Keywords
Parkinson´s Disease; Focus of attention; External focus of attention; Internal focus of attention; Motor learning; Balance; Gait
People with Parkinson’s disease (PwPD) suffer from a number of motor and non-motor disorders. Research in the field of motor learning suggests the superiority of an external focus of attention (EFA) compared to an internal focus of attention (IFA), including for PwPD. However, no systematic review with meta-analysis has yet examined the effectiveness of different attentional foci across medication states (ON & OFF) for this population. This study aims to evaluate the effectiveness of an EFA versus an IFA on balance, gait, and motor symptom severity in PwPD during both medication states.
The study design was a systematic review with meta-analysis. Four electronic databases were searched for eligible studies. Methodological quality was assessed with the ROBUST-RCT assessment.
Nine studies with a total of 240 subjects were used for the analyses. The meta-analysis for the ON/normal medication status did not indicate an effect in favour of any attentional focus for balance (SMD: 0.00; 95% CI between -0.46 and 0.46), gait (SMD: 0.11; 95% CI between - 0.30 and 0.53), and motor symptom severity (SMD: -0.16; 95% CI between -0.55 and 0.22). The meta-analysis for the OFF medication status did not indicate an effect in favour of any attentional focus for balance (SMD: 0.15; 95% CI between -0.24 and 0.54), gait (SMD: 0.16; 95% CI between -0.56 and 0.88), and motor symptom severity (SMD: -0.15; 95% CI between -0.53 and 0.24).
Neither attentional focus showed a significant benefit over the other for balance, gait, or motor symptom severity in PwPD regardless of medication state. In the absence of a group effect, the choice of attentional focus may be determined by a person-centered approach, considering aspects such as individual preferences, training duration, and medication. The results of this work should only be interpreted considering the risk of bias.
Parkinson´s Disease; Focus of attention; External focus of attention; Internal focus of attention; Motor learning; Balance; Gait
This revised version reflects substantial revisions made in response to peer review. Key methodological improvements include a more detailed assessment of heterogeneity across interventions, populations, and outcome measures, now presented in an expanded Table 2. We have clarified the criteria for outcome selection and use of standardised mean differences to enhance comparability across studies. Sensitivity analyses have been added to examine the influence of intervention duration, disease duration, and methodological quality on the primary outcome (balance), addressing concerns regarding wide confidence intervals and imprecision.
We have replaced the PEDro scale with the more robust ROBUST-RCT tool for risk of bias assessment, in line with current best practice. The rationale for separate analyses based on medication state (ON vs OFF) has been expanded, supported by recent neurophysiological evidence. Furthermore, the discussion has been strengthened to highlight the clinical implications of our findings, particularly the need for personalised rehabilitation strategies in Parkinson’s disease due to the observed heterogeneity in response to attentional focus strategies. Additional references have been included to support these revisions.
See the authors' detailed response to the review by Sachchida Rai and Payal Singh
People with Parkinson’s disease (PwPD) suffer from a number of motor and non-motor disorders. The main parkinsonian motor disorders are (Gibb & Lees, 1988; Kalia & Lang, 2015): Bradykinesia, muscle rigidity, resting tremors, postural and gait instability. The parkinsonian motor disorders are caused by a disruption of a motor circuit in the central nervous system. This motor circuit consists of a loop between the motor cortex, the basal ganglia and the thalamus. This loop is essential for the initiation, modulation, and execution of voluntary movements (Kandel et al., 2000). The motor cortex sends motor signals to the basal ganglia, which act as a processing centre for the planned movement to refine and modulate it (Young et al., 2023). Once the motor signals have been modulated by the basal ganglia, they are relayed by the thalamus and return to the motor cortex (Ludwig et al., 2017). Thus, the signals for planned movements are adequately transmitted to the brainstem and spinal cord to be executed by the muscles (AbuHasan & Munakomi, 2023).
The basal ganglia play an important role in Parkinson’s disease. This set of structures includes the striatum, the pallidum, the subthalamic nucleus, and the substantia nigra (pars compacta and pars reticularis) (Kandel et al., 2000). The pars compacta contains the cells responsible for dopamine production (Sonne et al., 2023). This neurotransmitter has neurochemical properties that affect motor control, cognitive functions, and limbic emotional functions (Sonne et al., 2023). The dopaminergic projections from the pars compacta (mainly to the striatum) have a regulatory function on the ganglionic circuit and thus on the motor circuit. In PwPD, the dopaminergic cells of the pars compacta in the substantia nigra degenerate early for an unknown reason. Due to the lack of dopamine in the basal ganglia, the motor circuit is disrupted, and parkinsonian motor disorders are one of the consequences of this dysfunction (Sonne et al., 2023).
The primary medical treatment for motor symptoms involves medications that increase intracerebral dopamine concentration or stimulate dopamine receptors (Kalia & Lang, 2015; Keus et al., 2014). Levodopa is the most effective medication for treating motor symptoms of PD (Connolly & Lang, 2014). The terms “ON” and “OFF” are used to distinguish phases characterized by motor and non-motor functions according to the person’s dopaminergic state. The onset of the “ON” phase depends on the response time to levodopa, which varies between 30 and 60 minutes depending on the stage of the disease (Sohn et al., 1994). The ON phase is marked by the improvement or even disappearance of motor or non-motor symptoms, which consequently increases the functional capacities of the PwPD. The OFF phase is characterized by the appearance or worsening of motor or non-motor symptoms, which consequently decreases the functional capacities of the PwPD (Chou et al., 2018). These two phases are influenced by the progression of the disease and are therefore specific to each individual.
Next to medical treatment non-medical interventions such as physiotherapy are recommended for motor disorders in PwPD (Keus et al., 2014). A wide range of physiotherapy interventions exists with little differences in treatment effects (Tomlinson et al., 2012). In their meta-analysis, Radder et al. (2020) found that conventional physiotherapy had positive effects on gait and motor symptoms, while strategy training specifically improved balance. Strategy training entails instructing patients in specific methods to enhance their motor skills and daily activities and may include external cues, cognitive movement strategies or compensatory strategies.
A relatively new approach utilising the focus of attention to improve motor skill acquisition was introduced by Wulf et al. (1998). The attentional focus is the place or concept to which a person directs their attention when performing a movement. Although there are several different attentional foci, Wulf et al. (1998) defined two of them.
An internal focus of attention (IFA) refers to concentrating on one’s own body movements. In contrast, an external focus of attention (EFA) directs attention towards the impact of those movements on the surrounding environment. A substantial body of research indicates that where an individual directs their attention greatly affects both the performance and acquisition of motor skills (Wulf, 2013). Favre-Bulle et al. (2024) conducted a systematic review with meta-analysis, concluding that employing an external focus of attention (EFA) provides benefits for both the learning and execution of motor skills in sports, as opposed to an internal focus of attention (IFA). The superiority of an EFA over an IFA is explained by the constrained action hypothesis. It is suggested that using an IFA tends to create interference between conscious and automatic motor processes (Wulf et al., 2001). Thinking about the positioning of one’s body parts reduces the ability to engage automatic processes. Thus, automatic processes are disrupted by conscious attempts to control movement.
The regulation between automatic movements and conscious voluntary movements is a major function of the basal ganglia (Hikosaka & Isoda, 2010). This structure is affected by the lack of dopamine in PwPD. Some studies suggest that when dopamine is replaced through medication (ON), PwPD may be able to benefit from the automatic processes activated by an EFA like healthy subjects (Piccoli et al., 2018; Wulf et al., 2009). Nevertheless, the opposite hypothesis is also proposed: using an EFA in medicated subjects (ON) would recruit automatic circuits that are still damaged, although under medications, and consequently decrease motor performance (Beck & Almeida, 2017).
Several primary studies have investigated the effectiveness of different attentional foci in people with PwPD (e.g. Beck et al., 2020; Chen et al., 2023), but existing systematic reviews either did not perform a meta-analysis (Piccoli et al., 2018) or did not specifically focus on PwPD (Chua et al., 2021). The aim of this systematic review was to evaluate the effect of specific attentional foci (external versus internal) on balance, gait, and the severity of motor symptoms in PwPD. Given that adequate medication is crucial for treating motor disorders in PD, this study examined the effects of both external and internal attentional foci in the ON and OFF states.
The study design was a systematic review with meta-analysis following the guidelines of the Cochrane Handbook (Higgins et al., 2022). The report was structured using the PRISMA statement (Moher et al., 2010) (RRID:SCR_018721).
The studies needed to assign participants to different intervention groups to allow for a comparison. This could be an intervention group and a control group or multiple different intervention groups. Studies based on a crossover design were also included. The following eligibility criteria for articles were defined:
Population: Individuals with PD or a parkinsonian disorder were included. The stage of the disease reported in Hoehn & Yahr stages (H&Y) (Hoehn & Yahr, 1967) was not an excluding factor, and no variation of the disease was excluded.
Interventions: Instruction of at least one attentional focus during acquisition of a motor task. Multiple attentional foci could be compared within the same study. This could involve either internal or external attentional focus. Other attentional foci were also eligible.
Comparison: Included studies needed to have a control group. This group could be instructed with a different attentional focus, receive no attentional instructions, or undergo usual treatment.
Outcomes: The effect of the attentional focus had to be measured on at least one of the following outcomes: balance, gait or the motor symptom severity. The medication status during the intervention and measurement had to be specified (ON, OFF, or normal). For ON and OFF states, the duration since the last medication intake had to be specified.
The search strategy was constructed using two search concepts (i.e. population and intervention). Multiple search terms for the population concept ‘Parkinson’s disease’ and the intervention concept ‘attentional focus’ were first combined using the Boolean operator ‘OR’. In the next step, both concepts were combined using the operator ‘AND’.
The search was conducted in four electronic databases (MEDLINE (OVID), Cochrane CENTRAL, Embase, and CINAHL Ultimate). For each database specific subject headings were added to the search terms if available. The design of the search string is presented in Table 1.
The selection process was divided into three phases. For each phase, the eligibility criteria were applied to decide on the inclusion of studies. All search results from each database were collated using RAYYAN software (RRID:SCR_017584) (Ouzzani et al., 2016) https://www.rayyan.com. First, duplicates were automatically removed using Rayyan’s deduplication functionality. Second, titles and abstracts were screened independently by GC and MK. Third, GC and MK independently reviewed the full texts of the studies that could potentially be included. In case of disagreements a third reviewer was used to solve the conflicts (KMS).
Data extraction was jointly performed by two reviewers, GC and MK. If data was only available in graphical form the WebPlotDigitizer (RRID:SCR_013996) (Rohatgi, 2017) was used to extract numerical data. We extracted data related to outcomes and outcome measures as well as demographic and clinical data.
The primary outcome in this review was “balance”, defined as the action of maintaining, achieving, or restoring a state of balance during posture or activity (Pollock et al., 2000). Outcome measures for this outcome included balance parameters like sway or balance tests (e.g. the Berg Balance Scale (BBS) or the Sensory Organization Test). When multiple outcome measures were present in a study, the most frequently used among included studies was selected. In cases of equal frequency, the BBS was prioritised due to its strong psychometric properties and widespread validation in people with Parkinson’s disease (Winser et al., 2019). The second outcome gait was defined as follows: Non-pathological gait is defined as a series of rhythmic, systematic, and coordinated movements of the limbs and trunk that result in the advancement of the body’s centre of gravity (Winter, 1987). Potential eligible outcome measures included gait parameters (e.g. walking speed, step variability, or symmetry) or gait tests like the Timed Up and Go (TUG). When multiple measures were present in a study, the most frequently used among included studies was selected.
The third outcome in this review was motor symptom severity. This included the expression of major motor symptoms related to PD, such as performing daily motor tasks, speech, facial expressions, muscle rigidity, or resting tremors (Goetz et al., 2008). Eligible outcome measures were the third part of the Unified Parkinson’s Disease Rating Scale (UPDRS-III) or its modified version (Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III)). When multiple measures were present in a study, the most frequently used among included studies was selected. Across all outcome domains, when no clearly established gold-standard measure existed, frequency of reporting served as the sole criterion for selection.
Given the well-established influence of dopaminergic medication on motor symptoms in Parkinson’s disease, data were analysed separately for ON and OFF medication states. Participants who maintained their usual medication regimen were considered to be in the ON state, typically marked by improved mobility, while the OFF state reflects a return of motor symptoms and reduced functional capacity (Chou et al., 2018). Differentiating these states was clinically important, as attentional focus strategies may yield different outcomes depending on medication status. Moreover, recent translational research highlights that underlying neural mechanisms (such as impaired synaptic plasticity and circuit dysfunction) vary with dopaminergic availability (Rai et al., 2024). Separate analyses therefore allowed for a more accurate assessment of how attentional focus interacts with medication-related motor fluctuations, which is critical for tailoring rehabilitation strategies in clinical settings.
Outcome data was extracted as close to the end of the intervention as possible. Some studies measured the effect of an attentional focus following a training program, in which case the measurement closest to the end of the training program was used. When studies reported outcomes in both medication states (i.e. ON and OFF state), measurements were taken as close to the end of the intervention on different days corresponding to different medications days.
Measurement and intervention conditions: Measurement conditions could vary between studies. If there was a choice among several conditions, such as measuring balance on a stable or unstable surface, the condition most frequently applied in all studies was used. For medication state, if subjects were instructed to maintain their usual medication, measurements were considered in a “normal” medication state and analysed together with the other data in ON state.
Risk of Bias was evaluated with the newly developed ROBUST-RCT tool. This tool addresses limitations in existing risk of bias assessment instruments and provides a structured approach for assessing bias in randomised controlled trials (Wang et al., 2025). Risk of bias was evaluated for the primary outcome balance. If a study did not report on this outcome, we based the rating on the outcome gait and if still not reported we based the rating on the outcome motor symptom severity.
The statistical analysis followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks et al., 2019). The effectiveness of an attentional focus was analysed for the outcomes balance, gait, and severity of motor symptoms in ON/normal and OFF states. Meta-analyses were conducted using Review Manager 5.4 software (RRID:SCR_003581). For each outcome, means, standard deviations, and the number of participants were used for the meta-analyses. If standard deviations were not available, they were calculated from other available variability measures using Review Manager’s calculator. An inverse variance model with random effects was used (DerSimonian & Laird, 1986). Efficacy was evaluated using standardized mean differences (SMD) and 95% confidence intervals (CI), allowing us to compare effect sizes across studies that used different scales to assess similar outcomes. The SMD expresses the intervention effect in units of standard deviation, which standardises the results and enables meaningful aggregation of data from studies using different measurement instruments (Deeks et al., 2019).
Effect sizes were classified based on Cohen’s (2016) guideline, with categories set at 0.2 for a small effect, 0.5 for a moderate effect, and 0.8 for a large effect. Statistical heterogeneity was evaluated using the I2 statistic, categorised as follows: 0% - 40% (potentially unimportant), 30% - 60% (moderate), 50% - 90% (substantial), and 75% - 100% (considerable) (Deeks et al., 2019). Funnel plots were employed to examine the potential for reporting bias.
A total of 726 records were identified from the four databases. After excluding duplicates, 558 records were screened based on their titles and abstracts. We excluded 543 records excluded in this process. Of the remaining 15 publications, the full texts of 14 studies were retrieved. The study not retrieved was a conference paper. Despite contacting the author, no response could be obtained. After examining the full texts according to the inclusion criteria, five studies were excluded for the following reasons: in one study, the intervention was not compliant; three manuscripts did not meet the comparison criteria; and one work was excluded because it did not measure the desired outcomes. In the end, nine studies were selected for this research. The selection process is illustrated in Figure 1 with a flow diagram.
The main characteristics of the included studies are presented in Table 2. The nine selected studies included four randomised controlled trials (RCTs) and five crossover studies. Chen et al. (2023), Beck et al. (2018, 2020), and Landers et al. (2016) are randomised controlled trials. Landers et al. (2005), Beck and Almeida (2017), Wulf et al. (2009), Martin et al. (2023) and Jazaeri et al. (2018) are crossover studies. A total of 240 subjects were included for analysis. The stage of the disease was given by the Hoehn & Yahr stages, (Hoehn and Yahr (II-III)) and the disease duration by the average number of years (6.52 years). In five studies, the intervention was conducted in a single session (Beck & Almeida, 2017; Jazaeri et al., 2018; Landers et al., 2005; Martin et al., 2023; Wulf et al., 2009). In the other four studies, training programmes with specific attentional foci for each group were analysed (Beck et al., 2018, 2020; Chen et al., 2023; Landers et al., 2016). The medication status during specific interventions is shown in the column corresponding to each endpoint. In three studies, outcomes were measured in both medication states (Beck et al., 2018, 2020; Chen et al., 2023).
| Study-ID | Design | Sample size, age | Stage (H&Y), disease duration (years) | Intervention | Endpoint assessment | Medication state & outcome (Measures) |
|---|---|---|---|---|---|---|
| Beck et al. 2017 | Crossover | N=19 Age=71.4 (SD=6.1) | Stage: II & III Time: M=7.6 | Participants on unstable platform. Measurements under both attentional conditions. EFA: Focus on minimising platform movements. IFA: Focus on minimising foot movements. | Two sessions (ON & OFF) with at least 48h apart. | ON & OFF Balance (Sway: PSI; angular excursion of the center of gravity on Biodex Balance System) |
| Beck et al. 2018 | RCT | N=39 EFA: Age=68.63 (SD=9.91) IFA: Age=73.05 (SD=7.84) | Stage: n.a. Time: EFA: M=7.0 (SD=5.01) IFA: M=6.70 (SD=4.16) | SAFEx program, for 11 weeks, 33 sessions of 60 min, ON. Two groups with different instructions. EFA: Focus on the movement of stickers on limbs. IFA: Focus on the movements of their limbs. | One week after the program. | ON & OFF Walking (Speed cm/s on Zeno Walkway—ProtoKinetics 9.75m long) Motor Symptoms (UPDRS-III) |
| Beck et al. 2020 | RCT | N=35 EFA: Age=65.4 (SD=6.21) IFA: Age=73.0 (SD=8.06) | Stage: n.a. Time: EFA: M=6.60 (SD=5.18) IFA: M=6.73 (SD=3.73) | Training program, 11 weeks, 33 sessions of 60 min, ON. Two groups: FAE (Sham): Attention directed to stickers on limbs (back of each hand, back of each foot, patella, and medial epicondyle of the humerus). FAI (SAFEx): Continuous attention on sensory feedback of limbs in space. | Two different days within the week after programme end. | ON & OFF Motor Symptoms (UPDRS-III) |
| Chen et al. 2023 | RCT | N=30 EFA: Age=68.93 (± 5.3) IFA: Age=69.40 (± 5.3) | Stage: EFA: M=2.8 (± 0.5) IFA: M=2.8 (± 0.4) Time: EFA: M=9.9 (± 8) IFA: M=10.1 (± 5.3) | Dual-task walking training program for 6 weeks, 12 sessions of 60 min, ON. Two groups with different instructions reminded of respective focus every 5 min. EFA: Focus on the movement of stickers on limbs. IFA: Focus on the movements of their limbs in space. | Within the week after the programme, 1-2 days between ON & OFF measures. | ON & OFF Balance (BBS) Walking (TUG) Motor Symptoms (MDS-UPDRS-III) |
| Jazaeri et al. 2018 HA-PD | Crossover | N=17 Age=63.06 (± 11.62) | Stage: M=2.41 (± 0.71) Time: n.a. | Two attentional conditions on a foam cushion. EFA: Focus on rectangular papers under feet without looking at them. IFA: Focus on feet without looking at them. | Single session: 2×70s per condition, 60s between trials, 5min between conditions. | OFF Balance (Postural sway on unstable platform) |
| Jazaeri et al. 2018 LA-PD | Crossover | N=17 Age=61.94 (± 8.40) | Stage: M=2 (± 0.61) Time: n.a. | Same as above. | Sam as above. | OFF Balance (Postural sway on unstable platform) |
| Landers et al. 2005 | Crossover | N=22 Age=72.7 | Stage: II & III Time: M=6.7 | Two attentional foci during the fourth condition of the Sensory Organization Test, normal medication: eyes open on unstable surface and fixed environment. EFA: “Keep the paper rectangles horizontal”; IFA: “Keep your feet horizontal.” | Single session: 20s per condition, 3 min between attentional conditions. | Normal Balance (Sensory Organization Test) |
| Landers et al. 2016 | RCT | N=22 EFA: Age=72.2 (± 4.4) IFA: Age=70.2 (± 4.4) | Stage: EFA: M=2.86 IFA: M=2.25 Time: n.a. | Balance program, for 4 weeks, 12 sessions of 45 min, normal medication. Two groups with different instructions. Example of EFA instruction: “Focus on keeping the platform level.” Example of IFA instruction: “Focus on maintaining equal pressure on the base of both feet.” | Immediately after the program. | ON Balance (BBS) Walking (Speed m/s Self-Selected Gait Velocity) |
| Martin et al. 2023 | Crossover | N=13 Age=68.46 (SD=9.11) | Stage: n.a. Time: M=5.38 (SD=3.3) | Sit To Stand (STS) under two attentional conditions; ON. EFA: “Touch my hand and stand up towards the ceiling.” IFA: “Lean forward at the hips and stand up until your back is straight.” | Single session: 30 seconds per condition. | ON Balance (Sway after STS) |
| Wulf et al. 2009 | Crossover | N=14 Age=71.1 | Stage: II & III Time: n.a. | Two attentional conditions on a balance cushion. EFA: Minimizing disc movements. IFA: Minimizing foot movements. | Single session: four 15-second trials per attentional condition. | ON Balance (Sway; inflated rubber disk placed on force platform measuring centre of pressure) |
The evaluation of the methodological quality of the included studies showed that the studies by Landers et al. (2016), with two items rated as having probable or definite risk of bias, and Chen et al. (2023), with three such items, had the lowest overall risk of bias among all selected studies. The studies by Landers et al. (2005) and Beck et al. (2017) had the lowest methodological quality, with each having five items rated as having a definitive or probable risk of bias. The criterion of sequence generation presented a risk of bias only in the Landers et al. (2005) study. All studies demonstrated a risk of bias related to the blinding of healthcare providers. However, the study by Landers et al. (2016) was the only one to report participant blinding, suggesting a probable low risk of bias for this criterion. The risk of bias assessment was based on the outcome ‘balance’ for all studies, except for Beck et al. (2018), which focused on ‘gait’, and Beck et al. (2020), which focused on ‘motor symptoms’. The assessment of methodological quality is shown in Figure 2.
In the following section, we present the syntheses for the outcomes: balance, gait, and motor symptoms. For each outcome, the analyses are reported separately based on the medication status of the participants (On/normal and OFF).
Balance
For the assessment of an attentional focus on balance in the ON/normal state, six studies could be included for the EFA-IFA comparison ( Figure 3). The EFA group comprised 93 subjects, while the IFA analysis included 94 subjects. Four studies involved single-session interventions (Beck & Almeida, 2017; Landers et al., 2005; Martin et al., 2023; Wulf et al., 2009) and the other two studies involved a training programme: 6 weeks with 12 sessions (Chen et al., 2023) and 4 weeks with 12 sessions (Landers et al., 2016). Three different balance outcome measures were used within this metanalysis. Body sway was assessed in three studies (Beck & Almeida, 2017; Martin et al., 2023; Wulf et al., 2009). Two studies assessed sway on an unstable surface (Beck & Almeida, 2017; Wulf et al., 2009). One study measured sway on a stable surface after sit-to-stand (Martin et al., 2023). The Berg Balance Scale (BBS) was used in two studies (Chen et al., 2023; Landers et al., 2016). The fourth condition of the Sensory Organization Test was used in one study (Landers et al., 2005). The meta-analysis showed no effect in favour of an attentional focus (SMD = 0.00; 95% CI between -0.46 and 0.46). Statistical heterogeneity was moderate (I2 = 58%).
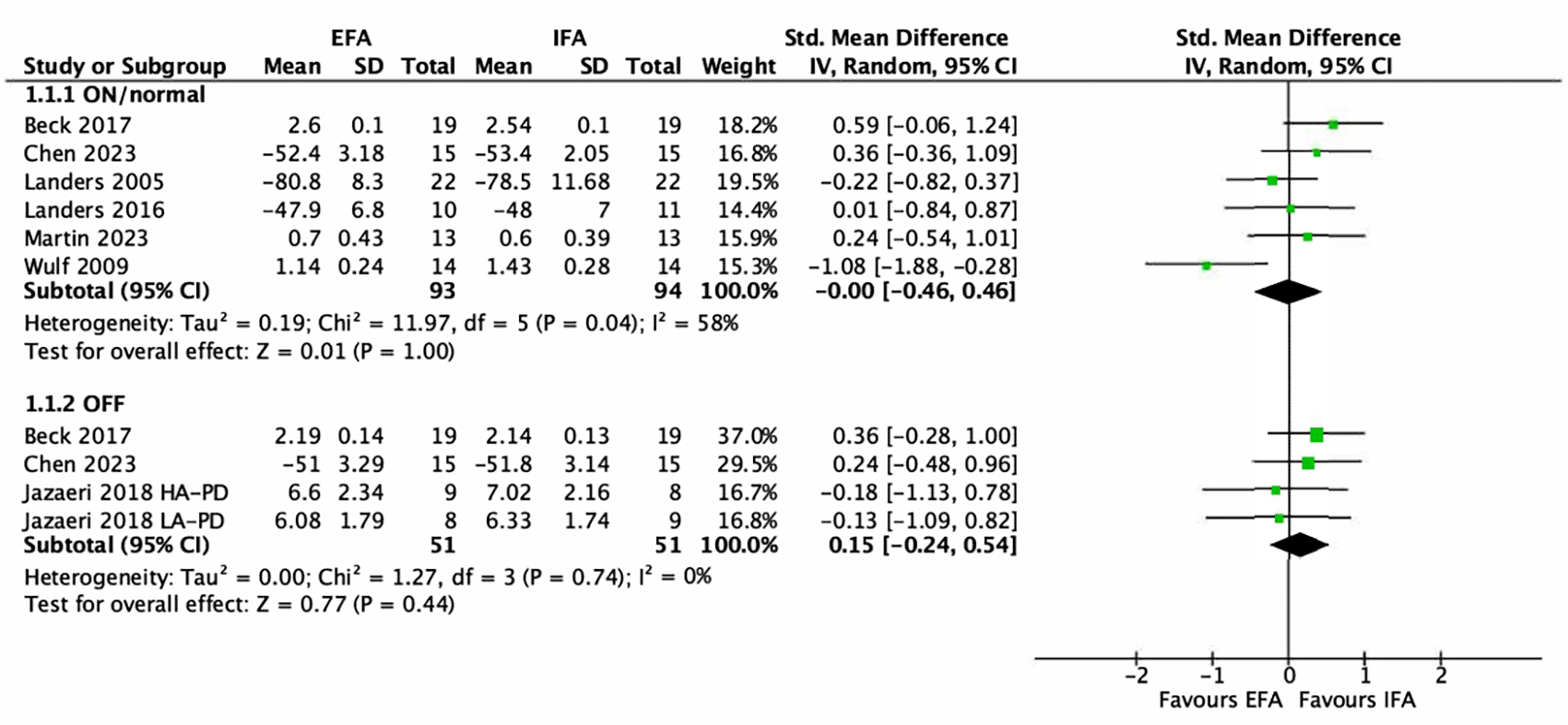
NB. EFA (external focus of attention), IFA (Internal focus of attention).
For the analysis of balance during an OFF-medication status, four studies were included ( Figure 3). Both groups (EFA and IFA) consisted of 51 participants each. Two studies reported single-session interventions (Beck & Almeida, 2017; Jazaeri et al., 2018) and one used a training programme (Chen et al., 2023). Two balance outcome measure were employed. Body sway was assessed in two studies (Beck & Almeida, 2017; Jazaeri et al., 2018). Both sway assessments were performed on an unstable surface.
The BBS was used in one study (Chen et al., 2023). The meta-analysis showed no effect in favour of an attentional focus (SMD = 0.15; 95% CI between -0.24 and 0.54). Heterogeneity was not important (I2 = 0%).
Gait
Concerning the evaluation of an attentional focus on gait in the ON/normal drug state, three studies could be included for the EFA versus IFA comparison ( Figure 4). The EFA group comprised 44 and the IFA group 46 participants. All three studies were interventions with a training programme (Beck et al., 2018; Chen et al., 2023; Landers et al., 2016). Two different gait outcome measures were used for the metanalysis. Walking speed was assessed in two studies (Beck et al., 2018; Landers et al., 2016). One measured walking speed in cm/s (Beck et al., 2018). The other study measured speed in m/s (Landers et al., 2016). The Timed Up and Go test was used in one study (Chen et al., 2023). The meta-analysis showed no effect in favour of an attentional focus (SMD = 0.11; 95% CI between -0.30 and 0.53). Heterogeneity was not important (I2 = 0%).
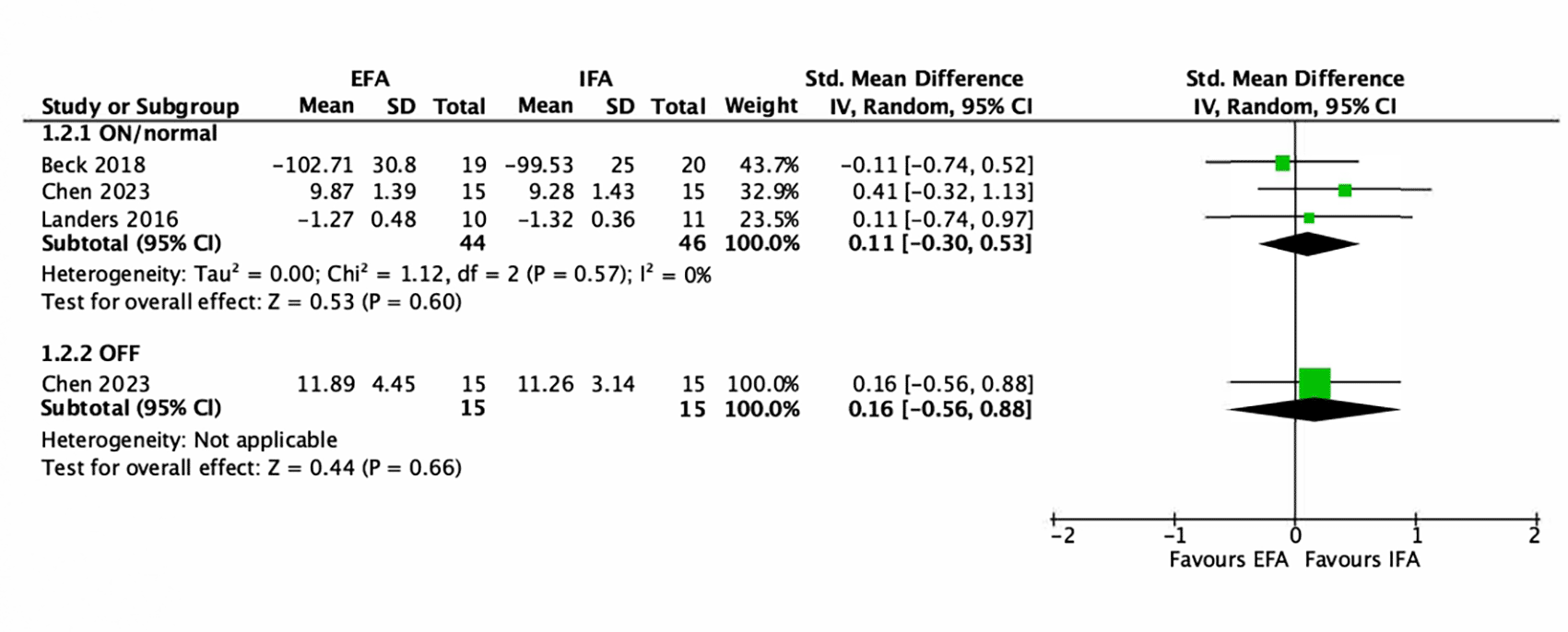
NB. EFA (external focus of attention), IFA (Internal focus of attention).
For the analysis of gait in the OFF-medication state, only the Chen et al. (2023) study was included for the EFA versus IFA comparison ( Figure 4). Both groups consisted of 15 participants. This study involved a training programme (6 weeks with 12 sessions). The Timed Up and Go (TUG) was used to measure walking. The meta-analysis showed no effect in favour of an attentional focus (SMD = 0.16; 95% CI between -0.56 and 0.88).
Severity of motor symptoms
Concerning the evaluation of an attentional focus on the severity of motor symptoms in the ON/normal drug state, three studies could be included for the EFA versus IFA comparison ( Figure 5). These were the studies by Beck et al. (2018, 2020) and Chen et al. (2023). All three studies were training programmes (duration: 11 weeks with 33 sessions for the Beck et al. studies and 6 weeks with 12 sessions for the Chen et al. study). The EFA analysis involved 51 participants, while the IFA analysis involved 53 participants. Two outcome measures were used to assess the severity of motor symptoms in the metanalysis. The Beck et al. (2018, 2020) studies used the third part of the Unified Parkinson’s Disease Rating Scale (UPDRS-III. Chen et al. (2023) used the third part of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III). The meta-analysis showed no effect in favour of an attentional focus (SMD = -0.16; 95% CI between -0.55 and 0.22). Heterogeneity was not important (I2 = 0%).

NB. EFA (external focus of attention), IFA (Internal focus of attention).
For the severity of motor symptoms in the OFF state, three studies could be included for the EFA versus IFA comparison ( Figure 5) (Beck et al., 2018, 2020; Chen et al., 2023). The EFA group comprised of 51 and the IFA group of 53 participants. In all three studies, a training programme was implemented, utilising the same two outcome measures as in the ON state analysis. The meta-analysis showed no effect in favour of an attentional focus (SMD = -0.15; 95% CI between -0.53 and 0.24). Heterogeneity was not important (I2 = 0%).
The presence of a possible reporting bias was analysed for the EFA versus IFA comparison for balance was analysed with a funnel plot ( Figure 6). We could not identify a reporting bias, but this may be caused by the limited number of included studies.
We explored the influence of the factors: duration of the interventions, disease duration, stage of the disease and methodological quality in more depth to identify whether they could have masked a potential effect on the primary outcome balance.
Sensitivity analysis – duration of the intervention
Within this analysis, we excluded studies with single-session interventions on the outcome balance. Only studies going over a longer duration were retained. For the ON/normal medication condition two studies remained within the analysis (Chen et al., 2023; Landers et al., 2016) reporting on 51 PwPD. Effectiveness was in favour of IFA (SMD: 0.22; 95% CI: -0.33 to 0.77). Heterogeneity was low with an I2 value of 0%. For the OFF condition only the study of Chen et al. (2023) remained. Effectiveness was in favour of IFA (SMD: 0.24; 95% CI: -0.48 to 0.96).
Sensitivity analysis – disease duration
Within this analysis, we excluded studies with a mean disease duration of less than 6 years on the outcome balance. Under the ON/normal medication condition, three studies (Beck et al., 2017; Chen et al., 2023; Landers et al., 2005) were included, comprising a total of 112 PwPD. The analysis indicated a small effect in favour of IFA, with a SMD of 0.22 and a 95% confidence interval ranging from –0.28 to 0.72. Heterogeneity was moderate, with an I2 value of 43%. For the OFF condition, two studies remained within the analysis (Beck et al., 2017; Chen et al., 2023) with a total of 68 PwPD. Effectiveness was in favour of IFA (SMD: 0.31; 95% CI: -017 to 0.79) with an I2 value of 0%.
Sensitivity analysis – stage of disease
Further exploration on the potential influence of the variable stage of the disease (i.e. the H&Y stage) was not possible, because all studies reported on PwPD with H&Y stages between 2 and 3 or did not report this variable at all.
Sensitivity analysis – methodological quality
With this analysis we explored whether the rated methodological quality of the included studies could have influenced the primary outcome balance. We excluded the studies with the highest amount of definitive or probable risk of bias ratings (i.e. Landers et al., 2005; Beck et al., 2017). Under the ON/normal condition four studies remained within the analysis (Chen et al., 2023; Landers et al., 2016; Martin et al., 2023; Wulf et al., 2009). The analysed effect size shifted slightly in favour of the EFA group. However, the magnitude did not reach the threshold for a small effect (SMD = -0.11; 95% CI: -0.75 to 0.54), indicating a statistically non-significant and clinically negligible difference. Heterogeneity was moderate (I2 = 63%). For the Off condition, the effect size was negligible (SMD = 0.03; 95% CI: -0.46 to 0.52), indicating no meaningful difference between groups and a statistically non-significant result.
The aim of this systematic review was to evaluate the effectiveness of an internal versus an external attentional focus on balance, walking, and the severity of motor symptoms in two dopaminergic states (ON and OFF) in PwPD.
The main finding was that neither type of attentional focus (internal or external) significantly affected balance, walking, or the severity of motor symptoms in PwPD, regardless of their dopaminergic status. The effect sizes were close to zero and did not reach the threshold for a small effect. Furthermore, the included studies showed inconsistent results, with some favouring an EFA and others showing benefits for an IFA.
Based on the primary outcome balance, we discuss different hypotheses in this section to contextualise the results. For the outcome of balance, two studies (Landers et al., 2005; Wulf et al., 2009) showed results favouring EFA during an ON state in PwPD. These findings are consistent with previous research on an EFA in healthy individuals (e.g. Favre-Bulle et al., 2024; Wulf, 2013). Since dopamine plays an important role in regulating the ganglionic circuit, these results may suggest that dopamine supplementation replaces the function of impaired dopaminergic cells, enabling the benefits of EFA to be realised (Young et al., 2023; Singh et al., 2024). With the help of medication, PwPD would therefore be able to initiate and use automatic processes as effectively as healthy individuals.
Conversely, the study by Beck and Almeida (2017) suggests that an IFA is superior to an EFA for balance in both dopaminergic statuses. Surprisingly, subjects showed better balance with an IFA in the OFF state than in the ON state.
This phenomenon might be explained by the assumption that dopaminergic replacement forces the involvement of a greater number of impaired structures in the motor process than in the OFF state. Without medication, conscious processes involving brain areas less affected by the lack of dopamine as required by IFA would be used (Beck & Almeida, 2017; Wu et al., 2015; Wu & Hallett, 2005). This might explain the superiority of IFA in the OFF states and support the hypothesis that an EFA engaging impaired neural circuits may be more useful in ON states in PwPD. However, the present metanalysis did not support this hypothesis. Dopaminergic medication may not be sufficient to fully compensate for the loss of motor automaticity in PwPD. Indeed, the action of levodopa on automaticity is not yet clear (Wu et al., 2015). PwPD would therefore think partly consciously about how they perform movements and this regardless of instructed attentional focus and medication status. The disadvantage of IFA compared to EFA is that it leads to interference between conscious and automatic processes as stated with the “constrained action hypothesis” (Wulf et al., 2001), which hypothesis that using an EFA allows the nervous system to generate motor commands in an automatic and efficient way. Instead, using an IFA can cause a disruption of the natural evolution of the motor system. However, given the current findings, it might be that in PwPD the positive effects of an EFA are not visible because of the above-mentioned involvement of impaired brain structures and deficits in automaticity. Thus, the disadvantages of an IFA compared with an EFA would disappear in PwPD, since both (EFA and IFA) would cause interference, regardless of the drug state.
In the following section, we interpret the results in the context of existing evidence from other systematic reviews. To our knowledge two systematic reviews on the effectiveness of different attentional foci in PwPD have been published (Chua et al., 2021; Piccoli et al., 2018).
In the study by Chua et al. (2021), an EFA is reported to be superior to an IFA in a wide range of populations (i.e. the analyses comprised PwPD but people with other clinical conditions were also included into the analyses). Within their analyses the influence of several potential factors on the effectiveness of the attentional foci were analysed. The potential factors were among others: age, level of motor skill, or health status. Health status was defined as a variable to observe if the ability to control a movement with an attentional focus could be altered by certain clinical conditions. Thus, PD was grouped with other clinical conditions that could influence the effect of attentional focus. However, the pathophysiological processes and the motor disorders in PD are unique and do not necessarily share similarities with conditions such as stroke or musculoskeletal problems.
Another systematic review that compared the effect of different attentional foci on PwPD was published by Piccoli et al. (2018). This review included four studies for the effectiveness of attentional foci in PwPD (Beck & Almeida, 2017; Kakar et al., 2013; Landers et al., 2005; Wulf et al., 2009). The systematic review by Piccoli and colleagues analysed the four studies qualitatively and did not perform a meta-analysis. The author confirmed the benefit of an EFA compared with an IFA during an upper limb motor task in the ON state in PwPD. However, this result is mainly based on the study by Kakar et al. (2013) where participants performed a dart-throwing task. Given the differences between the motor tasks of dart-throwing and the balance tasks in the current review, several factors may be relevant for the different findings: i) the nature of the tasks (dart-throwing involves a goal-oriented task with an external target, whereas balance tasks frequently do not involve such clear targets); ii) motor demands (dart-throwing involves fine motor skills with hand-eye coordination, whereas balance tasks require more gross motor skills and postural adjustments); and iii) cognitive load (maintaining balance may require higher cognitive loads compared to dart-throwing). We did not include the study by Kakar et al. (2013) as upper limb motor tasks were not considered in this review. Similar to this systematic review the analysis of Piccoli et al. (2018) found contradictory results for the balance outcome.
A limitation of this systematic review was that we included studies with different acquisition periods. We included both studies with a single training session and studies using a training period over several weeks. It is difficult to exclude a carry-over effect in the studies with a single training session. These studies used a crossover design. Participants received a training intervention with an EFA and IFA during the training period. Performance measured with a post-acquisition test might be biased because participants may have used a different attentional focus than what was intended by the test instructions. The wash-out period between the two interventions was generally very brief and never reached a duration where interference from the instructions could be ruled out. This risk of bias is not present in studies using only a single focus of attention during the acquisition phase.
Another limitation could be related to the dopaminergic state during the measurements in the study by Landers et al. (2005). The subjects in this study were instructed to maintain their usual medication regimen the day before and the day of the measurements. We assumed that the subjects were taking dopaminergic medications. The medication state was considered “normal” during these measurements. Unlike other studies that required about an hour of waiting after taking the medication for ON measurements, it is not possible to know the precise dopaminergic state of the subjects in the study by Landers et al. (2005).
The differences in duration and frequency of the training programs are another limitation. Indeed, the influence of the duration of a programme based on a specific attentional focus could be observed in the analysis of motor symptom severity (i.e. a longer training period causes a longer exposure to a certain attentional focus, which might increase the effectiveness of this focus). The studies by Beck et al. (2018, 2020) implemented a program of 33 sessions over 11 weeks (three sessions per week). The study by Chen et al. (2023) is based on a programme approximately half as long, lasting six weeks with a total of 12 sessions (two sessions per week). The two studies by Beck et al. (2018, 2020) suggest that the benefits of an EFA on the severity of motor symptoms may emerge from a certain frequency of training over a longer duration.
Another limitation of this study was that the confidence intervals are wide, and precision was low for most of the analyses. In order to identify possible sources of imprecision we analysed the influence of the clinical variables: duration of the intervention, disease duration and stage of the disease on the primary outcome balance with separate sub-group analyses.
We explored the influence of the duration of the intervention with a sensitivity analysis on the primary outcome balance. Within this analysis single session interventions were excluded. Analysis of the longer duration interventions indicated that an IFA was more effective for balance for ON/normal medication with a small effect size. A similar finding was seen for the OFF condition. Although both analyses had high uncertainty with large confidence intervals. This also indicates that an EFA might be more effective for single session interventions.
The hypothesis that individuals with Parkinson’s disease (PwPD) at different stages of disease duration might benefit from different types of attentional focus was also examined through a subgroup analysis. Within this analysis we found that PwPD with a disease duration of more than 6 years seemed to benefit more from an IFA when compared to the full-analysis with all studies. However, the effect remained small with a large confidence interval.
We tried to confirm these results with a sensitivity analysis of the variable stage of the disease (i.e. measured with the H&Y stage) but as all studies reported on the H&Y stages 2-3 a subgroup analysis was not possible.
The influence of the methodological quality of the included studies on the primary outcome was also explored through a subgroup analysis. In this analysis, we excluded the studies with the highest risk of bias rating on the ROBUST-RCT assessment. Effect estimates for both analyses (i.e. ON and OFF medication status) were imprecise, with large confidence intervals that were compatible with effect estimates favouring each group. Therefore, we could not establish an influence of the risk of bias rating on the effect estimates.
The results of the sensitivity analyses are exploratory. Given the small sample sizes and the limited number of included studies we were not able to investigate these variables in more detail using meta-regression.
A further limitation is that the meta-analysis of the balance outcome during the ON state showed a moderate heterogeneity. This was probably caused by a single study showing a large effect in favour of the EFA condition (Wulf et al., 2009). To identify potential reasons, we analysed several factors, including the number of participants, stage of the disease, age, order of focus testing, verbal instructions given to participants, and intervention conditions. After investigating these clinical data, we were unable to identify any differences that might explain this disparity compared with the other studies. More specifically, the study did not involve a longer training duration and comprised only a single session. Participants were considered in the ON state, the average age was 71 years, and the disease stage involved H&Y stages II and III.
Another limitation was the relatively small combined sample size of only 228 participants across all studies, which may compromise the statistical power of the findings.
Although the results of this work do not show superiority of a specific attentional focus in PwPD, it is important to note that they only concern the three analysed outcomes (i.e. balance, gait and motor symptom severity)
Regarding clinical implications, it is important for rehabilitation specialists to observe the patient’s attentional preferences in order to adapt the attention according to their needs. These may vary depending on the progression of the pathology as well as the biopsychosocial factors that are different for each PwPD. Indeed, some studies demonstrate the superiority of an EFA on balance in anxious PwPD or those with a history of falls (Jazaeri et al., 2018; Landers et al., 2005). Therefore, we believe that a patient-centred approach where strategies are developed considering the medication status, the duration of the interventions and in collaboration with the person’s preferences and their environment is essential given the current state of evidence. A patient-centred approach is further supported by the heterogeneity observed in the included studies. Despite the absence of significant group-level effects in the meta-analyses, individual studies reported varying responses to attentional focus strategies, suggesting that some PwPD may benefit more from one approach than another. For instance, Beck and Almeida (2017) found better balance outcomes with an internal focus in the OFF state, while Landers et al. (2005) and Wulf et al. (2009) reported benefits of an external focus in ON states. These inconsistencies may reflect underlying differences in disease stage, anxiety levels, or attentional preferences (i.e. factors that are not captured in group-level analyses but are critical in clinical practice).
This patient-centred approach is supported by expert opinion, such as that expressed by Okun (2017), who emphasises that due to the complexity and fluctuating nature of Parkinson’s disease, treatment should be tailored to individual patient needs. Other sources, such as Khanna and Jones (2023), argue that the wide range of distinct patient phenotypes (i.e. characterized by varying combinations of motor and non-motor symptoms) necessitates a personalised treatment approach in PwPD.
Clinically, this suggests that rehabilitation professionals should assess and incorporate individual attentional preferences, medication status, and psychosocial factors when designing interventions. Future RCTs should consider stratifying participants based on these variables or employing adaptive trial designs to better capture individual responsiveness. Moreover, exploring alternative attentional strategies (e.g., holistic or switching focus) and integrating neuroimaging to understand underlying mechanisms could further refine personalized interventions.
Further research is needed, specifically randomized controlled studies, to investigate the effectiveness of different attentional foci in larger sample sizes of PwPD undergoing similar rehabilitative interventions.
Furthermore, it would be interesting to investigate certain profiles of the disease separately. The choice of one attentional focus over another could be particularly useful in the stages of the disease where PwPD are still mobile. In these phases, it is important to maintain independence and safety during daily activities. In addition, recent research has demonstrated that other types of attentional focus, such as a holistic focus or a switching focus of attention, have shown promising results in supporting healthy individuals acquire sport-related motor skills (Abedanzadeh et al., 2022; Favre-Bulle et al., 2024). The effectiveness in of these principle in PwPD should be investigated in future studies.
Lastly, further neuroimaging studies could be dedicated to understanding the impact of medications on the engagement of brain areas and networks associated with different attentional foci in PwPD.
The results of this work do not demonstrate the superiority of either EFA or IFA for balance, walking, and the severity of motor symptoms in PwPD. For all outcomes there was no superiority of an attentional focus irrespective of the medication status of the participants (i.e. ON or OFF status). Furthermore, the results do not support the generalisation of an EFA superiority observed in other populations to the population of PD. Therefore, it is recommended to select the attentional focus based on individual preference in PwPD and by considering potential other key elements, such as the training duration and medication status. The results of this work should be interpreted considering the risk of bias and its methodological limitations.
Figshare: Evaluation of external vs. internal focus of attention in Parkinson’s disease: A systematic review and meta-analysis during on and off medication states; DOI: https://doi.org/10.6084/m9.figshare.28334363 (Sattelmayer, 2025).
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We used the proprietary software Rayyan (https://www.rayyan.com) for the screening process. An open access software with similar functionality, which can used for the screening in systematic reviews is ASReview (https://asreview.nl).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Yes
References
1. Paily VP, Ramakrishnan S, Sidhik A, Girijadevi RR, et al.: Cervical Insufficiency Management with Elective Transvaginal Cervicoisthmic Cerclage.J Obstet Gynaecol India. 2025; 75 (2): 142-147 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 21 Jul 25 |
read |
|
Version 1 06 Mar 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)