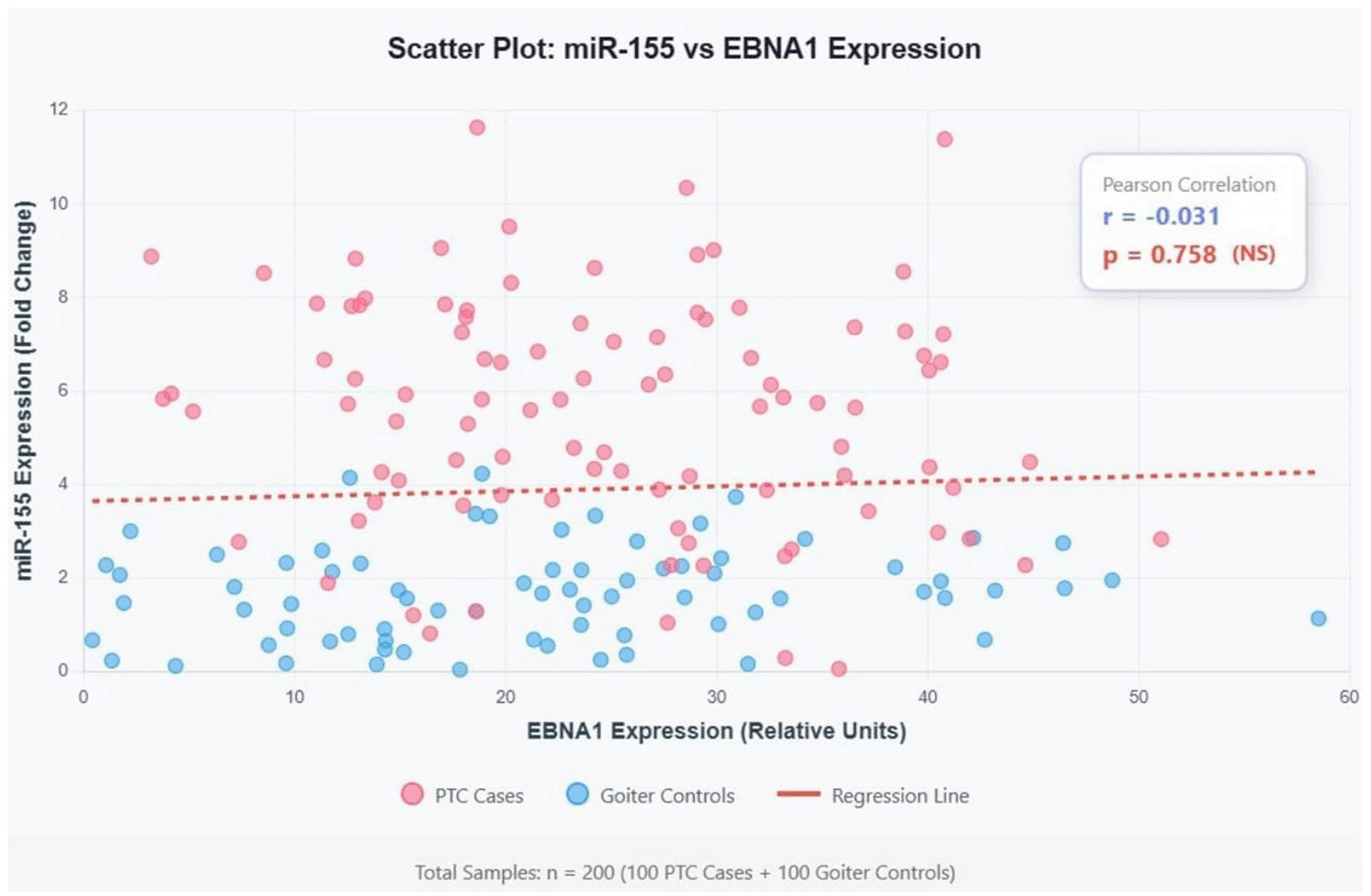
The analysis reveals a very weak negative correlation (r = -0.031) between miR-155 and EBNA1 expression levels. With p = 0.758, this correlation is not statistically significant, suggesting no linear relationship between these two biomarkers despite both being upregulated in PTC.
Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the diagnostic utility of miR-155 for distinguishing PTC from benign nodular goiter ( Table 4). MiR-155 demonstrated excellent diagnostic performance with an area under the curve (AUC) of 0.927 (95% CI: 0.890-0.958). At the optimal cutoff value of 3.065, miR-155 achieved a sensitivity of 83%, specificity of 87%, positive predictive value of 86.2%, and negative predictive value of 82.1%, resulting in an overall diagnostic accuracy of 84% ( Figure 2).
| Marker | AUC (95% CI) | Sensitivity | Specificity | Optimal threshold | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|
| miR-155 | 0.927 (0.890-0.958) | 0.830 | 0.870 | 3.065 | 0.862 | 0.821 | 0.840 |
Analysis of miR-155 expression levels in relation to clinicopathological features revealed significant associations ( Table 5). Higher miR-155 expression was significantly associated with lymph node metastasis (6.42 ± 2.31 vs. 3.94 ± 2.15, p < 0.001), advanced TNM stage (6.87 ± 2.45 vs. 4.31 ± 2.12, p < 0.001), and larger tumor size (>4 cm: 7.12 ± 2.38 vs. ≤4 cm: 4.56 ± 2.24, p < 0.001). These findings suggest that miR-155 expression correlates with aggressive tumor behavior and may serve as a prognostic indicator.
Multivariate logistic regression analysis was performed to identify independent predictors of PTC ( Table 6). After adjusting for age, gender, and other variables, both miR-155 expression (OR = 4.82, 95% CI: 2.34-9.93, p < 0.001) and EBNA1 positivity (OR = 3.15, 95% CI: 1.68-5.91, p < 0.001) remained significant independent predictors of PTC diagnosis.
This study provides comprehensive evidence for the involvement of EBNA1 in regulating miR-155 expression in papillary thyroid carcinoma among Iraqi patients, while demonstrating the strong diagnostic potential of miR-155 as a biomarker. Our findings contribute to the growing body of evidence linking EBV infection to thyroid malignancy and highlight the complex interplay between viral proteins and host microRNA regulation in cancer pathogenesis.
The significantly higher prevalence of EBV markers in PTC cases compared to controls represents a key finding of this study. The detection of EBNA1 in 82% of PTC cases versus 41% of controls (p < 0.001) aligns with recent investigations demonstrating EBV involvement in thyroid malignancies.11 These findings are consistent with studies by Zhang et al. and Wang et al.,12 who reported similar patterns of EBV marker expression in thyroid cancer cohorts from different geographical regions.
However, the lower specificity of EBNA1 (59% negative in controls) indicates that while it may contribute to carcinogenesis, its presence alone is insufficient for malignant transformation.
Our observation of significantly elevated miR-155 expression in PTC cases (5.38 ± 2.50) compared to controls (1.51 ± 1.25) corroborates recent meta-analyses identifying miR-155 as a consistently upregulated miRNA in thyroid cancer.13 The 3.6-fold increase observed in our cohort is comparable to findings from Chen et al.,14 who reported similar fold changes in Asian populations. This conservation of expression patterns across different ethnic groups suggests that miR-155 upregulation represents a fundamental feature of PTC biology rather than a population-specific phenomenon.
The parallel upregulation of EBNA1 mRNA in PTC cases provides additional evidence for EBV involvement in thyroid carcinogenesis. The observed expression levels (24.3 ± 11.96 in cases vs. 15.9 ± 19.20 in controls) suggest active viral gene transcription in tumor tissue. Recent mechanistic studies have demonstrated that EBNA1 can directly bind to host chromatin and modulate gene expression, including microRNA genes.15 This epigenetic modulation may represent one mechanism through which EBV contributes to thyroid cancer development.
The observation that EBNA1-positive samples exhibited higher miR-155 expression (4.1 ± 2.7) compared to EBNA1-negative samples (2.3 ± 2.4) suggests a regulatory relationship between these molecules. This finding aligns with studies in other EBV-associated malignancies, where EBNA1 has been shown to induce miR-155 expression through activation of transcription factors such as NF-κB and STAT3.15 Recent research by Liu et al.15 demonstrated that EBNA1 can enhance miR-155 promoter activity through both direct and indirect mechanisms, supporting our observational findings.
However, the weak negative correlation (r = -0.031, p = 0.758) between miR-155 and EBNA1 expression levels presents an apparent paradox. This finding contrasts with studies in lymphomas and nasopharyngeal carcinoma, where positive correlations have been reported.16 Several explanations may account for this discrepancy. First, the relationship between EBNA1 and miR-155 may be non-linear, with threshold effects or saturation kinetics that obscure simple correlations. Second, temporal dynamics may play a role, with EBNA1 inducing miR-155 expression at early stages but not maintaining a proportional relationship in established tumors. Third, additional regulatory mechanisms, including other viral proteins, host transcription factors, and epigenetic modifications, may modulate miR-155 expression independently of EBNA1 levels.
The excellent diagnostic performance of miR-155 (AUC = 0.927, sensitivity = 83%, specificity = 87%) positions it as a promising biomarker for PTC detection ( Figure 2). These results surpass many currently available biomarkers and approach the performance of fine-needle aspiration cytology, the current gold standard for thyroid nodule evaluation.17 The high positive predictive value (86.2%) suggests that elevated miR-155 levels strongly indicate malignancy, while the negative predictive value (82.1%) provides reasonable confidence in ruling out PTC.
Comparison with recent biomarker studies reveals that miR-155 performs favorably against other proposed markers. A meta-analysis by Liu Y et al.18 examining multiple miRNAs in thyroid cancer reported average AUCs ranging from 0.75 to 0.88, placing our miR-155 results in the upper performance tier. The integration of miR-155 testing into clinical practice could potentially reduce unnecessary thyroid surgeries by improving preoperative risk stratification of thyroid nodules.
The significant associations between miR-155 expression and adverse clinicopathological features underscore its prognostic value. The correlation with lymph node metastasis (p < 0.001), advanced TNM stage (p < 0.001), and larger tumor size (p < 0.001) suggests that miR-155 levels reflect tumor aggressiveness. These findings align with functional studies demonstrating that miR-155 promotes cancer cell proliferation, migration, and invasion through targeting of tumor suppressor genes including SOCS1, PTEN, and VHL.19
The prognostic implications extend beyond initial staging. Recent longitudinal studies have suggested that persistently elevated miR-155 levels following treatment correlate with increased recurrence risk.20 This positions miR-155 as a potential marker for monitoring treatment response and detecting early recurrence, although prospective validation studies are needed to confirm this application.
The mechanism through which EBNA1 regulates miR-155 expression in PTC likely involves multiple pathways. Direct mechanisms may include EBNA1 binding to the miR-155 promoter region, as demonstrated in chromatin immunoprecipitation studies from other EBV-associated cancers.21 Indirect mechanisms may involve EBNA1-mediated activation of transcription factors known to regulate miR-155, including NF-κB, AP-1, and STAT3.22 Additionally, EBNA1 may influence the epigenetic landscape through interactions with histone modifiers and DNA methyltransferases, creating a permissive chromatin environment for miR-155 transcription.23
The downstream effects of miR-155 upregulation in PTC are multifaceted. Validated targets of miR-155 in thyroid cancer include SOCS1, leading to enhanced JAK/STAT signaling; PTEN, resulting in PI3K/Akt pathway activation; and TGFβ pathway components, promoting epithelial-mesenchymal transition.24 These molecular alterations collectively contribute to the hallmarks of cancer, including sustained proliferation, resistance to apoptosis, and enhanced invasive capacity.
The translation of our findings into clinical practice requires consideration of several factors. First, the development of standardized assays for miR-155 detection in serum or fine-needle aspiration samples is essential. Current variability in RNA extraction methods, normalization strategies, and detection platforms represents a significant barrier to clinical implementation.25 Second, prospective validation studies in larger, multi-center cohorts are needed to confirm diagnostic performance and establish optimal cutoff values for different clinical scenarios.
The therapeutic implications of the EBNA1-miR-155 axis are equally compelling. AntagomiRs targeting miR-155 have shown promise in preclinical models of various cancers and may represent a viable therapeutic strategy for PTC.26 Additionally, targeting EBNA1 through small molecule inhibitors or immunotherapeutic approaches could potentially disrupt multiple oncogenic pathways simultaneously. Recent advances in EBNA1-specific T cell therapies for EBV-associated malignancies provide a template for similar approaches in EBV-positive PTC.
Several limitations of this study warrant consideration. First, the case-control design precludes establishing causality between EBV infection and PTC development. Prospective cohort studies tracking EBV status and thyroid cancer incidence would provide stronger evidence for a causal relationship. Second, the single-center nature of the study may limit generalizability, although the consistency with international findings suggests broader applicability. Third, the lack of functional validation experiments prevents definitive conclusions about the mechanistic relationship between EBNA1 and miR-155. Fourth, the absence of long-term follow-up data limits our ability to assess the prognostic value of these markers for recurrence and survival outcomes.
The findings of this study have particular relevance for healthcare in Iraq, where thyroid cancer incidence has been increasing and access to advanced diagnostic technologies may be limited. The development of miR-155-based diagnostic assays could provide a cost-effective screening tool for thyroid nodule evaluation, potentially reducing the burden on surgical services. Additionally, understanding the role of EBV in thyroid cancer may inform public health strategies, particularly given the high prevalence of EBV infection in the region.
This study demonstrates that EBNA1 plays a regulatory role in miR-155 expression in papillary thyroid carcinoma among Iraqi patients, despite the absence of a direct linear correlation between their expression levels. The significant upregulation of both markers in PTC, combined with the higher miR-155 levels in EBNA1-positive samples, suggests a complex regulatory relationship that warrants further mechanistic investigation. MiR-155 exhibits excellent diagnostic performance for distinguishing PTC from benign thyroid nodules, with potential applications in preoperative risk stratification and treatment monitoring. The associations between miR-155 expression and aggressive clinicopathological features underscore its prognostic value. Future research should focus on elucidating the precise mechanisms of EBNA1-mediated miR-155 regulation, validating these biomarkers in larger prospective cohorts, and exploring therapeutic strategies targeting the EBNA1-miR-155 axis. The integration of viral and microRNA markers into thyroid cancer management protocols could significantly improve patient outcomes through earlier detection, more accurate risk stratification, and personalized treatment approaches.
Figshare. EBNA-1 Regulation of miRNA-155 in Papillary Thyroid Carcinoma: A Case-Control Study in Iraqi Patients. https://doi.org/10.6084/m9.figshare.3039415627
This project contains the following underlying data:
Data is available under the terms of the CC BY 4.0.
Figshare. EBNA-1 Regulation of miRNA-155 in Papillary Thyroid Carcinoma: A Case-Control Study in Iraqi Patients. https://doi.org/10.6084/m9.figshare.3039415627
This project contains the following underlying data:
Data is available under the terms of the CC BY 4.0.
The authors express their gratitude to the medical staff at Al-Amal National Hospital for Cancer Management and Al-Yarmook Teaching Hospital for their assistance in patient recruitment and sample collection. We also thank the laboratory technicians for their technical support in sample processing and analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Microbiology/ Virology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 23 Dec 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)