Keywords
Iron-deficiency, anaemia, immune response, iron supplements, pregnancy, public health, vaccine efficacy
Abstract*
Iron deficiency is the most common nutritional deficiency worldwide, affecting more than 1 billion people. Recent evidence has highlighted iron status as a key requirement for effective immune responses. Iron deficiency is common in pregnant women and vaccines are mandated at this stage of life. In the antenatal clinics (ANC) of the Shoklo Malaria Research Unit on the Thailand-Myanmar border, all pregnant women receive nutritional iron supplements at prophylactic or treatment dosages, as determined by haematocrit. Migrant pregnant women have been offered vaccination against diphtheria-tetanus since 1997, and since April-2022 COVID-19 vaccination, as indicated by vaccine history. In this setting, nearly 10% of pregnant women are anaemic at their first ANC visit; approximately 50% of anaemic women receiving treatment doses of nutritional supplements respond by increasing haematocrit after one month of supplementation.
This study aimed to investigate whether response to iron supplementation is associated with improved vaccine responses in pregnant women with anemia in this setting. We will monitor three groups of pregnant women vaccinated at their first ANC visit before 28 weeks of gestation receiving nutritional iron-containing multiple micronutrient supplements (Sangobion®, Procter&Gamble): 1) non-anaemic women who receive preventive doses of supplementation, 2) anaemic women who display a haematocrit response to treatment doses, and 3) anaemic women who do not respond to treatment doses. The primary endpoint is the difference in the geometric mean titer ratio (rGMT, comparing baseline to 28 days post-vaccination) of diphtheria-tetanus and COVID vaccine-specific antibodies between anaemic women who respond and those who fail to make a haematocrit response to iron-containing interventions.
Iron deficiency in different populations is associated with poor vaccine efficacy, and nutritional iron supplementation may improve the immune response to vaccines. Interventions that significantly increase vaccine efficacy would represent a major benefit to public health.
NCT05385042, 17-05-2022, https://clinicaltrials.gov/study/NCT05385042
Iron-deficiency, anaemia, immune response, iron supplements, pregnancy, public health, vaccine efficacy
The reviewer had 3 minor points, all important. The first was a query on handling blood samples and a new section has been added under the heading “Sample handling”. The second was a query on detailed dietary information as “certain foods and the way iron is consumed and then absorbed can be affected by food stuffs”. Unfortunately, no detailed dietary information (a statement added to this effect) was planned although smoking and betel consumption data will be collected. The final query suggested non-pregnant women as a comparator group which would require more matching and more budget than available. If anaemia or iron deficiency anaemia are important to vaccine response it is likely to be similar in non-pregnant women.
See the authors' detailed response to the review by David Churchill
Iron deficiency (ID) is the most common nutritional deficiency worldwide, affecting more than one billion people, and is the most common medical problem in pregnancy.1,2 Although anaemia is the most prominent manifestation of ID, it may also impair adaptive immune responses.3 Evidence from human genetics, molecular mechanistic and animal studies, and clinical trials has been combined to identify iron status as a previously unrecognized key modulator of immune responses.4–6 Mutations in the TFRC gene that encodes the transferrin receptor, the protein that cells take up iron, cause profound immunodeficiency in children, although only mild anaemia.7,8 Preclinical studies show that low serum iron disables T- and B-cell responses to vaccination, because activated antigen-specific lymphocytes need iron in order to support their proliferation and function.5 Conversely, iron supplementation improved the immune responses to vaccination in iron-deficient animal models.4 In humans, increasing evidence has linked iron deficiency with suppressed adaptive immunity. For example, a study evaluating the antibody response to influenza vaccines in the elderly found that pre-vaccination haemoglobin (Hb) and serum iron levels were significantly lower in poor responders than in responders.9 In Kenyan infants, anaemia and iron deficiency at the time of vaccination were associated with suboptimal antibody responses to diphtheria, pertussis, and pneumococcal vaccines, whereas nutritional iron supplementation improved the antibody response to measles vaccine in iron-deficient children.10 Together, these findings warrant deeper investigation of whether iron deficiency in different populations is associated with poorer vaccine efficacy and, in particular, whether nutritional iron supplementation can improve immune responses to vaccines.
Globally, immunization programs have achieved high coverage and provide tremendous benefits, but vaccines underperform in low- and middle-income countries (LMIC); they frequently achieve lower seroconversion rates than in high-income countries.11 Why vaccines do not work as well in LMIC remains uncertain.12 We hypothesized that ID is a key contributory and potentially correctable factor. Any intervention that significantly increases vaccine efficacy would represent a major public health benefit.
One population in whom ID is prevalent and vaccines are mandated is pregnant. Of note, nutritional status has been shown to influence maternal response to immunization and placental transfer of antibodies, although the results are variable.13 Iron deficiency anaemia in pregnant Sri Lankan women is associated with lower transplacental antibody transfer after maternal immunization against diphtheria, tetanus, and varicella zoster virus (VZV), but the effect of iron supplements in this context has not yet been examined.14 All pregnant women are offered maternal vaccination against diphtheria and tetanus; COVID-19 vaccination is safe and effective during pregnancy.15
Multiple micronutrient supplements, rather than iron and folate, are now recommended by the World Health Organization for pregnant women, in addition to a healthy diet.16 In the antenatal clinics of SMRU on the Thailand-Myanmar border, all pregnant women receive nutritional iron supplements at dosages determined by their haematological status.17 One in five women followed up until childbirth have anaemia during pregnancy.17 At their first antenatal clinic visit, pregnant women have haematological parameters assessed and are deemed anaemic if haematocrit15 is below 33% (Hb 11 g/dL) in the first trimester (<14 weeks gestation) and below 30% (Hb 10 g/dL) thereafter. Women not anaemic at their first antenatal clinic visit receive prophylactic nutritional supplements, including iron, to help maintain Hb levels. In the first six months of 2021, nearly 10% of pregnant women attending their first antenatal clinic were anaemic and treated with the assumption of iron deficiency. This is significantly lower than the 27% observed in 2011, which is largely attributable to successful falciparum malaria elimination efforts in the greater Mekong sub-region.18 Anaemic women are prescribed higher doses of nutritional iron, and previous data from the site indicate that approximately 50% experience an increase of 3% Ht (1 g Hb) after 28 days of nutritional supplementation. The increase in Ht is dependent on gestational age; in the first trimester, there is a physiological drop in Ht of approximately 1-2% and later in the last trimester of pregnancy, physiological haemoconcentration occurs, so treatment response must account for gestation, as demonstrated in a population of approximately 10,000 women from this setting ( Figure 1).17 However, many anaemic women remain anaemic with multiple factors common in resource-limited settings, including nutritional deficiencies, infection (intestinal worms, malaria), inherited red blood cell (IRBC) disorders such as thalassaemia and glucose-6-phosphate dehydrogenase (G6PD) deficiency,19 non-compliance, poor absorption and utilization of iron (or both, for example due to concomitant inflammation),20 or other causes of anaemia that render it non-responsive to iron.
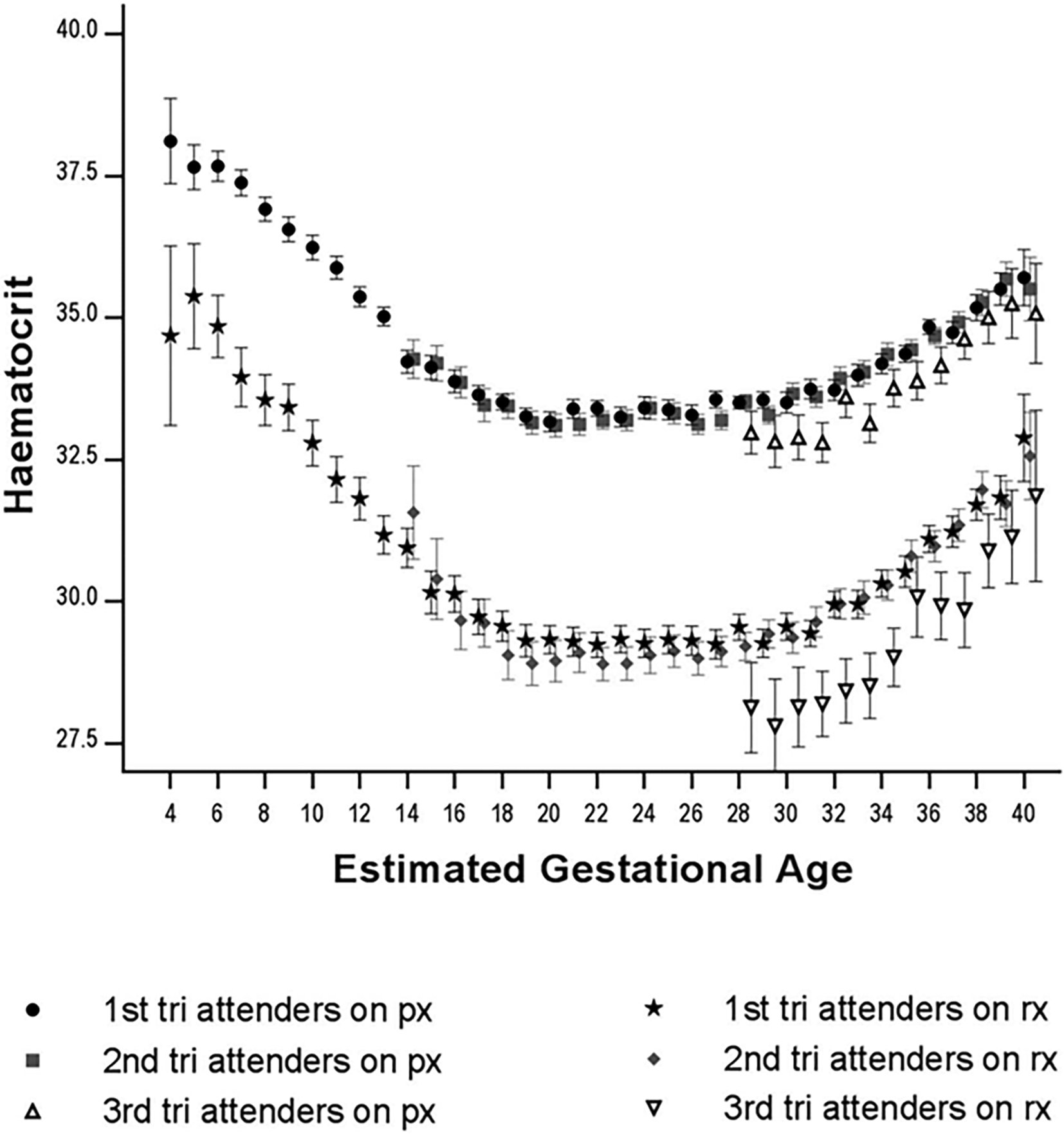
N: 1st tri px 4,371; 2nd tri px 3,199; 3rd tri px 811; 1st tri rx 1,039; 2nd tri rx 941; 3rd tri rx 198.
Abbreviations: px-prophylaxis, rx-treatment, Tri-trimester.
This figure has been reproduced from an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited. This figure was first published online in 2019 in Global Health Action, 12:1, “Evaluation of a treatment protocol for anaemia in pregnancy nested in routine antenatal care in a limited-resource setting’ (DOI: 10.1080/16549716.2019.1621589 Figure 2 of 2.17
Mean (95%CI) haematocrit according to the haematinic group (px – prophylaxis, rx – treatment), gestation (4–40 weeks), and trimester of the first antenatal visit (n = 10,559).
Thus, three groups of pregnant women can be monitored in this setting: 1) non-anaemic women receiving prophylactic iron-containing multiple micronutrient supplements; 2) anaemic women receiving higher doses of iron-containing multiple micronutrient supplements and who are haematologic responders within 28 days; and 3) anaemic women receiving higher dose supplements who do not make a haematologic response within 28 days.
The aim of the present study was to investigate whether antibody responses to maternal diphtheria-tetanus and SARS-CoV-2 vaccination are increased in anaemic pregnant women who make a haematocrit response to nutritional iron-containing interventions compared to those who do not. The planned study flow deviates little from real-world practice for these marginalized populations, apart from the provision of SARS-CoV-2 immunization and study-related procedures (type of nutritional supplement, blood sampling, and questionnaires). The enrolled women will be administered vaccines at their first antenatal clinic visit and followed longitudinally during pregnancy. Hence, by following the development of immune responses to vaccination in these groups as a prospective intervention study, we can test whether improvements in iron and haematological status are also correlated with improvements in the immune response to diphtheria and tetanus, SARS-CoV-2 vaccines, and transplacental antibody transport.
Protocol
Trial registration: NCT05385042, 17-05-2022, https://clinicaltrials.gov/study/NCT05385042/
The SMRU provides antenatal care during childbirth to the migrant population of Burmese and Karen ethnic groups who reside on the Thailand-Myanmar border. This marginalized population has been extensively employed in the agricultural sector in the border areas of Tak Province for decades. The estimated population is between 200,000 and 300,000, and most of this population are of reproductive age. Migrants frequently live together as family units and relationships occur naturally in this age group. Health care for documented migrants is covered by Thai Public Health but remains a grey zone for undocumented migrants who are required to pay in full, causing delays or non-attendance for needed health care. SMRU has provided health care to this undocumented population in rural areas since 1997. Initially antenatal care clinics tackled the high maternal-related mortality of P. falciparum malaria,21 and the issue of multidrug-resistant strains of P. falciparum22,23 and in the most recent decades, elimination of P. falciparum24 and treatment of recurrent P. vivax25 in an area of G6PD deficiency.19,26 Both falciparum and vivax malaria have a significant impact on pregnant women and the fetus, increasing adverse birth outcomes, such as preterm birth and small for gestational age.27
The SMRU continues to provide sexual and reproductive care on a humanitarian basis for this marginalized population. Yearly, more than 3,000 women are enrolled to care for SMRU clinics. At the first antenatal care visit, all women are offered a routine dating scan by a trained sonographer and again at 22 weeks. Women are screened for mother-to-child transmission of diseases, including HIV, Hepatitis B and Syphilis. Malaria is screened by a finger prick for a blood smear read by trained microscopists, and anaemia is screened from the same finger prick through collection into an Ht capillary tube.17 All women requiring treatment are provided with counseling and medication, as required. Women are encouraged to give birth to skilled birth attendants. The WHO partogram is used to monitor labor, and women who require caesarean section are transferred to the nearest Thai Hospital.
The time schedule of enrolment, interventions, assessments, and visits for participants is summarized as a study flow ( Figure 2).
The investigators will enrol pregnant women attending SMRU clinics in the Tak Province, Thailand. The vast majority of patients using SMRU clinical services are Burmese and Karen migrant workers resident along the Thailand-Myanmar border. Women will only be enrolled during their first antenatal care visit. There will be no advertisements or flyers, as health literacy is challenging in this setting.28
The consent process will be carried out by staff (trained counselors) from a separate study team, not involved in routine antenatal care procedures, and in a space designated for study procedures to minimize the risk that patients feel coerced or obligated to participate. Participants will be given the opportunity to verbally confirm or withdraw their consent at any time. Every effort will be made to ensure that the study considers local cultural sensitivity. Community engagement teams are involved in communities served by SMRU for many of its activities, and feedback from these teams is instrumental in the development of consent materials. Specifically, the consent process will be developed to support the meaningful transfer of information to potential participants with low health literacy.
Participants in this study will be pregnant women living in a malaria-endemic region, aged 18 years or older, who have a viable singleton fetus (<28 weeks gestation), attending their first antenatal care visit at Shoklo Malaria Research Unit, who accepted an invitation to be screened, and will be included if they met all eligibility criteria. The criteria not already mentioned will include willingness and ability to comply with the study protocol for the duration of the study, ability to understand information about the study and provide consent, and provision of written informed consent (including copy for patient) for COVID-19 vaccination (if unvaccinated).
The participant may not enter the study if any of the following apply: dT vaccine within the previous 2 years; a history of allergic reaction to dT or COVID-19 vaccine; Ht <21% or >50% (or Hb <7.0g/dL or >14.0g/dL by HemoCue); known severe haemoglobinopathy (HbE/beta-thalassaemia syndrome, beta-thalassaemia major or HbH syndrome) or G6PD deficiency; HIV-positive; slide confirmed malaria; fever (defined at >37.5°C); symptoms of COVID-19 (women will be tested by rapid tests as routine in clinic); known severe medical or obstetric complications, for example valvular heart disease, placenta praevia, known or clinical vitamin B12 deficiency as indicated by megaloblastic (pernicious anaemia or clinical symptoms). Iron supplementation is known to be safe in pregnant women with haemoglobin E, alpha-thalassaemia, or beta-thalassaemia trait.29
Screening and enrolment will take place during the first antenatal care visit. All information required to define eligibility will be available by reviewing the records of new pregnant women and will be carried out by trained members of the study team. Eligible women will be invited to join a participant information session (Supplementary file 1) in which they are entitled to refuse. The number of eligible, invited, and refusal individuals will be tracked.
This is not a randomized study, and nutritional supplements are routinely used in this setting. The assignment of study groups based on the level of Ht or Hb is the same as that routinely practiced in this setting. At their first antenatal clinic visit, pregnant women will have haematological parameters assessed and will be deemed anaemic if Ht is <33% (Hb < 11 g/dL) in the first trimester and <30% (Hb < 10 g/dL) in the second trimester and thereafter.30 Standardized micronutrient nutritional supplements (Sangobion®, Procter&Gamble) ( Table 1) will be provided for the purposes of this study. In line with standard practice in antenatal care at the study site, women with anaemia will be prescribed a higher dose of nutritional supplements than women without anaemia. All women will be advised on how to take the supplements correctly (Supplementary file 2), what side effects are expected, and how they can minimize the side effects of oral iron. Women will be asked to opt for a once-daily dosing time of supplements (one tablet for non-anaemia, three for anaemia) based on when they are most likely to remember taking the supplement, for example, with morning or evening meal, with the rationale that remembering more doses is better than optimal timing (i.e., morning, when hepcidin levels are lower and fractional iron absorption is likely higher).31 Treatment duration will be 12 weeks, after which they will be provided with standard prophylactic nutritional supplements until delivery. Women who are not anaemic at 12 weeks will receive prophylactic nutritional supplements until delivery. Women who are non-responders (according to gestation) will be investigated.
| Non-anaemic* | Anaemic*^ | |
|---|---|---|
| Product (Sangobion®) | 1 Capsule | 3 Capsules |
| Iron Salt | 250mg | 750mg |
| (elemental iron) | (30mg) | (90mg) |
| Folic Acid | 1000mcg | 3000mcg |
| Copper Sulfate | 200mcg | 600mcg |
| Manganese Sulphate | 200mcg | 600mcg |
| Vit C/Ascorbic Acid | 50mg | 150mcg |
| Sorbitol | 25mg | 75mg |
| Vit B12/Cyanocobalamin | 7.5mcg | 22.5mcg |
Immunization for diphtheria and tetanus32 is routine at antenatal care visits because the expanded program of immunization (EPI) in these populations is weak. dT will be provided based on the woman’s history of immunization from previous pregnancies (if any) and verified by referring to past records whenever available. A history of immunization with dT in the last two years is an exclusion criterion. Importantly, as part of the present study, all pregnant women will receive COVID-19 vaccines because the routine supply of this vaccine for migrants is not yet available. At the time of writing the approved protocol, SARS-CoV-2 immunization for undocumented migrants was limited in Thailand but became easier to obtain in 2023. A history of SARS-CoV-2 immunization will not prevent admission to the study; the vaccine will be administered if indicated by a negative history of vaccination, with verification of records when available.
Screening procedures at baseline will include a) information available from routine antenatal care, which will be verified by the study team after confirmation of eligibility and provision of informed consent, and b) study-specific assessment. Demographics included age, gravidity, parity, weight, height, ethnic group, smoking, and betel nut use; contact details of the participant will be recorded; confirmation of viability and gestational age and potential complications such as placenta praevia; a medical and obstetric history including the history of vaccination (past dates, including dT and COVID-19 vaccination), alongside birth interval in multigravidae; and use of hormonal contraceptives before the current pregnancy. No detailed dietary information will be collected. Any concomitant medications will be recorded. Physical and obstetric examinations by medically trained personnel will include vital signs (pulse, respiratory rate, blood pressure, liver and spleen sizes, and temperature), symphysis fundal height, fetal lie, and fetal heartbeat will be confirmed. The results of point-of-care laboratory tests offered at the first antenatal care visit were reviewed, and counseling for treatment arranged as required.
Study-specific assessment will include a revision of gastrointestinal symptoms (Supplementary file 3) before taking the nutritional supplement, a venous blood draw of 9.5mL of blood, provision of immunizations, dT and SARS-Cov-2 (if indicated, and the type provided will be recorded, e.g., Pfizer, Moderna), provision of nutritional supplements and guidance on how and when to take them and their expected effects.
After the baseline (day 0) study visit, there will be seven further planned study visits: at day 7, day 28, month 2, and month 3 post-enrolment, at 36-38 weeks of gestation, at childbirth, and at 2 months postpartum ( Table 2). Visits at days 7, 28, 2, and 3 are similar and comprise physical and obstetric examinations, review of Adverse Events and gastrointestinal symptoms, compliance checked using pill count and the modified ASK-12 (Supplementary file 4), provision of nutritional supplementation and strategies to reduce gastrointestinal symptoms if any, and Ht and malaria smear from capillary blood (except day 7). Day 7, day 28 and week 36-38 visits will include a venous blood draw of 9.5mL of blood; in these first two visits, a request for a stool sample for examination of intestinal parasites will be made. If it is not possible to provide a stool sample on day 7, a stool kit will be provided, and women will be encouraged to collect it on the morning of the next planned study visit. For samples provided on day 7, antiparasitic treatment will be provided if indicated according to the type of helminth infection after the blood sample at day 28. At day 28, dT and COVID vaccination will be provided as required: either dT1 on day 0 and dT2 on day 28, or dT1 on day 0 followed by no dose at d28 if the day 0 dT dose was 3rd dT dose in her life, since no further dose is required on day 28; a similar schedule will be used for the COVID vaccine). Following the month 3 visit, the woman will follow routine antenatal care until week 36-38 visit.
| Procedures | Visits | ||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Month 1 | Monthly (2,3) | 36-38 weeks | Delivery | Post- partum (2 month) | |
| Baseline | Visit 1 | Visit 2 | Visit 3-4 | Visit 5 | Visit 6 | Visit 7 | |
| Informed consent | X | ||||||
| Demographics | X | ||||||
| Medical and Obstetric History | X | ||||||
| Physical and obstetric examination | X | X | X | X | X | X | X |
| Vaccine history | X | ||||||
| Ultrasound | X | ||||||
| Malaria and Haematocrit/Haemoglobin | X | X | X | X | X | X | |
| HIV and Hepatitis B | X | ||||||
| Eligibility assessment | X | ||||||
| Vaccination dT | X | X* | |||||
| Vaccination SARS-CoV-2 | X | X* | |||||
| Provision of nutritional supplements | X | X | X | X | |||
| Adverse Events, Gastrointestinal symptoms | X | X | X | X | |||
| ASK-12 modified and pill count | X | X | X | ||||
| Venous blood draw | X | X | X | X | X^ | ||
| Cord blood draw | X | ||||||
| Stool test | X | X | |||||
During labor, the mother will have Ht and malaria smears (from capillary blood) checked, a stool sample for examination of intestinal parasites, and, after delivery, 9.5mL of cord blood will be collected for study purposes. The last visit was planned at 2 months postpartum with mother samples including Ht and malaria smear (from capillary blood), a venous blood draw of up to 9.5mL of blood, and an infant venous blood draw (0.5mL) and Ht (60μL) to check for anaemia (routine method in this area). The total blood volume from the mother was up to 48.06mL, from the cord was up to 9.5mL and from the infant 0.56mL.
Women with anaemia may be considered non-responders to oral multiple micronutrient nutritional supplements at day 28 and will be based on the trimester of enrolment and whether inclusion was on the Ht or Hb. Women enrolled in the first trimester (<14 weeks) will be considered non-responders if their Ht falls by ≥3% (or Hb by ≥1 g/dL) below baseline with 4 weeks of supplementation, based on a review of Ht response to haematinics in 10,559 pregnant women from the same study area.17 Women enrolled in 2nd trimester will be considered non-responders if their Ht does not increase by ≥3% (or Hb does not increase by ≥ 1 g/dL) after 4 weeks of supplementation. If women are non-responders, they will be further investigated via serum ferritin quantification; if this is low (<15ng/mL), intravenous (i.v) iron supplementation will be provided, a treatment that has previously been implemented at the clinic. The dose of intravenous iron sucrose (Venofer®) was 3 × 200 mg by slow intravenous administration. Infusions will be spaced over days of weekly at patient convenience (maximum of three doses in one week), with resuscitation equipment and doctor on standby, as per current practice. Following intravenous iron sucrose treatment, the supplements will be continued at a prophylactic dose. Ht can vary, and if there are concerns about response after day 28, a blood sample to determine the serum ferritin level will be checked along with Complete Blood Count (CBC) and C-reactive protein (CRP), as a measure of inflammatory status, to determine the treatment plan.
The primary endpoint is the difference in geometric mean titer ratio (rGMT), comparing baseline to 28 days post-vaccination, of dT vaccine-specific antibody between anaemic women who remain anaemic (non-responders) versus anaemic women who experience a recovery (responder) as defined above.
This study aims to test a number of secondary outcomes, comparing women who were not anaemic at enrolment, women who were anaemic at enrolment and made a haematocrit response, and women who were anaemic at enrolment but failed to make a haematocrit response. The secondary outcomes will include the following.
1) Longitudinal analysis of iron parameters (serum iron, serum ferritin, soluble transferrin receptor, hepcidin);
2) Evaluation of difference in proportion of women in each of the three groups that achieve a protective titre (defined as anti-diphtheria toxoid Ab, >0.10 IU/mL; anti-tetanus toxoid Ab, >0.10 IU/mL) 28 days after vaccination and at birth;
3) Evaluation of differences in frequencies of circulating immune cell subsets identified using mass cytometry (CyTOF), notably plasmablasts and activated T and B cell subsets, at days 7 and 28 post-vaccination (expected to be induced following vaccination);
4) Systems immunology: data integration for assessment of longitudinal changes in immune phenotypic profiles during pregnancy in this setting
5) Transplacental antibody transfer: Assessment of the effect of iron status on antigen-specific transplacental IgG transfer.
6) Evaluation of the use of Ht as an inexpensive method, in comparison to Hb (assessed from CBC and/or Hemocue), to identify women who need treatment and will respond adequately to treatment.
7) Investigation of whether betel nut consumption, associated with anaemia, interferes with response to iron33,34 and Ht and Hb trajectories.
8) Assessment of vitamin A status as a potential confounder that could influence immune responses.
9) Compliance: investigating whether high compliance is associated with recovery from anaemia (treatment group) and maintenance of Ht (prophylaxis group);
10) Analysis of Adverse Effects, predicting that these will not segregate with group;
Secondary analysis will also be performed on the associations of outcome parameters with continuous measures of iron status and iron deficiency status (using ferritin-based definitions, adjusted for concurrent inflammation).
Malaria Smear
Malaria Smear (MS) and parasite count (thin and thick films stained by Giemsa’s method) will be performed in accordance with the SMRU standard operating procedures. Parasite density was calculated by counting the number of asexual parasites per 500 leukocytes in the thick blood film based on an assumed white blood cell (WBC) count of 8,000/μl.
Blood Haematocrit
Haematocrit will be measured using a standard test by finger prick sampling of 60μL of blood. Anticoagulated blood capillary tubes will be centrifuged at 10,000 RPMs for 3 min and read using a Hawksley Micro-haematocrit reader.
HemoCue
Haemoglobin concentration will be quantified at screening in field sites using either the HemoCue 301+ or 201+ test systems.
Complete blood count and other haematologic analyses
Venous blood collected in EDTA will be used to perform the following laboratory analyses: complete blood count using an automated haematology analyzer (Sysmex NX-550), C-reactive protein (CRP) by Nyco Cards (Abbott, UK), Hb typing by Capillary Electrophoresis (Capillarys 2Flex, Sebia, France), or HPLC. The G6PD phenotype will be analyzed by the fluorescent spot test (R&D Diagnostics, Greece), as is the routine for all new pregnant women. The buffy coat will be stored for human DNA extraction and genotyping of common Asian alpha-thalassaemia and G6PD mutations according to established standard operating procedures.19
Iron status assessment
Serum samples for iron and immunological markers will be collected in clot-activator tubes. Analyses of serum iron (Fe), transferrin saturation (TSAT), hepcidin, and retinol-binding protein (RBP, as a measure of vitamin A status) will be performed by accredited laboratories, and hepcidin levels will be assessed using a validated ELISA.
Vaccine serology
Serum IgG concentrations against the diphtheria CRM197 toxoid antigen, tetanus, and SARS-CoV-2 spike protein will be measured by immunoassay.
Cellular Immune Profiling and Responses
Whole blood samples for studies of cellular immune phenotypes, including the plasmablast response, will be collected in EDTA and preserved using Stable-Lyse/Stable-Store v2 reagents (Smart Tube Inc., USA) at site laboratories. Cellular immunological analysis will be performed using Mass Cytometry (CyTOF). Briefly, fixed whole blood samples will be temporarily stored at 4 °C at the trial site before archiving at -80 °C in SMRU laboratories. At the time of analysis, the samples will be thawed prior to barcoding to enable red cell lysis. Barcoded samples will be then stained in batches (in parallel with batch control samples to enable correction of batch-to-batch variation if necessary) with a pan-leukocyte targeted panel of >45 metal-tagged antibodies; staining intensity data will be acquired using a Helios mass cytometer (Standard Biotools).
Stool test
The stool test will be performed using fecal material (~500 mg) and the formalin ethyl-acetate concentration technique (FECT) method at SMRU for soil-transmitted helminths and food-borne trematodes.35
Samples will be processed at the site clinic according to their type. CBC samples collected in EDTA tubes will be stored at 4°C until shipment in ice packs on the same day to the central laboratory in Mae Ramat (around 2h transportation time). Sample collected without anticoagulant will be left to clot and will be spun down at the site clinic. Serum will be frozen at -20°C and shipped in dry ice within 2 months of collection. At the central laboratory, serum samples will be kept at -80°C. Samples treated with StableLyse/Stable store will be processed at the site clinic and transported on the same day to the central laboratory to be kept at -80°C.
Temperature control will be managed following GLP guidelines according to SMRU SOPs which include calibration, daily record or continuous monitoring of fridges and freezers temperature, regular preventive maintenance and de-icing. Shipment of samples from Thailand to UK will be performed in dry-ice with continuous temperature monitoring.
The calculated sample size is 100 anaemic participants (Ht <33% or Hb < 11 g/dL in trimester one, or Ht <30% or Hb < 10 g/dL in trimester two) and 50 non-anaemic subjects. SMRU has published an example of dose-dependent antibody-boosting responses to tetanus vaccination in this population. Based on these data, we estimated that a sample size of N=50 per arm (50 anaemic pregnant women will be responders to treatment and 50 anaemic pregnant women will be non-responders to treatment) will be required to detect a mean difference in log10 antibody titer at day 28 post-vaccination of 0.4 (equivalent to geometric mean titer ratio = 2.5). This sample size is based on a standard deviation in log10 titer of 0.6, at 80% power, 5% significance level (2-sided), and 10% dropout. The total number of included women will be 180 (120 anaemic and 60 non-anaemic) to allow any potential withdrawals to be replaced.
Descriptive statistics will be used to describe the demographic characteristics of the two groups. Clinical outcome data will be analyzed depending on the type of variables; for continuous variables, the difference in the means and corresponding 95% confidence intervals and Analysis of Covariance, adjusting for baseline values, will be used for comparing the two groups. For categorical variables, the number and percentage of participants in each category will be reported, and chi-square tests were used to compare the two treatment groups. A suitable transformation will be applied if the variables were not normally distributed. Analyses will be conducted on the intention-to-treat (analyzed as randomized) and per-protocol (as ITT, but with protocol violators excluded) populations.
Mass cytometry data will be normalized, de-barcoded, and cleaned prior to identification and analysis of immune cell subsets using unsupervised clustering methods based on adaptations of existing workflows.36 Transplacental IgG transfer titers and ratios will be calculated following quantification in maternal blood samples taken at 36-38 weeks of gestation and in paired cord blood samples.
As this is a small study, treatment effects are likely to have wide confidence intervals; consequently, inferences will be tentative and reported as such. Tests will be two-sided and considered to provide evidence for a significant difference if p-values are less than 0.05. Reasons for missing data, loss to follow-up and participant withdrawals will be carefully considered, reported and assessed for what type of random ‘missingness’ is present. Missing data will be minimized by collecting the minimum amount of data required, and data queries will be resolved prior to the analysis. Adherence to nutritional supplements will be monitored by pill count and participants who completed six or more of seven days per week at the correct number of tablets will be classified as adherent, over the 12 weeks period, while those who take supplements fewer than six days per week, and/or fewer than their prescribed number of tablets will be classified as non-adherent.
All study data will be recorded using standard Case Report Forms (CRFs). Data will be entered into a secure database in accordance with standard operating procedures. Data may be used alone or in combination with data from related studies in the secondary analyses. All paper forms will be tracked by a unique study identity (ID) number, which can be linked back to the patient ID (PID) in the SMRU’s password-protected internal clinical database. All clinical laboratory results will be entered into an electronic, password-protected study database. Raw and processed data files from mass cytometry analysis will be held on secure servers at the MRC WIMM, University of Oxford. Electronic study records will be de-identified upon completion of data collection. Electronic records will be maintained indefinitely in the databases and remain password-protected.
This study will be conducted in accordance with the current approved protocol, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice, Good Clinical Laboratory Practice, relevant regulations, and standard operating procedures. A risk assessment and monitoring plan will be prepared before the study opens, and will be reviewed as necessary over the course of the study to reflect significant changes to the protocol or outcomes of monitoring activities.
Regular monitoring will be performed according to ICH Good Clinical Practice. The data will be evaluated for compliance with the protocol and accuracy in relation to the source documents. Following written standard operating procedures the monitor verifies that the clinical study is conducted, and data are generated, documented, and reported in compliance with the protocol, Good Clinical Laboratory Practice, and the applicable regulatory requirements.
The main severe risk in this interventional study is the risk of severe COVID-19 vaccine-induced thrombotic thrombocytopenia (VITT), the incidence of which is unknown but appears exceedingly rare.32,37 Multiple professional societies worldwide have endorsed SARS-CoV-2 immunization for pregnant women, including Thailand, based on evidence of a higher risk of severe morbidity and mortality in this population group.15 There are data on the co-administration of diphtheria-tetanus combined with COVID-19 vaccination on the same day. The CDC has stated that COVID-19 vaccines can be administered at any time in relation to other non-COVID-19 vaccines and, if needed, can be administered on the same day as other vaccines.
Further risks include the mild adverse events expected from the oral intake of nutritional supplements containing iron. Participants will be followed closely for their response to nutrition supplements, which can provide individual benefits and support as side effects will be anticipated, and strategies to reduce them will be provided at the study outset and as adverse events will be assessed at study visits. The benefits of mixed micronutrient supplements outweigh the risks and follow the international and local guidelines for antenatal care.
Vaccines can protect the mother (and hence the fetus) from complications including mortality and prolonged hospitalization due to severe COVID-19. Pregnant women infected with COVID-19 carry a greater risk of morbidity and mortality than their non-pregnant counterparts. The benefit of vaccination outweighs the risk of not receiving the COVID-19 vaccine in this current pandemic; currently, there is no fixed supply of vaccine to migrants in this part of Thailand and at the time of writing this protocol (August 2023), 3+ years into the pandemic, there were migrant women who had never been offered COVID-19 vaccination. The combined dT (diphtheria and tetanus) is routine and would be provided if the woman participated in the study.
A further benefit to the participants in this study is individualized monitoring for anaemia. More generally, there will be broad benefits from increasing knowledge of how iron status relates to responses to ‘routine’ vaccination during pregnancy; this will be especially important for pregnant women with anaemia and others taking iron-containing nutritional supplements globally.
After the analysis, the results will be prepared for open-access peer-reviewed international journals with a view to publication. The study team will be compliant with the standards and guidelines regarding open access of research data. The results of the study will be explained to the Tak Province Border Community Ethics Advisory Board and to health care workers who support pregnancies and do the work of constant counseling and encouraging women to take iron supplements.
Iron deficiency in different populations is associated with poor vaccine efficacy, and nutritional iron supplementation may improve the immune response to vaccines. This study may be the first to characterize vaccine responses in anaemic pregnant women who remain anaemic versus anaemic women who experience recovery of their haematological concentrations in a low-resource setting. This study provides information on the role of nutritional iron supplementation in improving immune responses to vaccines in iron deficiency. This study fills a research gap by profiling the changes in cellular immune phenotypes throughout pregnancy in a low-resource setting. Interventions that significantly increase vaccine efficacy would represent a major benefit to public health.
This proposal was presented to the Tak Province Border Community Ethics Advisory Board (T-CAB), which consists of members of the local community, and their suggestions were incorporated into the current protocol and associated documents. The study was submitted to two independent institutional review boards and both approved the study: the Oxford University Tropical Research Ethics Committee (Oxtrec10-22) and the Faculty of Tropical Medicine Ethics Committee (TMEC 22-013).
Informed consent will be sought from each individual participant in their own language (Karen or Burmese). There will be a written participant information sheet informing of the purpose, procedures, potential risks and benefits which participants could read at their own pace; or for those who cannot not read a literate witness from the antenatal clinic will assist the participant through the document. Participants and their witness, will be informed that participation is voluntary, they can withdraw at any time without affecting their clinical care, and their data will be kept confidential.
Participants will be asked to sign a separate consent form explaining that the specimens will be stored and may be used for purposes other than those described in the study protocol. If the participant does not sign the consent for biobanking, their specimens will be discarded after the analysis described in this protocol is finalized.
No data or software are associated with this article.
ORA: Protocol for a study on iron supplementation and immune responses to maternal vaccination in pregnant women (IRONMUM). https://ora.ox.ac.uk/objects/uuid:d0feae48-efce-47b9-bdb1-d65c032c79a5.38
The contents include
Supplementary files
Supplementary 1. Pictorial of Study for Explanation to Participants before enrolment
Supplementary 2. Number of tablets to take daily in the IRONMUM study by group (Anaemic, Not Anaemic)
Supplementary 3. Review for adverse effects (GIT) and pill taking
Supplementary 4. Modified ASK-12 compliance
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
This study is funded by Procter & Gamble who manufactures Sangobion, which can be interpreted as a conflict of interest. For this reason all data will be made open access upon publication. A citation for the de-identified database, will may be made freely available to other researchers via the Oxford University Research Archive.
ORA: Protocol for a study on iron supplementation and immune responses to maternal vaccination in pregnant women (IRONMUM). https://ora.ox.ac.uk/objects/uuid:d0feae48-efce-47b9-bdb1-d65c032c79a5.38
Supplementary 5. SPIRIT checklist for Protocol for a study on iron supplementation and immune responses to maternal vaccination in pregnant women (IRONMUM).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank the laboratory and clinical staff who critically reviewed the protocol and the Karen and Burmese translators for their work on PIS and ICF. Significant thanks also go to the Clinical Trials Support Group at MORU who were persistent and supportive throughout the submission of the various documents related to this study. No artificial intelligence or medical writer has been used in the writing of this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Iron deficiency anaemia in pregnancy - rare diseases in obstetrics and diabetes in pregnancy.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 11 Nov 25 |
|
|
Version 1 27 Mar 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)