Keywords
Primary pancreatic lymphoma, non-Hodgkin’s lymphoma, Diffuse large B cell lymphoma, Hepatitis C virus, Antiviral therapy
This article is included in the Oncology gateway.
Primary pancreatic lymphoma (PPL) is an exceptionally rare malignancy, often misdiagnosed as pancreatic adenocarcinoma due to overlapping and non-specific clinical and radiological features. Hepatitis C virus (HCV) infection has been increasingly associated with the development of lymphoproliferative disorders, particularly B-cell non-Hodgkin’s lymphoma (NHL). Herein, we present a case of a 63-year-old male with chronic HCV infection and F2 hepatic fibrosis who presented with abdominal pain, pruritus, and weight loss. Radiological imaging revealed a pancreatic mass which was subsequently confirmed via histopathological examination to be diffuse large B-cell lymphoma (DLBCL). Despite the initiation of antiviral therapy, the patient succumbed before starting chemotherapy. This case underscores the potential association between HCV infection and PPL, highlighting the importance of early antiviral treatment to prevent severe extrahepatic complications.
Primary pancreatic lymphoma, non-Hodgkin’s lymphoma, Diffuse large B cell lymphoma, Hepatitis C virus, Antiviral therapy
Primary pancreatic lymphoma (PPL) is a scarce form of extranodal non-Hodgkin lymphoma (NHL), accounting for merely 0.1% of all malignant lymphomas, 0.6% of extranodal lymphomas, and 0.2% of pancreatic malignancies.1 The most common histological subtype is diffuse large B-cell lymphoma (DLBCL),2 which often presents in the pancreatic head and predominantly affects males in their fifth or sixth decades of life.3 Chronic hepatitis C virus (HCV) infection has been epidemiologically associated with an increased risk of developing B-cell NHL, particularly marginal zone lymphoma and DLBCL.4 However, a direct link between HCV and PPL has not been clearly established, with only a limited number of case reports hinting at a possible association. In this context, we present the case of a 63-year-old male with chronic early-stage HCV infection who developed PPL located in the tail of the pancreas. This case contributes to the limited literature on the potential association between HCV infection and PPL.
A 68-year-old male with a history of a recent myocardial infarction one month before hospitalization, with no modifiable cardiovascular risk factors and managed on dual antiplatelet therapy, was admitted following a two-month history of intermittent abdominal pain, bloating, pruritus, and substantial unintentional weight loss of 15 kg. The patient also reported marked asthenia and anorexia. His Eastern Cooperative Oncology Group (ECOG) performance status was assessed as 2, signifying that he was ambulatory and capable of full self-care but unable to engage in work-related activities, remaining active for more than 50% of his waking hours. A physical examination revealed stable vital signs.
The abdomen was soft and non-distended, with mild epigastric tenderness. No palpable masses or peripheral lymphadenopathy were noted. Splenomegaly was observed, extending 2 fingerbreadths below the left costal margin.
Laboratory investigations revealed hypochromic, microcytic anemia, with a hemoglobin level of 12.7 g/dL, a mean corpuscular volume of 72.6 fL, and a mean corpuscular hemoglobin of 25.6 pg. Inflammatory markers were elevated, with a C-reactive protein of 57 mg/L and a ferritin level of 407 ng/mL, findings consistent with anemia of inflammation. The white blood cell and platelet counts were within normal ranges. Renal function parameters were preserved, and liver enzyme levels were unremarkable. Lactate dehydrogenase (LDH) was elevated at 245 U/L (normal range: 125–245 U/L). Additionally, serum albumin was reduced to 32 g/L.
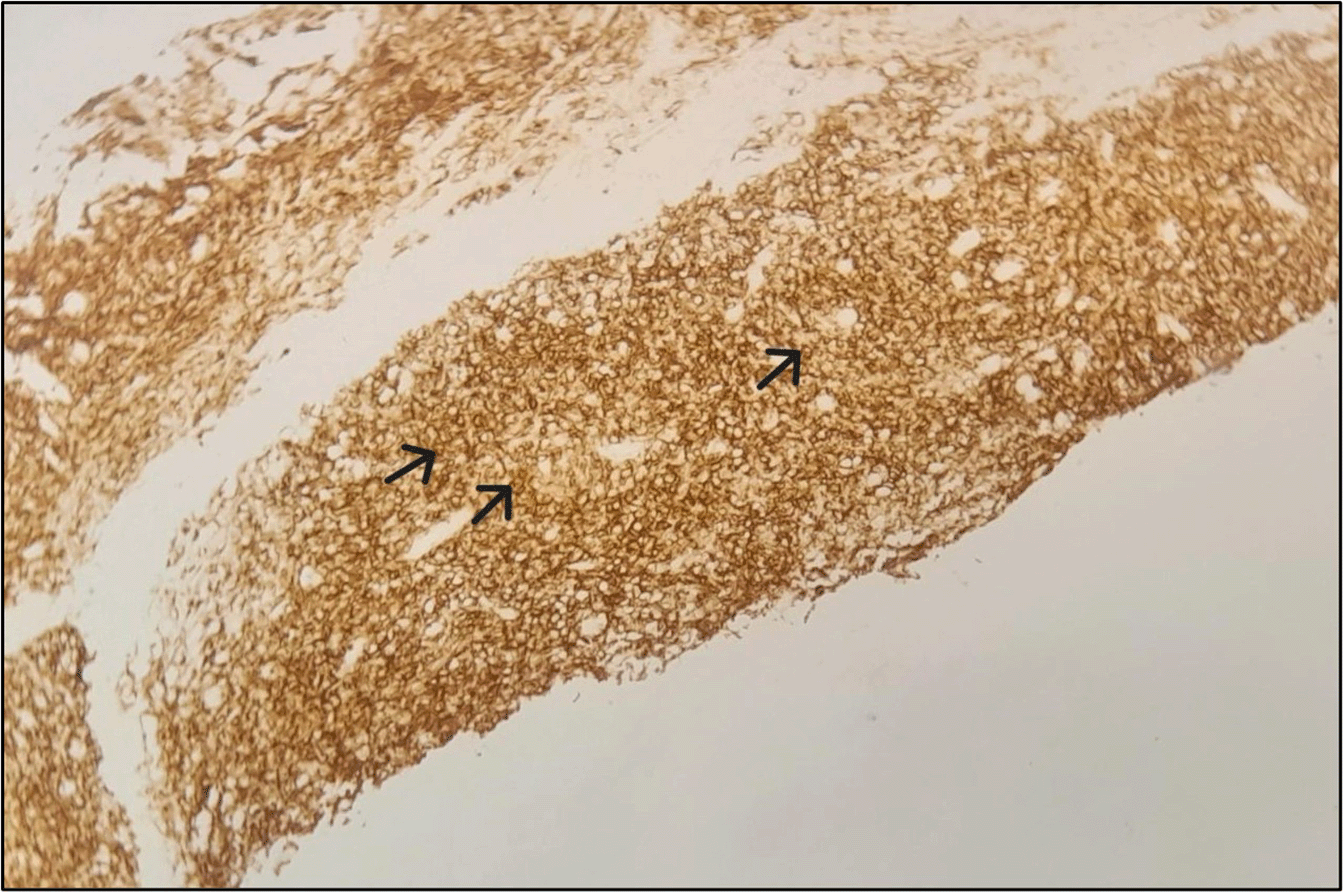
Abdominal ultrasound identified the presence of a pancreatic mass along with a small volume ascitic effusion. A follow-up abdominal computed tomography (CT) scan further characterized the lesion ( Figure 1), revealing a locally advanced mass in the pancreatic tail measuring 85 × 72 mm. The tumor was found to encase the splenic artery, obstruct the splenic vein, and invade adjacent structures, including the spleen (splenomegaly of 16 cm with multinodularity), stomach, left diaphragmatic crus, left adrenal gland, and left colon. There was minimal ascites. Subsequently, carbohydrate antigen 19-9 (CA 19-9) was measured, and its level was within normal limits. Confirming the diagnosis was challenging due to the recent myocardial infarction, dual antiplatelet therapy, and the proximity of the tumor to the spleen. An endoscopic trans-gastric ultrasound-guided biopsy was recommended but could not be performed due to limited resources. Consequently, a CT-guided percutaneous fine-needle biopsy of the pancreatic mass was performed via a posterior trans-splenic approach, following a temporary withdrawal of antiplatelet therapy. Histopathological examination revealed an undifferentiated malignant tumor, composed of sheets and isolated cells with indistinct borders and scant cytoplasm. The nuclei were round to oval, hyperchromatic, and contained small nucleoli. No glandular differentiation or necrosis was observed. One biopsy core showed infiltration into the splenic parenchyma. These features suggested a lymphomatous process ( Figure 2). Immunohistochemical analysis demonstrated intense and diffuse expression of CD20 and Bcl-2 in the tumor cells, with focal expression of CD10. CD3 and CD5 marked only reactive small lymphocytes, and cytokeratin was not expressed ( Figure 3). These findings are consistent with a diagnosis of follicular lymphoma, grade 1-2, as per the World Health Organization classification criteria.
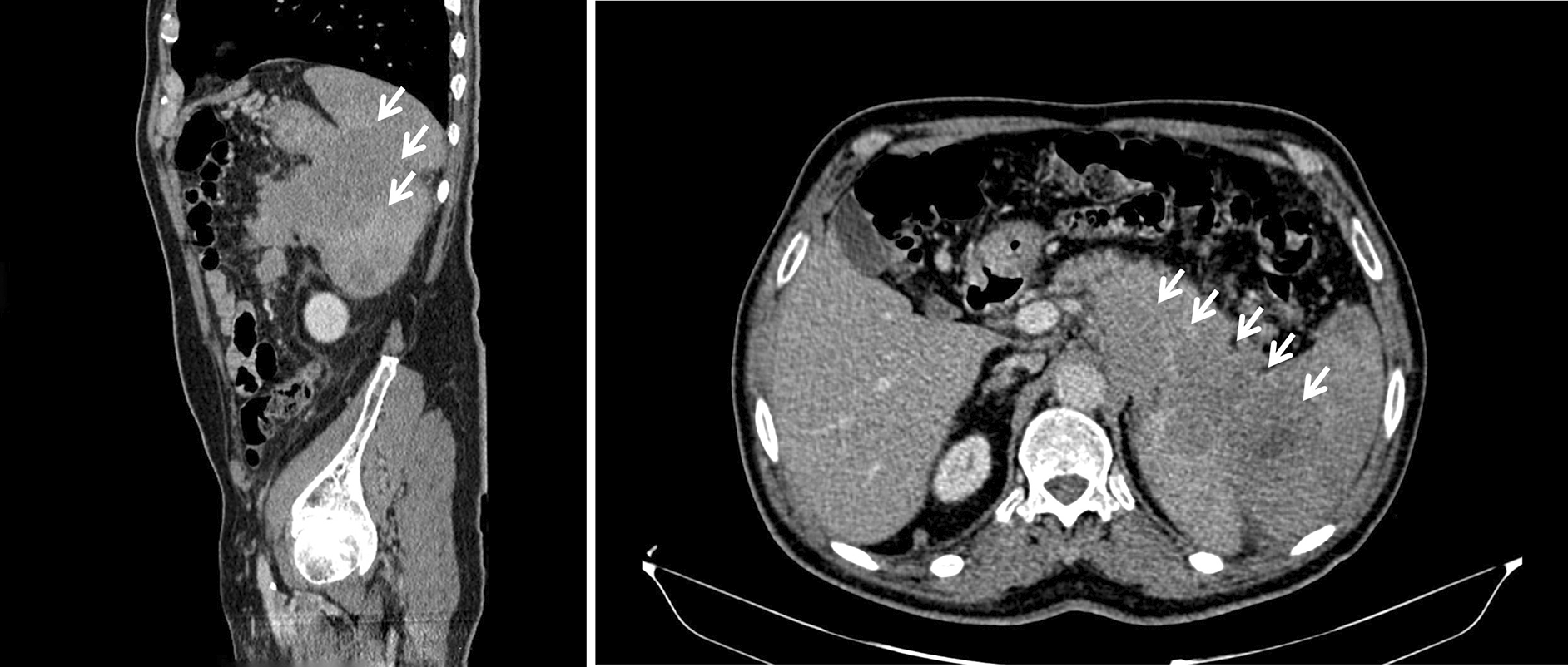
The white arrows indicate the locally advanced mass in the pancreatic tail. From left to right: sagittal and axial views of the tumor.

Serological testing confirmed chronic HCV infection with positive HCV RNA at 5.99 log IU/mL (996,000 IU/mL), genotype 1b. Serological testing for hepatitis B virus (HBV) and human immunodeficiency virus (HIV) returned negative results. Transient elastography (FibroScan®) indicated a liver stiffness measurement of 8.7 kPa, corresponding to F2 fibrosis.
As part of the lymphoma staging workup, a thoracic CT scan revealed infra-mediastinal posterior left adenopathy (43×32 mm), left hilar adenopathy (32×18 mm), and interbronchial left adenopathy (38×30 mm). Additionally, a left lobar nodule compressing the left bronchial lumen was noted. Bronchoscopy with cytological analysis of bronchial lavage showed only inflammatory cells, with no evidence of malignancy. An esophagogastroduodenoscopy (EGD) identified suspicious infiltrative folds in the gastric fundus, raising concern for possible tumoral invasion. However, two separate biopsies of the fundic mucosa yielded normal histological results. Further assessments, including an otorhinolaryngological examination, revealed no abnormalities.
Given the advanced stage of pancreatic lymphoma and the patient's compromised performance status, a decision was made to initiate antiviral therapy (AVT) for HCV before proceeding with chemotherapy. The patient received only an 8-week course of direct-acting antivirals before experiencing a rapid clinical deterioration. A follow-up CT scan revealed extensive tumor progression, with widespread lymphomatous involvement affecting the cervical, axillary, mediastinal, hilar, celiac-mesenteric, retroperitoneal, and bilateral inguinal lymph nodes. Additionally, the disease extended to the lungs, stomach, and spleen, further worsening the initial pancreatic involvement. The patient ultimately succumbed to the disease before completing the antiviral treatment regimen.
Lymphomas are a heterogeneous group of cancers originating from the clonal expansion of lymphocytes, including B cells, T cells, and natural killer (NK) cells. These malignancies represent approximately 5% of all new cancer cases in the United States.5
NHL commonly exhibit extranodal involvement. The gastrointestinal tract is the most frequently affected site, particularly the stomach and small intestine.6 However, primary involvement of the pancreas by NHL is exceedingly uncommon. PPL is an exceptionally rare subtype, constituting about 0.1% of all malignant lymphomas, and less than 1% of pancreatic tumors. The most prevalent histological variant of PPL is DLBCL, representing approximately 80% of cases.1
Clinically, PPL typically manifests with nonspecific symptoms, making its diagnosis challenging. The most common presenting symptom is abdominal pain, reported in approximately 83% of cases. This is often associated with the presence of an abdominal mass, observed in 58% of patients, as well as significant weight loss, occurring in 50% of cases, and jaundice, noted in 37% of patients. Additional clinical manifestations may include acute pancreatitis, small bowel obstruction, and diarrhea.6
Although pruritus is not a common symptom of PPL, it was observed in our patient. The pathogenesis of pruritus in PPL may be multifactorial, potentially arising from biliary obstruction due to tumor compression, paraneoplastic cytokine release, histamine-mediated mechanisms, or hepatic involvement leading to impaired bile metabolism.7 Additionally, systemic lymphoma symptoms such as fever and night sweats are less common in PPL compared to other types of NHL.8
The majority of reported cases predominantly involve the head of the pancreas, though occurrences in the body and tail have also been well-documented,9 as demonstrated in the present case.
Laboratory findings in PPL are nonspecific and can vary significantly. Elevated LDH levels may be observed, reflecting high tumor turnover. In contrast, tumor markers such as CA19-9 typically remain within normal limits unless biliary obstruction is present.3
Imaging is crucial for the identification of pancreatic masses, with CT scans being the most commonly used modality for detecting PPL.10 Advances in multi-phase CT imaging, particularly in the arterial and portal venous phases, have significantly improved the visualization of pancreatic neoplasms and their vascular involvement.11PPL can manifest in different forms, such as a large bulky mass, a focal nodular lesion, or a poorly defined infiltrative tumor exhibiting uniform contrast enhancement.12 One of the key imaging features suggestive of PPL is the absence of pancreatic ductal dilatation, vascular occlusion, necrosis, and calcifications, despite the tumor’s considerable size. Similarly, our patient exhibited none of these imaging findings, aligning with the established criteria for PPL.
However, while imaging provides valuable diagnostic clues, a definitive diagnosis of PPL requires histopathological examination, complemented by immunohistochemical analyses to distinguish it from other pancreatic neoplasms, particularly pancreatic adenocarcinoma and neuroendocrine tumors.13
The treatment approach and prognosis of PPL are influenced by the tumor's stage and grade.
Chemotherapy alone has led to long-term remission in several cases.14 The first-line treatment involves a regimen of cyclophosphamide, doxorubicin, vincristine, and prednisolone, with rituximab added for CD20-positive cases, improving remission rates.14 Radiation therapy, typically up to 40 Gray, has been used in combination with chemotherapy, though its effectiveness is still uncertain.15 PPL patients treated with chemotherapy have better outcomes than those with pancreatic adenocarcinoma, with 5-year survival rates ranging from 26% to 66%.16
The link between chronic HCV infection and the onset of B-cell NHL, including marginal zone lymphoma and DLBCL, is well-established.4
Pozzato et al.17 and Ferri et al.18 were the pioneers in identifying the correlation between HCV and NHL. The initial impetus to investigate this association stemmed from the well-documented high prevalence of HCV infection in lymphoma patients. Huang et al19 reported a twofold higher risk of pancreatic cancer in HCV-infected individuals, identifying, 34 cases over 340,819 person-years within an HCV cohort in comparison to the general Swedish population. Although the association between HCV and pancreatic cancer is not yet fully understood, strong evidence supports its role in lymphoma development, as evidenced by the regression of B-NHL after HCV eradication through antiviral therapy (AVT). Notably, in cases of indolent HCV-associated B-NHL, AVT may be considered a first-line treatment instead of immunochemotherapy.20,21 Studies have shown that AVT for HCV can lead to the regression of NHL and other lymphoproliferative diseases. After successful antiviral treatment, up to 75% of HCV-infected patients with NHL or other lymphoproliferative disorders may achieve complete or partial remission.20,21 Despite these clinical observations, the molecular mechanisms of HCV-induced lymphomagenesis remain poorly understood. Three main theories have been proposed: Chronic antigenic stimulation resulting from the continuous activation of lymphocyte receptors by viral antigens, leading to unregulated proliferation; viral replication within B cells exerts oncogenic effects through intracellular HCV proteins; and the “hit and run” mechanism, where transient intracellular viral presence causes permanent B-cell damage, such as mutations in tumor suppressor genes.4
In the presented case, our patient was diagnosed with active hepatitis C infection with early-stage liver disease (F2 fibrosis). He also exhibited cardiovascular manifestation as evidenced by a prior myocardial infarction with no modifiable cardiovascular risk factors, and developed PPL located in the tail of the pancreas. This highlights the necessity of including PPL in the differential diagnosis of pancreatic masses, particularly in patients with HCV -infected patients. Early antiviral treatment in HCV-infected individuals may be crucial to prevent lymphomagenesis and other potential extrahepatic manifestations. Further research is warranted to elucidate the pathophysiological mechanisms linking HCV infection to pancreatic lymphomas and to establish optimal management strategies for these patients.
The association between chronic HCV infection and B-cell lymphoproliferative disorders is well-established in the literature; however, its direct link to PPL remains exceedingly rare. Early recognition and accurate differentiation from other pancreatic malignancies are critical, as PPL demonstrates a favorable response to AVT and chemotherapy, in contrast to the poor prognosis typically associated with pancreatic adenocarcinoma.
Written informed consent for the publication of clinical details and/or images was obtained from the patient's son as the legal representative.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)