Keywords
alcohol use disorder, viral infection, biomarkers, ROC analysis, liver enzymes, popula-tion-specific reference values
This study examined biomarker signatures in men with alcohol use disorder (AUD) in Uzbekistan, with and without viral infections.
A cross-sectional study included 292 males with stage II AUD (virus-negative: n = 251; virus-positive: n = 41) and 49 alcohol-free controls. Clinical, hematological, and biochemical parameters were measured, and ROC analysis evaluated diagnostic performance.
Virus-negative patients showed the clearest biomarker profile of alcohol dependence, with reduced glucose, creatinine, and urea, and elevated total protein, α-amylase, De Ritis ratio, and direct bilirubin. ROC analysis confirmed strong diagnostic value for AST (AUC = 0.951), FIB-4 (0.877), MAP (0.817), and creatinine (0.711). Leukocytes (AUC = 0.790) and lymphocytes (0.735) best differentiated viral status. Fibrosis risk in virus-positive patients was 1.5-fold higher, with splenomegaly in 7.3%. Mild thrombocytopenia, absence of granulocytopenia, and rare delirium (<5.5%) distinguished this cohort from European groups, resembling East Asian patterns.
Liver enzymes, α-amylase, bilirubin, MCV, FIB-4, and MAP provide strong diagnostic value for AUD. Multimarker panels including leukocyte, lymphocyte, and creatinine levels support viral status differentiation. Findings emphasize population-specific biomarker signatures in Central Asians and the utility of multimarker strategies for personalized AUD management.
alcohol use disorder, viral infection, biomarkers, ROC analysis, liver enzymes, popula-tion-specific reference values
Alcohol use disorder (AUD) is a serious public health problem with significant social, economic, and medical consequences, as it primarily affects individuals of working and reproductive age. Globally, 5.3% of deaths are attributable to alcohol consumption, and more than 2.4 million people suffer from alcohol-related cirrhosis.1
Alcohol abuse damages multiple organs and systems, including the liver, pancreas, heart, kidneys, nervous system, and hematopoietic cells.2 Reliable diagnostic and prognostic biomarkers provide a minimally invasive approach—most commonly blood-based—for early detection and management. The integration of biomarker screening with instrumental and clinical data forms the basis of modern diagnostics.
Biomarker variability is strongly influenced by sex, age, ethnicity, and geography,3 reflecting environmental and cultural factors such as diet, lifestyle, and alcohol consumption habits. Even closely related ethnic groups with distinct dietary patterns and attitudes toward psychoactive substances demonstrate significant biochemical differences.4 These phenotypic traits are reinforced at the genetic level through diverse functional combinations of single nucleotide polymorphisms (SNPs), which regulate physiological and pathological processes.5 This underscores the importance of population- and ethnicity-specific research.
The present cross-sectional study assessed hematological and biochemical parameters in alcohol-dependent patients with and without viral infections in Uzbekistan, compared with healthy controls.
A cross-sectional study was conducted at the Republican Specialized Scientific and Practical Medical Center for Mental Health (RSSPMCMH, Uzbekistan). To minimize readmissions, the enrollment period was restricted to 3 months, yielding 292 male patients with stage II alcohol use disorder (AUD) and 49 alcohol-free controls. Female patients (n = 8) and mild AUD cases (n = 2) were excluded. One control was excluded after hepatitis B detection. Final groups: Cohort 1 (AUD without viral infection, n = 251), Cohort 2 (AUD with hepatitis B, C, or HIV infection, n = 41), and Cohort 3 (controls, n = 49).
Inclusion: men aged 20–65 with ICD-10 F10.2 diagnosis of stage II chronic alcoholism. Controls: age-matched males without alcohol or psychoactive substance use, confirmed clinically and by laboratory tests. Exclusion: females, mild withdrawal symptoms, acute infections, and controls with previously undiagnosed viral infections.
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Local Ethics Committee of the Republican Specialized Scientific and Practical Medical Center for Mental Health (RSSPMCMH), Tashkent, Uzbekistan (Approval No. 1/2025, 18 March 2025). Written informed consent was obtained from all participants.
Somatic status was assessed by clinical examination and instrumental diagnostics. Hematological and biochemical parameters were measured using automated analyzers (Mindray, China) with standardized enzymatic and colorimetric methods (detailed description provided in Supplementary File S1 — Supplementary Methods). Composite indices included FIB-4, De Ritis ratio, mean arterial pressure (MAP), and body mass index (BMI), calculated by standard formulas.
Diagnostic accuracy of biomarkers was evaluated by ROC analysis. AUROC values with 95% confidence intervals were estimated using bootstrap resampling (n = 1000). Optimal cut-offs were determined by Youden’s Index, with corresponding sensitivity and specificity reported.
Analyses were performed using Python 3.11 (scikit-learn, numpy, pandas, matplotlib). Nonparametric tests (Mann-Whitney U, Kruskal-Wallis H, χ2 with Bonferroni correction) were applied; significance was set at p < 0.05. A retrospective power analysis confirmed ≥85% power for detecting medium-to-large effect sizes despite the smaller control group.
Among the 292 alcohol-dependent male patients examined, 14% (n = 41) were identified as carriers of viral infections, including hepatitis C (n = 18), hepatitis B (n = 18), combined hepatitis B and C (n = 1), triple hepatitis B/C/D (n = 1), HIV (n = 2), and HIV with hepatitis B (n = 1). Despite variation within groups, average age did not differ significantly across cohorts (see Table 1). Patients over 50 years old typically had alcohol use histories exceeding 10 years.
Values are presented as medians (Q1–Q3) or n (%).
Severe withdrawal symptoms were reported in 9% (n = 23) of patients, all from the virus-negative group, whereas only 1.15% (n = 3) of virus-positive individuals were affected. Most patients (85.5%) exhibited moderate withdrawal symptoms.
The study population was predominantly Turkic-speaking (94.5%, n = 276), mainly of Uzbek ethnicity. Both alcohol-dependent patients (n = 292) and alcohol-free controls (n = 49) demonstrated comparable ethnic composition, ensuring cohort homogeneity. Notably, psychotic symptoms (delirium and/or hallucinations) were observed in 5.5% (n = 16) of alcohol-dependent patients. Among these, 43.8% (n = 7) were of Slavic origin and 18.8% (n = 3) were Tatar, despite these groups comprising only 6.1% and 2.7% of the total alcohol-dependent cohort, respectively. This disproportion suggests a potential ethnogenetic vulnerability to alcohol-related neuropsychiatric complications.
Elevated FIB-4 scores (≥4) and MAP were more frequent among virus-positive patients, reflecting advanced fibrosis and vascular dysregulation.
The prevalence of somatic comorbidities varied across study groups (see Figure 1).
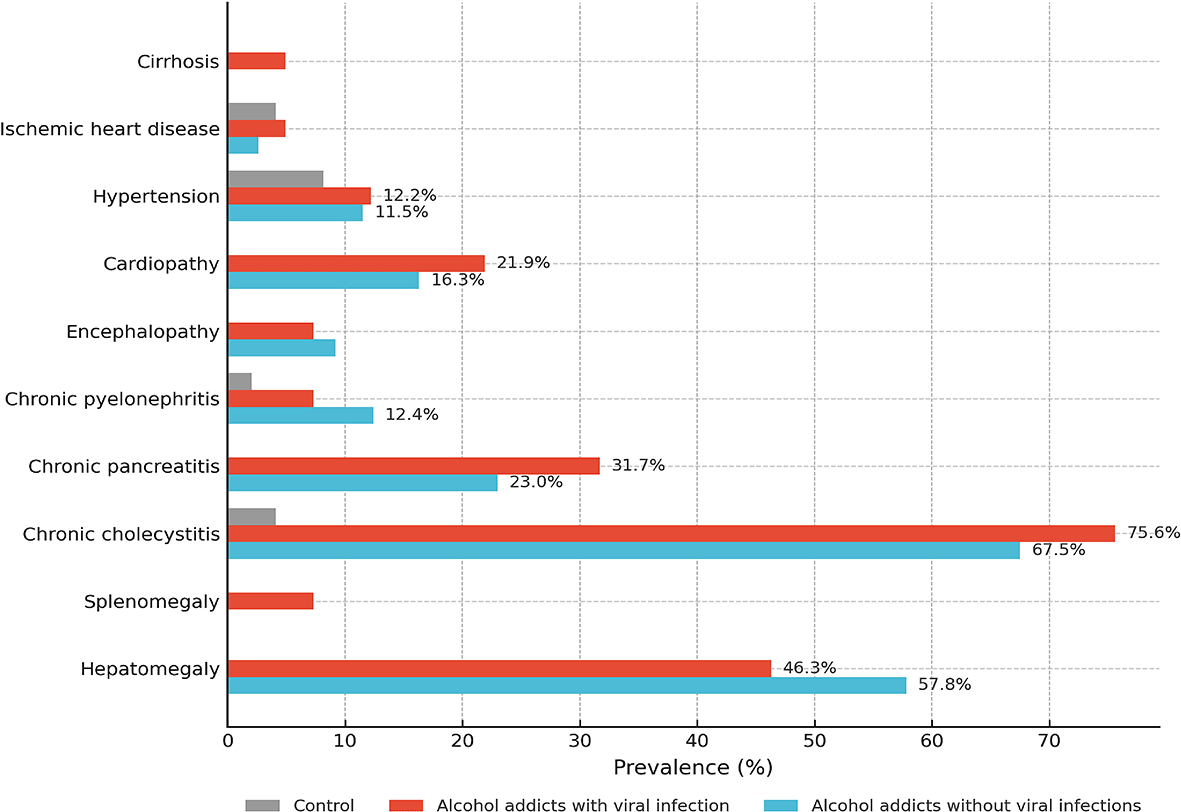
Hypertension, cardiopathy, chronic pancreatitis, cholecystitis, encephalopathy, splenomegaly, and cirrhosis are shown as relative frequencies (%).
Hypertension was more frequent in alcohol-dependent patients (11.5% without viral infection; 12.2% with viral infection) compared to controls (8.2%) (p < 0.05 for both patient groups vs. controls). Cardiopathy occurred in 21.9% of virus-positive patients and 16.3% of virus-negative patients versus 0% in controls (C vs. V, **p < 0.001; C vs. N, **p < 0.001). Chronic pancreatitis was observed in 31.7% of virus-positive and 23.0% of virus-negative patients compared to 0% in controls (C vs. V, **p < 0.001; C vs. N, **p < 0.001). Chronic cholecystitis was the most prevalent condition, affecting 75.6% of virus-positive and 67.5% of virus-negative patients versus 4.1% in controls (C vs. both patient groups, **p < 0.001). Encephalopathy occurred in 7.3% of virus-positive and 9.2% of virus-negative patients, while it was absent in controls (C vs. V, *p < 0.05; not significant for C vs. N or V vs. N). Splenomegaly was found only in virus-positive patients (7.3%, p < 0.01). Cirrhosis was also observed exclusively in this group (4.9%), although differences did not reach statistical significance. No significant variation was identified for ischemic heart disease, hepatomegaly, or chronic pyelonephritis (all p > 0.05).
A comparative analysis of hematological parameters was conducted among virus-positive (n = 41) and virus-negative (n = 251) alcohol-dependent patients, and an alcohol-free control group (n = 49). Only values outside physiological reference ranges were considered. Hematological parameters are summarized in Supplementary Table S1.
Hemoglobin (Hb) levels were significantly reduced in both alcohol-dependent groups, falling below normal in 78% of virus-positive and 45% of virus-negative patients. Macrocytosis, defined as MCV > 100 fL, was observed in 17–29% of alcohol-dependent individuals versus 6.1% of controls (p < 0.001), supporting its association with chronic alcohol exposure.
Erythrocyte sedimentation rate (ESR) was elevated in 63.4% of virus-positive and 57.4% of virus-negative patients (p < 0.001), compared to 10.2% of controls, indicating systemic inflammation, especially with viral comorbidity.
Leukocytosis was more common in virus-positive patients (36.6%) than in virus-negative patients (15.1%). A right shift in the differential count—with increased segmented neutrophils showing nuclear hypersegmentation—was noted in 7.6–9.8% of patients, suggesting megaloblastic anemia or hepatic/renal impairment. Lymphocytosis, likely reflecting antiviral immune activation, was present in 9.2% of virus-positive and 5% of virus-negative individuals, while lymphocytopenia occurred in 16.3% of controls. Unexpectedly, monocytosis was more frequent in controls (44.9%) than in alcohol-dependent individuals (20–26.8%), possibly reflecting transient immune responses.
Thrombocytopenia was identified in 13.5–17.1% of alcohol-dependent patients versus 4% of controls (p = 0.029). Mean platelet volume (MPV), a marker of platelet activation and turnover, was elevated in 41.5% of virus-positive patients, compared to 16.7–22.4% in the other groups (p < 0.001).
Biochemical parameters were evaluated in virus-positive (n = 41) and virus-negative (n = 251) alcohol-dependent patients, as well as in an alcohol-free control group (n = 49). Detailed biochemical characteristics across the three study cohorts are presented in Supplementary Table S2.
As shown in Supplementary Table S2, glucose levels were slightly lower in alcohol-dependent groups compared to controls. However, within alcohol-dependent cohorts, virus-positive patients demonstrated 1.2-fold higher glucose levels than virus-negative individuals (p = 0.002), possibly reflecting altered gluconeogenesis and insulin sensitivity. Creatinine concentrations were reduced in both alcohol-dependent groups by ~1.5-fold vs. controls, likely indicating reduced muscle mass or altered renal tubular function. α-Amylase (AAMY) levels were significantly higher in alcohol-dependent patients, with mean values 1.7 times those of controls (p < 0.001; Dunn’s test; r = 0.01). However, elevated enzyme activity was found in only 2.4–8.2% of individuals. This likely reflects total α-amylase activity, not limited to pancreatic isoforms, which is common in chronic alcohol users.
Liver enzymes showed marked alterations. ALT levels were 2.1 times higher in both alcohol-dependent cohorts versus controls (p < 0.001), and 1.2 times higher in virus-positive vs. virus-negative patients—suggesting additive liver injury from viral and alcohol-related mechanisms. AST levels were approximately threefold higher in alcohol-dependent groups, with slightly greater elevation in the virus-negative cohort, possibly due to dominant alcohol-related damage.
Bilirubin profiles varied: conjugated bilirubin was elevated in 72% of virus-negative and 65.9% of virus-positive patients. However, virus-positive individuals also demonstrated elevated unconjugated bilirubin, implying compromised hepatic conjugation capacity.
Thus, according to the obtained data, statistically significant differences (p < 0.001) in blood biomarker profiles between alcohol-dependent patients and individuals without alcohol abuse (control cohort) were observed among biochemical markers, specifically liver enzymes (ALT, AST), bilirubin fractions, creatinine, glucose, and urea, while among hematological indicators, the most pronounced distinctions were noted for hemoglobin, erythrocyte sedimentation rate, and mean corpuscular volume of erythrocytes. Detailed distributions of biochemical biomarkers across the three cohorts are shown in Supplementary Figure S1.
To evaluate the diagnostic potential of clinical and laboratory markers, Receiver Operating Characteristic (ROC) analysis was performed (see Table 2).
Class coding: (a) Positive = Cohorts 1+2, Negative = Cohort 3; (b) Positive = Cohort 2, Negative = Cohort 1.
Two comparisons were made: (а) Cohort 3 (controls) vs. Cohorts 1+2 (all alcohol-dependent patients); (b) Cohort 1 (virus-negative) vs. Cohort 2 (virus-positive alcohol-dependent patients).
The area under the curve (AUC) quantifies discrimination ability (1.0 = perfect). Sensitivity and specificity reflect correct identification of true positives and true negatives.
Key findings (Cohorts 1+2 vs. controls): AST: AUC = 0.951 (95% CI: 0.915–0.987), 94% sensitivity, 89% specificity at 73 U/L; FIB-4: AUC = 0.877 (95% CI: 0.820–0.935), 76% sensitivity, 96% specificity at 1.16; MAP: AUC = 0.817, 96% sensitivity, 59% specificity at 97 mmHg; ALT: AUC = 0.808; AAMY = 0.802; direct bilirubin = 0.799; Poor discriminators: BMI (0.276), Creatinine (0.060).
Key findings (virus-negative vs. virus-positive): WBC: AUC = 0.790 (95% CI: 0.705–0.876), 95% sensitivity, 59% specificity; Lymphocytes: AUC = 0.735, 90% sensitivity, 64% specificity; Monocytes: AUC = 0.682, 98% sensitivity, 37% specificity; Creatinine: AUC = 0.711, 88% sensitivity, 46% specificity.
This study investigated biochemical, hematological, and somatic characteristics in two alcohol-dependent cohorts—with and without viral infections—compared to controls. Statistically significant differences were observed across multiple parameters, underscoring the impact of chronic alcohol use and viral coinfection on systemic physiology. Although the relatively small control group is a limitation, post hoc power analysis for key markers such as ALT indicated sufficient statistical power (≥85%), supporting the robustness of the findings.
Non-invasive indices including BMI, MAP, and the FIB-4 score were employed to assess metabolic and hepatic status. The FIB-4 index identified fibrotic changes in 42% of patients with viral infections and in 27% of virus-negative alcohol-dependent individuals, consistent with long-term alcohol exposure and previously reported fibrosis rates.6
Our results demonstrate a substantial burden of somatic complications in alcohol-dependent men, with notable differences between those with and without viral comorbidity. The high prevalence of chronic cholecystitis and pancreatitis highlights cumulative hepatopancreatic injury, amplified in virus-positive patients. Cardiopathy was disproportionately common in virus-positive patients, supporting the notion that viral infection aggravates cardiovascular vulnerability. Splenomegaly was observed exclusively in virus-positive individuals (7.3%), suggesting greater risk of portal hypertension and advanced hepatic involvement. Cirrhosis was also detected only in virus-positive patients, though without statistical significance, likely reflecting sample size limitations rather than absence of association. Encephalopathy occurred in both patient cohorts but was absent in controls, suggesting it is primarily linked to alcohol exposure rather than viral status. The lack of difference between groups supports AUD as the principal driver of neurotoxic complications.7 In contrast, ischemic heart disease, hepatomegaly, and chronic pyelonephritis showed no significant differences, implying closer relation to lifestyle or cumulative alcohol exposure than to viral comorbidity.
Significant reductions in hemoglobin (Hb) were detected in both alcohol-dependent cohorts, with subnormal levels in 75% of virus-positive and 50% of virus-negative individuals. Ethanol and acetaldehyde exert cytotoxic effects on erythroid precursors and promote oxidative stress.8 Individuals with reduced aldehyde dehydrogenase (ALDH2) activity—common in Asian populations—accumulate more acetaldehyde, leading to enhanced hemoglobin-acetaldehyde adduct (HbAA) formation.9 This mechanism may partly explain higher susceptibility to liver injury and anemia in Asian alcohol users.
Our data are consistent with Russian findings showing reduced hemoglobin in alcohol-dependent individuals, although baseline values in the Russian cohort were higher, potentially due to ethnic-genetic or environmental differences.10 Inflammatory markers including ESR, leukocyte count, and lymphocytes were elevated in alcohol-dependent groups, particularly in those with viral coinfection, suggesting ongoing systemic inflammation. Increases in Candida albicans-specific Th17 cells, as reported by Zeng et al., may reflect fungal colonization and immune activation in alcohol-associated liver disease.11
Unexpectedly, monocytosis was more frequent in controls, possibly reflecting immune responses to non-viral stimuli. Chronic alcohol use induces both immune suppression and hyperinflammation through epigenetic alterations of monocyte/macrophage function.8 Despite this, granulocytopenia was rare and comparable between groups, differing from some prior studies.12
Thrombocytopenia was also confirmed, with significantly lower platelet counts (PLT) in alcohol-dependent patients. While low PLT has been associated with withdrawal-related neuropsychiatric complications,13 no such manifestations were observed in patients with severe thrombocytopenia here. Interestingly, platelet counts were near normal in individuals with delirium or hallucinations, suggesting thrombocytopenia may not be the primary driver of these symptoms.
The mean platelet volume (MPV), an indicator of platelet activation, was elevated in 41.5% of virus-positive patients, reflecting increased turnover or stress-induced megakaryopoiesis, potentially mediated by acetaldehyde toxicity and ALDH2 variants.14,15
Our findings on macrocytosis are consistent with prior studies. MCV was elevated in up to 29% of alcohol-dependent patients, supporting its role as a biomarker of chronic alcohol exposure.16–18 Mechanistically, macrocytosis may reflect direct erythrocyte toxicity or deficiencies in folate, vitamin B12, and liver function.19
Serum α-amylase was significantly higher in alcohol-dependent patients than in controls, despite overall lower activity than reported in Brazilian studies.20 Elevated amylase may reflect pancreatic/hepatic inflammation21 or sympathetic activation, as suggested by King and Nater.22,23
The AST/ALT ratio (De Ritis index) followed expected patterns, being higher in alcohol-dependent individuals and slightly more elevated in those without viral infections, consistent with alcoholic liver disease and corroborated by Iluz-Freundlich et al.24 Elevated direct bilirubin further supported cholestasis. In contrast, unconjugated hyperbilirubinemia was more frequent in virus-positive individuals, suggesting impaired conjugation and hepatocellular function.
Elevated bilirubin in 19–24% of controls may reflect subclinical liver dysfunction, possibly driven by poor diet and sedentary lifestyle. Similar trends were documented in Indian and Chinese populations.25,26
Creatinine was significantly lower in alcohol-dependent individuals, consistent with prior reports,27 possibly reflecting reduced muscle mass or impaired renal function. These findings support including creatinine in the MELD score, a validated mortality predictor in end-stage liver disease.28
Glucose and total protein showed paradoxical trends: hyperglycemia and hypoproteinemia were more common in controls, possibly due to obesity. In contrast, elevated globulin fractions in alcohol-dependent individuals may reflect chronic inflammation.29,30
Hypoglycemia was present in 27.1% of virus-negative alcohol-dependent patients, consistent with alcoholic ketoacidosis. This warrants expanded biochemical assessment in future studies, including ketone body and osmolality measurements.31–34
Neuropsychiatric symptoms (e.g., delirium, hallucinations) were present in 5.5% of alcohol-dependent patients. Strikingly, 36.8% of Slavic individuals in the alcohol group exhibited such symptoms, despite representing only 6.1% of the cohort. These observations may reflect population-specific genetic differences in enzymatic systems involved in alcohol metabolism.10
Finally, ROC analysis highlights the effectiveness of liver-specific biomarkers (AST, ALT, FIB-4) and systemic parameters (MAP, AAMY) in distinguishing alcohol-dependent patients from controls. Elevated AUC values for AST and FIB-4 align with prior research indicating hepatic injury and fibrotic transformation.35,36 MAP elevation is consistent with alcohol-induced sympathetic activation.37 In subgroup analysis, immune-inflammatory markers (WBC, lymphocytes, monocytes) were more relevant for differentiating virus-positive from virus-negative patients, suggesting viral coinfection contributes additional immunologic changes.38,39 The findings reinforce the utility of combining biochemical, hematological, and physiological markers to enhance diagnostic accuracy. However, the low efficiency of some markers (e.g. platelets, hemoglobin, BMI) suggests their limited role and is most likely due to ethnopopulation characteristics.
This study has several limitations. First, only male patients were included, as the small number of female participants and sex-related biomarker variability precluded meaningful subgroup analysis. Second, the recruitment period was restricted to 3 months to minimize patient recirculation and ensure the dataset reflected primary screening; while methodologically justified, this limited the overall sample size. Third, the control group was relatively small compared to the patient cohorts. Despite these limitations, the study provides novel and valuable population-specific insights into biomarker profiles in alcohol use disorder with and without viral comorbidity. Importantly, this work represents the first systematic investigation of such biomarkers in a poorly studied population of alcohol-dependent individuals in Uzbekistan, highlighting the need for further research in this unique setting.
ROC analysis confirmed the strong diagnostic value of liver enzymes (AST, ALT), α-amylase, MCV, direct bilirubin, the De Ritis ratio, FIB-4 index, and mean arterial pressure (MAP) in identifying alcohol dependence. Leukocyte, lymphocyte, and creatinine levels were the most informative for distinguishing patients with viral comorbidity. These findings highlight the clinical utility of multimarker panels for early detection, differentiation, and risk stratification in alcohol use disorder.
Importantly, deviations between Uzbek control values and international reference ranges underline the need for population- and ethnicity-specific interpretation of biochemical and hematological markers in clinical practice. Establishing regionally validated diagnostic thresholds could improve accuracy of screening and personalized management of patients with alcohol use disorder in Central Asia.
The authors used ChatGPT (OpenAI, 2025) to improve the language. After using this tool, the authors reviewed and edited the content and take full responsibility for the publication’s content.
Repository: Population-Specific Biomarker Dataset for Alcohol Use Disorder in Uzbekistan.
DOI: https://doi.org/10.5281/zenodo.1767383640
The project contains the following underlying data: Raw Dataset – Hematology_Biochemistry_Somatic.xlsx (raw anonymized patient-level hematological, biochemical, somatic, and clinical data for all three cohorts).
Repository: Population-Specific Biomarker Dataset for Alcohol Use Disorder in Uzbekistan.
DOI: https://doi.org/10.5281/zenodo.1767383640
This project contains the following extended data:
- Supplementary File S1 — Supplementary Methods (detailed description of laboratory methods, formulas for derived indices, and ROC analysis procedures).
- Supplementary Table S1 – Hematological biomarkers (group-level distributions and statistical comparisons of hematological parameters across cohorts).
- Supplementary Table S2 – Biochemical biomarkers (group-level distributions and statistical comparisons of biochemical parameters across cohorts).
- Supplementary Figure S1 – Box plot distributions of biochemical biomarkers in the study cohorts.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC BY 4.0).
The work was carried out in accordance with the research plan for the current year and approved by the Ministry of Health of the Republic of Uzbekistan. The work was carried out on the basis of the Republican Specialized Scientific and Practical Medical Center for Mental Health.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)