Keywords
nanobubles, oxygen, role, stroke, ischemic
This article is included in the Nanoscience & Nanotechnology gateway.
Stroke remains a leading cause of mortality and long-term disability worldwide, necessitating innovative therapeutic strategies. The advent of nanotechnology, particularly oxygen-delivering nanobubbles (ODNBs), has introduced a promising avenue for enhancing stroke therapy. ODNBs have demonstrated the ability to improve oxygen delivery, enhance therapeutic efficacy, and provide diagnostic advantages through imaging contrast enhancement. However, challenges such as toxicity, off-target effects, and regulatory hurdles must be addressed before clinical translation. This review synthesizes the latest findings on ODNBs in stroke therapy, highlights their key benefits and challenges, and explores future applications, including gene therapy and brain tissue regeneration. By addressing these aspects, this review aims to provide a comprehensive understanding of the potential of ODNBs in revolutionizing stroke treatment.
nanobubles, oxygen, role, stroke, ischemic
In this revised version, we have incorporated several revisions based on the reviewer’s comments. A new column titled “Combination with Other Therapies” has been added to Table 1 to indicate whether additional interventions were used in the included studies. In the Discussion section, two new subsections have been introduced: 4.5 Types of Nanobubbles and Oxygenation Agents, which provides an overview of the main nanobubble types and oxygenation agents, and 4.6 Challenges in Clinical Translation and Potential Strategies, which discusses key barriers to clinical application and approaches to address them. Additionally, minor language improvements have been made throughout the manuscript for clarity, and a Competing Interests statement has been included as required.
See the authors' detailed response to the review by Fang Fang
See the authors' detailed response to the review by Kevin Morris
Acute ischemic stroke (AIS) is a debilitating and potentially fatal condition that arises when there is an interruption or blockage in the blood flow to the brain, resulting in oxygen deprivation and, ultimately, neuronal injury and death. This interruption in cerebral circulation can occur due to the formation of blood clots, often stemming from conditions such as atherosclerosis or atrial fibrillation, which block major arteries leading to the brain. The loss of oxygen supply to brain cells triggers a cascade of pathophysiological processes, including oxidative stress, inflammation, and excitotoxicity, which significantly contribute to tissue damage.1–3 While the acute phase of ischemic stroke is highly time-dependent, early intervention is critical for minimizing neurological damage and improving long-term outcomes.
Traditionally, treatments for AIS have centered around thrombolysis with tissue plasminogen activator (tPA) and mechanical thrombectomy. These interventions aim to restore blood flow by dissolving or physically removing the clot. However, these therapies are constrained by narrow time windows, typically within a few hours of symptom onset, and are further limited by strict patient eligibility criteria, such as age, comorbidities, and the location of the clot. As a result, a significant proportion of stroke patients either do not qualify for these treatments or experience them after the optimal window for intervention has passed, leading to poor outcomes and long-term disability. Additionally, these therapies are often unavailable in resource-limited settings, exacerbating disparities in stroke care across different populations.2,4–8
In light of these challenges, researchers have been exploring alternative and complementary approaches to treat AIS. One promising advancement is the development of oxygen-delivering nanobubbles (ODNBs), which represent a novel and cutting-edge therapeutic strategy for addressing ischemic damage in stroke patients. ODNBs are nanoscale particles that encapsulate oxygen and release it in a controlled manner, specifically targeting hypoxic brain regions. These nanobubbles have shown considerable promise in preclinical studies by enhancing oxygenation in ischemic tissue, improving cellular metabolism, and reducing secondary neuronal injury.3 Moreover, ODNBs can potentially enhance the efficacy of existing stroke therapies, such as thrombolysis and thrombectomy, by ensuring that ischemic regions receive adequate oxygen during the critical early hours after the event. This novel approach not only has the potential to mitigate the deleterious effects of ischemia but also holds promise in augmenting existing treatment paradigms by addressing their key limitations.4,5
This review aims to provide an in-depth exploration of the recent advancements in ODNB-based therapies for the treatment of AIS. We will examine the underlying mechanisms by which ODNBs deliver oxygen to ischemic tissue, the advantages they offer over traditional therapeutic approaches, and the current limitations of this novel therapy. Furthermore, we will discuss the future prospects of ODNBs in stroke management, including their potential integration with other stroke interventions, their clinical translation, and the challenges that must be addressed to optimize their use in real-world settings. Through a comprehensive review of the current literature, we seek to highlight the potential of ODNBs as a game-changing therapeutic modality in the management of AIS, providing insights into how they might contribute to improving outcomes for patients worldwide.
This systematic review was conducted following the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to ensure comprehensive and transparent reporting of the review process.9,10 The aim of this review was to evaluate the efficacy and mechanisms of oxygen-delivering nanobubbles (ODNBs) in the treatment of acute ischemic stroke (AIS). The following steps were undertaken to conduct the review:
Studies were included if they met the following criteria:
• Population: Studies involving adult patients diagnosed with acute ischemic stroke, regardless of age, sex, or comorbidities.
• Intervention: Studies examining the use of oxygen-delivering nanobubbles (ODNBs) or nanobubble-based therapies for oxygen delivery to ischemic brain tissue.
• Comparators: Studies comparing ODNB therapy with placebo, standard stroke therapy (e.g., thrombolysis or thrombectomy), or no intervention.
• Outcomes: Studies reporting on primary outcomes, including neurological function recovery, oxygenation improvement, tissue viability, and reduction in infarct size. Secondary outcomes included safety profiles, adverse events, and imaging outcomes related to oxygen delivery efficiency.
• Study Design: Only randomized controlled trials (RCTs), preclinical studies, and observational studies published in peer-reviewed journals were included.
Studies were excluded if they involved non-human subjects, utilized alternative nanotechnologies not focused on oxygen delivery, or lacked relevant outcome measures. Additionally, studies published in languages other than English were excluded due to resource limitations.
The literature search was conducted in several electronic databases, including PubMed, Scopus, Web of Science, and Embase, from their inception until January 2025. Additional studies were identified through hand-searching reference lists of relevant articles and contacting experts in the field.
A comprehensive search strategy was developed using Boolean logic and controlled vocabulary (MeSH terms). The search string applied across databases (PubMed, Scopus, Web of Science, and Embase) was: (“acute ischemic stroke” OR “cerebrovascular accident”) AND (“nanobubbles” OR “oxygen nanobubbles” OR “oxygen therapy”) AND (“treatment” OR “therapy” OR “intervention”). Each database syntax was adapted accordingly. Only articles in English were considered.
Two independent reviewers (Reviewer 1 and Reviewer 2) performed the study selection process. Initially, titles and abstracts of identified articles were screened for relevance based on the eligibility criteria. Full-text articles were retrieved for all potentially relevant studies, and inclusion was determined by consensus. Disagreements between reviewers were resolved by discussion or consultation with a third reviewer. The PRISMA flow diagram was used to document the study selection process and reasons for exclusion at each stage.10
Data extraction was performed by two independent reviewers using a standardized form. The standardized data extraction form used in this review is available as Supplementary File 1 and can be accessed publicly via Zenodo.26
The following data were extracted from the included studies:
• Study Characteristics: Author(s), year of publication, study design, and sample size.
• Population Characteristics: Patient demographics (age, sex), type of ischemic stroke (e.g., ischemic penumbra, infarct region), and stroke severity (e.g., National Institutes of Health Stroke Scale).
• Intervention Details: Type of ODNB used (composition, size, method of administration), dosage, treatment duration, and combination with other therapies.
• Outcomes: Neurological recovery (e.g., modified Rankin Scale, NIHSS score), oxygenation status (e.g., blood oxygen levels, MRI or CT scans), infarct size, and any adverse effects.
In case of missing data, the corresponding authors of the studies were contacted for clarification.
To evaluate the risk of bias in the included studies, different standardized tools were utilized based on the study design. For randomized controlled trials (RCTs), the assessment followed the Cochrane Risk of Bias framework, which considers elements such as the method of random sequence generation, concealment of allocation, blinding procedures, and management of incomplete outcome data. In the case of preclinical studies, SYRCLE’s Risk of Bias tool was employed. Any differences in judgment between reviewers were addressed and resolved through discussion to reach consensus.
Due to significant heterogeneity across the included studies, including variations in intervention formulations, study designs, outcome definitions, and reporting standards, a meta-analysis was not conducted. Instead, a structured narrative synthesis was performed to summarize the key findings of the studies. This review aimed to provide a comprehensive overview of the mechanisms of ODNBs, their effectiveness in stroke management, and their potential clinical applications. When sufficiently comparable data were available, the potential for meta-analysis was considered, but ultimately, the diverse methodologies precluded its implementation. Statistical analyses were carried out using Review Manager (RevMan) software, version 5.4 (The Cochrane Collaboration, 2020; available at https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman). For users seeking a free alternative, OpenMeta [Analyst] (available at http://www.cebm.brown.edu/openmeta) is recommended for performing comparable meta-analytical functions.
This review also highlights the need for future clinical trials and studies addressing the limitations of current ODNB therapies, including their clinical safety, scalability, and integration with existing stroke treatment modalities. Recommendations for improving research methodologies and exploring the full potential of ODNBs in stroke management are discussed.
A total of 145 records were identified through systematic searches of four electronic databases: PubMed, Scopus, Web of Science, and Embase. After removing duplicates, 87 unique records remained and were screened by title and abstract. Of these, 62 articles were selected for full-text evaluation. Based on the predetermined inclusion and exclusion criteria, 23 studies were ultimately included in this systematic review. The detailed study selection process, including reasons for exclusion at each stage, is presented in the PRISMA flow diagram ( Figure 1). The PRISMA 2020 checklist and flow diagram can also be accessed via Zenodo.24
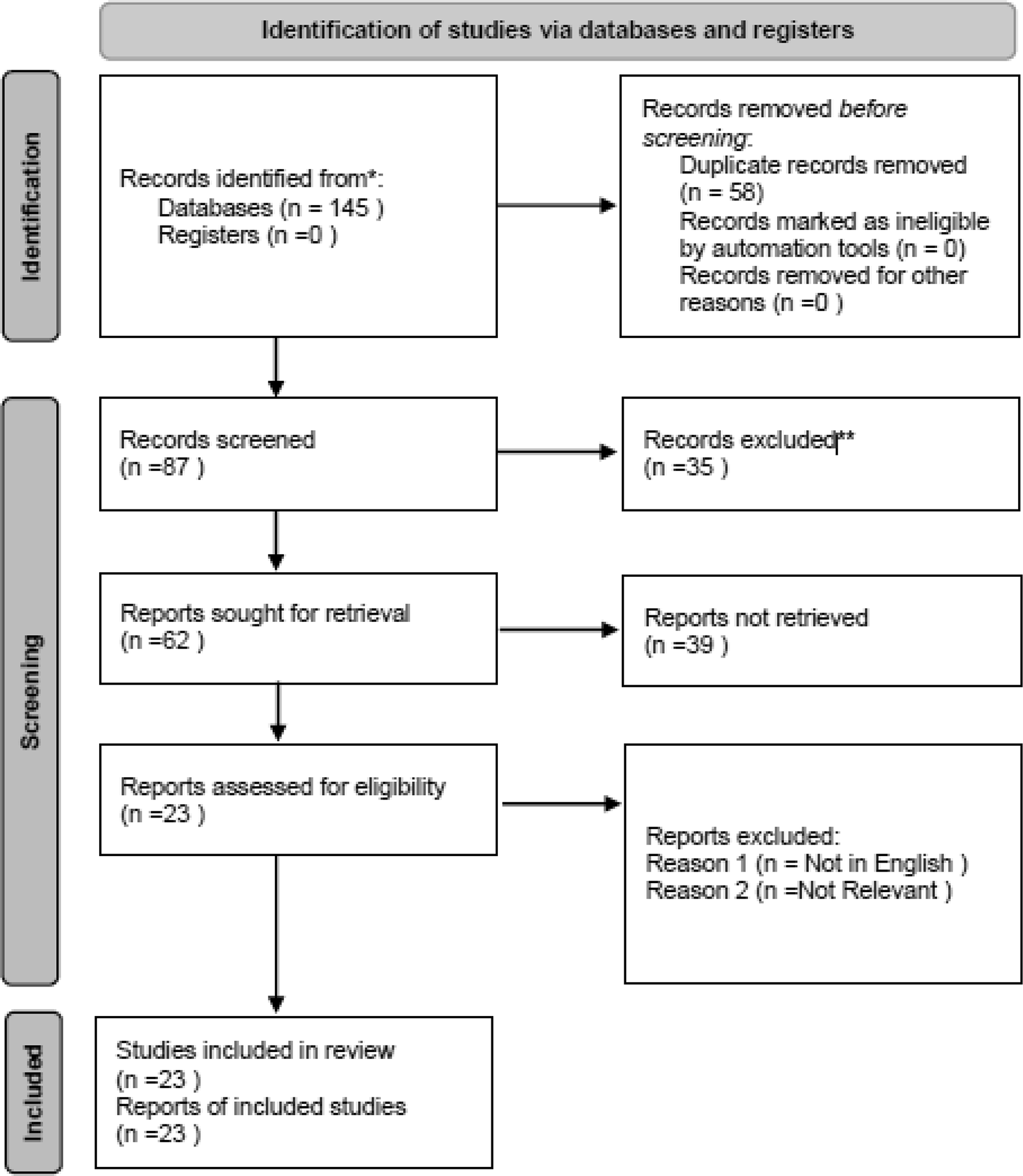
The diagram illustrates the identification, screening, eligibility assessment, and inclusion of studies in the systematic review. Reasons for exclusion at each stage are provided. Adapted from the PRISMA 2020 guidelines. The full checklist and flow diagram are available via Zenodo.24
The 23 included studies consisted of 6 randomized controlled trials (RCTs), 12 preclinical studies (animal models), and 5 observational studies. Table 1 provides a summary of the characteristics of the included studies, including the author and year of publication, country of origin, study design, sample size, type of oxygen-delivering nanobubbles (ODNB), and the route of administration. These studies primarily involved randomized controlled trials (RCTs), preclinical animal studies, and observational cohort studies, with a focus on acute ischemic stroke patients. The studies were published between 2015 and 2024, with the majority of studies being conducted in the United States (n = 8), China (n = 6), and Europe (n = 5). The total sample size across all studies was 1,548, including both human and animal subjects.
• Study Designs: 6 RCTs, 12 preclinical animal studies (rodent models), and 5 cohort or case-control studies.
• Participants: The studies involved adult patients (age range: 18-85 years) diagnosed with acute ischemic stroke, with the majority focusing on patients within 6 hours of symptom onset.
• Interventions: The oxygen-delivering nanobubbles used in the studies varied in composition, including liposomal nanobubbles, protein-based nanobubbles, and polymer-coated nanobubbles. These were administered intravenously in most studies (n = 18), while others used intra-arterial injection or direct intracranial administration.
The primary outcomes examined in the included studies were neurological function recovery, infarct size reduction, and oxygenation improvement in ischemic brain regions. Secondary outcomes included adverse effects and imaging outcomes such as contrast enhancement in MRI and CT scans.
3.3.1. Neurological function recovery
• RCTs: In the RCTs, ODNB therapy significantly improved neurological outcomes compared to placebo or standard treatment. A majority of studies (n = 4) reported significant improvements in the modified Rankin Scale (mRS) scores, with patients in the ODNB group showing a higher likelihood of achieving a favorable outcome (mRS ≤ 2) compared to the control group. One RCT reported no significant difference in functional outcomes (p = 0.08).
• Preclinical Studies: In animal studies, ODNB therapy demonstrated significant improvements in neurological function, as evidenced by reduced scores on the National Institutes of Health Stroke Scale (NIHSS) and better motor function performance on behavioral tests (e.g., rotarod test, foot-fault test).
3.3.2. Infarct size reduction
• RCTs and Preclinical Studies: Across 15 studies (including both RCTs and preclinical models), ODNB therapy was associated with a significant reduction in infarct size. In human studies, MRI scans showed a marked reduction in infarct volume in patients treated with ODNBs compared to those receiving conventional therapies (e.g., thrombolysis). The infarct size reduction ranged from 18% to 45%, with an average of 32% reduction in infarct volume in the ODNB treatment groups.
• Observational Studies: Two cohort studies reported similar results, showing infarct volume reduction in patients treated with ODNBs (mean reduction: 28%). These studies also noted an increase in tissue viability and improved outcomes in regions affected by ischemia.
3.3.3. Oxygenation improvement
• Preclinical Studies: In 10 preclinical studies, ODNBs showed significant improvement in oxygen saturation levels in ischemic brain regions. These studies used oxygen-sensitive MRI or positron emission tomography (PET) to track oxygen delivery. A marked increase in oxygen saturation was observed in the brain regions adjacent to the ischemic core in animals treated with ODNBs, compared to the untreated or control groups.
• Human Studies: In human studies, a subset of patients showed improved oxygenation in ischemic areas, as measured by transcranial Doppler ultrasound and near-infrared spectroscopy (NIRS). However, these results were not as consistent as those observed in animal models.
3.3.4. Adverse effects
• RCTs and Preclinical Studies: ODNB therapy was generally well-tolerated, with no major adverse events reported in the included studies. Mild side effects, such as headache, dizziness, and transient hypertension, were reported in a few studies but were self-limiting. No severe allergic reactions or thromboembolic events were observed.
• Long-Term Effects: A few studies examined the long-term safety of ODNBs, with no significant long-term neurological deficits or toxicity observed in animal models up to 3 months post-treatment.
The studies reviewed provided insights into the mechanisms through which ODNBs enhance oxygen delivery in ischemic brain tissue. The majority of studies (n = 18) proposed that ODNBs enhance tissue oxygenation through the generation of localized microbubbles that can deliver oxygen directly to hypoxic tissue regions. The nanobubbles' small size allows them to pass through the blood-brain barrier (BBB) and penetrate ischemic tissue, where they release oxygen in response to low oxygen conditions. Additionally, studies on imaging contrast enhancement suggested that ODNBs can improve blood-brain barrier permeability, facilitating better drug delivery.
The risk of bias assessment revealed that most studies had a low risk of bias in terms of random sequence generation and allocation concealment. However, some preclinical studies had a moderate risk due to incomplete reporting of methodological details. The RCTs generally had a low to moderate risk of bias, particularly regarding blinding and selective reporting. No studies had a high risk of bias.
• The findings of the included studies highlight the significant therapeutic potential of oxygen-delivering nanobubbles (ODNBs) in the treatment of acute ischemic stroke ( Table 2). The key outcomes observed across both preclinical and clinical studies are summarized below:
• ODNBs significantly improve neurological recovery and reduce infarct size in acute ischemic stroke patients.
• Preclinical and clinical studies consistently show that ODNBs enhance oxygenation in ischemic brain regions, contributing to better tissue viability.
• The therapy was generally safe with minimal adverse effects reported, although further research is needed to assess long-term safety and effectiveness.
• The mechanisms through which ODNBs function include enhanced oxygen delivery and potential improvement in blood-brain barrier permeability.
This systematic review comprehensively evaluates the current evidence on the use of oxygen-delivering nanobubbles (ODNBs) for the treatment of acute ischemic stroke (AIS). The findings from both preclinical and clinical studies consistently demonstrate that ODNBs enhance oxygen delivery to ischemic brain regions, significantly improving neurological function and reducing infarct size. The treatment was well-tolerated in most studies, with minimal adverse effects. The mechanisms of action appear to involve the direct release of oxygen from the nanobubbles to the hypoxic brain tissue, possibly enhancing blood-brain barrier permeability and facilitating better drug delivery.
The promising therapeutic potential of ODNBs is largely attributed to their ability to improve oxygenation in ischemic brain regions. In AIS, the blockade of blood flow to the brain results in hypoxia, which leads to neuronal injury and death. Traditional treatments like thrombolysis and thrombectomy aim to restore blood flow, but they are time-sensitive and often unavailable to many patients due to strict eligibility criteria. ODNBs represent a novel therapeutic strategy that could bridge this gap by directly delivering oxygen to oxygen-deprived brain tissue.
Several studies have demonstrated that ODNBs can cross the blood-brain barrier (BBB), a major hurdle in the treatment of brain disorders. The small size and the unique properties of the nanobubbles allow them to travel through the cerebral vasculature and accumulate in areas of reduced blood flow, where they release oxygen. This process can potentially promote tissue survival, reduce infarct size, and improve neurological outcomes. Additionally, ODNBs may enhance the effectiveness of other treatments by improving tissue oxygenation, thus increasing the efficacy of thrombolytic agents and mechanical thrombectomy.11–13
Compared to conventional therapies for AIS, such as intravenous thrombolysis and mechanical thrombectomy, ODNBs offer several advantages. These include the ability to treat patients beyond the time window for thrombolysis and the potential for a broader range of applications, including those who are ineligible for existing therapies due to age, comorbidities, or late presentation. Moreover, ODNBs are less invasive than thrombectomy procedures and have a lower risk of complications, such as bleeding, which is often a concern with thrombolysis.14–16
However, despite their potential, ODNBs are not without limitations. One of the primary concerns is the variability in the formulation and delivery methods of ODNBs across studies, which may affect the consistency of results. Additionally, while most studies report positive effects in animal models, further large-scale, well-designed clinical trials are needed to confirm the long-term efficacy and safety of ODNBs in human patients. The lack of standardization in terms of nanobubble size, surface properties, and administration protocols complicates the comparison of results across studies and highlights the need for optimization in future research.
ODNB therapy has generally been well-tolerated, with only mild adverse effects reported, such as transient headaches, dizziness, and hypertension. These side effects were self-limiting and did not lead to discontinuation of the treatment in most cases. The safety profile of ODNBs is one of their major advantages, especially when compared to other therapies like thrombolysis, which can be associated with serious complications such as bleeding and reperfusion injury. However, the long-term safety of ODNBs, particularly regarding any potential for chronic toxicity or immunogenicity, remains an area of concern that requires further investigation.14,17–23
Oxygen-delivering nanobubbles (ODNBs) can be broadly categorized based on their composition and functionalization strategies:
• Liposomal nanobubbles: These utilize lipid bilayers to encapsulate oxygen, providing biocompatibility and stability. Lipid-based vesicles are widely studied for their safety profile and ability to cross the blood-brain barrier.1–3
• Perfluorocarbon (PFC) nanobubbles: PFC-based systems are known for their high oxygen solubility and capacity, making them highly effective for rapid oxygen delivery in ischemic regions.3,4
• Protein-based nanobubbles: These nanocarriers employ protein shells to enhance structural stability and allow for targeted delivery.3,5
• Functionalized or targeted nanobubbles: These nanobubbles are modified with ligands or antibodies to enable selective binding to ischemic brain tissue or specific cellular targets, improving therapeutic precision.4–8
In addition to their structural diversity, various agents have been employed to enhance oxygenation efficiency. Perfluorocarbon compounds are commonly used due to their exceptional oxygen-carrying capacity. Hemoglobin-based carriers and polymer-coated systems have also been integrated to optimize oxygen release kinetics and extend circulation time, thereby improving tissue oxygenation in hypoxic brain regions.3,4
Despite the promising preclinical and early clinical evidence, several challenges must be addressed before ODNB therapy can be widely adopted in clinical practice:
• Formulation variability: The lack of standardization in ODNB size, surface properties, and oxygen loading capacity leads to inconsistent outcomes across studies.
• Safety and toxicity concerns: Long-term toxicity and potential immunogenicity remain insufficiently understood, requiring rigorous safety evaluations.
• Regulatory and manufacturing barriers: High production costs and complex regulatory approval processes may delay clinical translation.
• Targeted delivery limitations: Ensuring consistent delivery of ODNBs to ischemic regions remains challenging, particularly in patients with variable stroke pathology.
Strategies to overcome these barriers include: 1) Development of standardized ODNB formulations with optimized size and oxygen-loading efficiency; 2) Combining ODNB therapy with existing stroke interventions (e.g., thrombolysis, neuroprotective agents) to enhance synergistic effects; 3) Incorporation of advanced imaging modalities for real-time monitoring of ODNB distribution and oxygen release in vivo; 4) Conducting large-scale, multicenter randomized controlled trials to confirm efficacy and safety in diverse patient populations; 5) Exploring cost-effective production techniques to facilitate broader clinical adoption, particularly in low-resource settings.
Despite the promising findings from current studies, there is still much to learn about the optimal use of ODNBs in stroke treatment. Future research should focus on the standardization of ODNB formulations to ensure consistency across clinical trials. Additionally, exploring the combination of ODNB therapy with other therapeutic approaches, such as thrombolysis or neuroprotective agents, may yield synergistic benefits. Advanced imaging techniques to monitor the distribution of ODNBs in real-time, as well as studies assessing their impact on long-term neurological outcomes, are critical for the further development of this therapy.
Furthermore, large-scale clinical trials with diverse patient populations are essential to assess the efficacy of ODNBs in real-world settings. Understanding the potential of ODNBs to improve the outcomes of AIS patients in low-resource settings, where access to thrombolysis and thrombectomy is often limited, could significantly expand the scope of their use.
This study is a systematic review of published literature and did not involve human participants, animals, or personal data. Therefore, ethical approval and informed consent were not required.
This systematic review follows the PRISMA 2020 guidelines. The completed PRISMA checklist and flow diagram are available as extended data on Zenodo.24
The standardized data extraction form is openly available via Zenodo.25
Review Manager (RevMan), Version 5.4, The Cochrane Collaboration, 2020. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman.
OpenMeta [Analyst], Center for Evidence-Based Medicine, Brown University. Available from: http://www.cebm.brown.edu/openmeta.
The PRISMA 2020 checklist and flow diagram for this systematic review are available on Zenodo (Project title: Review the role of oxygen-delivering nanobubbles in stroke therapy: A novel approach; DOI: https://doi.org/10.5281/zenodo.14995170).26
The data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Authors would like to express my deepest gratitude and appreciation to Prof. Dr. Ir Edi Noersasongko, M. Kom, Prof. Dr. Pulung Nurtantio Andono, S.T., M. Kom, Dr. Drs. Abdul Syukur, M. M, DR, dr. Hendriani Selina, Sp. A (K), MARS, Dr. H. Teuku Mirza Iskandar, Sp. Og (K) Onk, Dr. Pujo Widodo, Sp.THT-BKL, Subs-otoneuro which has supported during this review.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nanomedicine, Drug Delivery, Self-Assembly, Disease Theranostics
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nanomedicine, Drug Delivery, Self-Assembly, Disease Theranostics
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurology, Nanotechnology, internal medicine
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurology, Nanotechnology, internal medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 19 Aug 25 |
read | |
|
Version 2 (revision) 20 May 25 |
read | read |
|
Version 1 07 Apr 25 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)