Keywords
Antibacterial, anti-inflammatory, dental care, herbal mix, gingivitis, plaque, toothpaste
Gingivitis is an inflammation of the gums caused by the buildup of bacterial plaque along the gum line. It can be reduced and prevented by regularly brushing your teeth with a good quality toothpaste. Recently, toothpaste made from natural ingredients has been developed which tends to be popular because it has minimal side effects and a pleasant odor. The aim of this study was to evaluate the herbal toothpaste mix in gingivitis model rats.
The product profile of toothpaste mix extracts (TPME) was carried out with organoleptic tests that pay attention to odor, color, taste and texture. in vivo testing was carried out on a sample of 30 Wistar rats modeling gingivitis with tooth brushing treatment twice a day for 7 days in the negative control group (base toothpaste), positive control (commercial toothpaste), toothpaste mix extract (TPME). Profiles of fibroblasts, collagen, angiogenesis counts, monocyte cell counts, polynuclear cell counts were observed by Haematoxylin-eosin staining (HE) and Masson Trichome (MT) staining, with immunohistochemical (IHC) markers CD163 and CD86. Proinflammatory gene expression was also observed in Interleukin-1 Beta (IL-1β) and Interleukin-6 (IL-6) genes.
TPME had a positive response to odor, color, taste and texture characters. Histological observations on days 0, 1, 3, 5 and 7 showed the most significant results on day 7 with an increase in the number of collagen, fibroblasts, angiogenesis, and M2 on CD163 which was in line with the decrease in the number of polynuclear cells, monocytes, M1 on CD86, expression of pro-inflammatory genes IL-1β, and IL-6 which were significantly different from other treatments (p<0.050).
TPME is able to become a toothpaste product that can treat gingivitis with twice daily application which has the potential to be a promising product to be applied in the future.
Antibacterial, anti-inflammatory, dental care, herbal mix, gingivitis, plaque, toothpaste
Gingivitis is a common and mild form of periodontal disease characterized by inflammation of the gum tissues, primarily caused by plaque buildup. The onset of gingivitis is often characterized by symptoms such as bleeding gums, which can occur with minimal stimulation. This bleeding is an important indicator of gingival inflammation and an early warning sign of potential periodontal disease.1–3 Untreated gingivitis can progress to periodontitis, a more severe form of gum disease that can lead to tooth loss and has been linked to systemic conditions such as cardiovascular disease and diabetes.4,5 In terms of its epidemiology, gingivitis affects most people. Studies show overall prevalence rates ranging from 90% to more than 95% in different populations, with the highest prevalence of inflammation in adults due to unhealthy lifestyles.6 In certain age groups, especially in adolescents aged 12-15 years, studies report gingivitis prevalence rates between 29.6% and 47.3%. A recent survey found that 47.3% of school-aged children had plaque-induced gingivitis.7
The main etiologic factor of gingivitis is the presence of bacterial plaque, which causes the inflammatory response seen in the gingival tissue.8,9 Studies show that certain bacterial species, particularly those belonging to the Streptococcus and Porphyromonas genera, are significantly associated with gingival inflammation. For example, the attachment and coagulation of Streptococcus species on tooth surfaces facilitates the formation of complex microbial communities that can lead to increased inflammation.10 The presence of these bacteria not only contributes to plaque formation, but also enhances the inflammatory response through the production of virulence factors that exacerbate tissue damage.11
The presence of bacterial interactions that cause gingivitis promotes the expression of pro-inflammatory genes such as IL-1β and IL-6. IL-1β is a key cytokine involved in the inflammatory response associated with gingivitis. IL-1β is produced by various cell types, including macrophages and gingival fibroblasts, in response to bacterial stimuli. The presence of pathogenic bacteria, such as Porphyromonas gingivalis, triggers the release of IL-1β, which in turn promotes inflammation and tissue damage.12,13 Studies show that IL-6 levels are significantly elevated in the gum tissue of patients with gingivitis, indicating its role in the inflammatory process.14,15 Bacterial components, such as lipopolysaccharide (LPS) from gram-negative bacteria, can activate Toll-like receptors (TLRs) on host cells, leading to the production of IL-1β and IL-6.16,17Activation of these TLRs is important for initiating the inflammatory response, as it increases the expression of these cytokines, thereby strengthening the immune response to bacterial challenge.
The inflammatory phase in wound healing is characterized by the presence of pro-inflammatory cytokines, including IL-1β and IL-6. Although these cytokines are essential for initiating the healing response, their prolonged expression can lead to chronic inflammation, which inhibits the transition to the proliferative phase of healing.18 Decreased levels of IL-1β and IL-6 can facilitate the resolution of inflammation, allowing the transition to the repair phase, which is characterized by increased fibroblast activity, collagen deposition, and angiogenesis.19 This transition is critical for effective wound healing in gingivitis, as it promotes tissue regeneration and restoration of gum architecture.
Factors such as poor oral hygiene and certain habits such as mouth breathing contribute to the incidence of gingivitis.20,21 The main pillar of gingivitis prevention is maintaining optimal oral hygiene. Regular brushing with fluoride-containing toothpaste is essential, as it helps to remove plaque and prevent its buildup. Studies show that a combination of tooth brushing and interdental cleaning, such as using dental floss or an interdental brush, significantly reduces plaque levels and, consequently, the incidence of gingivitis.22,23 It is recommended that individuals brush their teeth at least twice a day and incorporate interdental cleaning into their daily routine to maximize oral health.24 Research shows that toothpastes containing certain active ingredients, such as herbal extracts or antimicrobial agents, can effectively reduce gum inflammation. For example, a randomized clinical trial found that toothpaste containing Miswak extract significantly reduced both the level of gum inflammation and the amount of plaque after three weeks of use.25 Similarly, another study showed that herbal toothpastes, such as those containing neem leaf extract, were effective in reducing plaque index (PI) and gum bleeding index (PBI), indicating their potential as an alternative to conventional toothpastes.26 Several studies have compared the effectiveness of herbal and non-herbal toothpastes in managing gingivitis. For example, a clinical study found that both herbal and non-herbal toothpastes were effective in controlling plaque and gingivitis, with some herbal formulations showing comparable effectiveness to traditional fluoride toothpastes.27,28 This suggests that toothpaste selection may influence the effectiveness of brushing in reducing gum inflammation.
This toothpaste is formulated from multiple extracts such as ginger, black cumin, royal jelly, and cinnamon.29–33 The synergistic effects of these herbal ingredients in toothpaste formulations have been explored in various studies. For example, the combination of multiple herbal extract has shown stronger antibacterial and anti-inflammatory effects, making it a promising candidate for improving oral health.34 Toothpaste formulations containing these herbal ingredients not only target plaque and gingivitis reduction, but also support overall gum health through their combined properties.
Toothpaste with single extracts has been widely reported, while toothpaste with multi-extracts is still limited, so this study aims to evaluate mixed extract toothpaste in reducing gingivitis in animal models of gingivitis by observing gene expression of IL-1β, IL-6, and histopathology of gingival tissue in inflammation-related cells (monocytes and polynuclears), angiogenesis, fibroblasts as well as immunohistochemical markers CD86 and CD163.
TPME is carried out organoleptic test on 34 respondents with observing parameters through aroma, color, taste and texture. Respondents' assessments will be carried out descriptive statistical tests and classical assumptions on the parameters tested.
This study is an experimental study with a randomized control trial research design using 30 Wistar rats calculated by the previous Federer formula. The rats selected were male white rats of the Wistar strain, weighing 150-250 grams, aged 6-8 weeks and in a healthy condition characterized by active movement, responding well when shocked, no anatomical abnormalities, and no hair loss. Rats that experienced weight loss, illness and death during acclimatization were not included in this study.
This study has received ethical approval from the Health Research Ethics Committee of the Faculty of Medicine, Maranatha Christian University with number 007/KEP/H/2024. The use of experimental animals in this study takes into account three main points of interest in the use of animals, namely (Replacment), the determination of restrictions on the number of animals used (Reduction), and the treatment of test animals that correctly or ethically fulfill the concept of experimental animals that avoid pain (Refinement). In accordance with ethical guidelines, all efforts were made to minimize animal suffering during the course of this experiment. The study was conducted with the approval of the relevant animal ethics committee, and all procedures were carried out under the supervision of qualified personnel. Efforts to ameliorate suffering included the use of appropriate anesthesia and analgesia, as well as close monitoring of animal well-being throughout the study. Additionally, humane endpoints were established, and animals were observed for signs of distress or discomfort, with timely interventions taken when necessary.
Before entering animal testing, a total of 30 rats were acclimatized for a week in Individual Ventilated Cages (IVC) measuring 30.5 x 20.5 x 15.5 cm with a 2 cm high sawdust mat, which was replaced every 2-3 days. These test animals were housed in a 12-hour light/12-hour dark cycle, room temperature of 22 ± 2°C, and humidity level of 50 ± 10% with regular feed and water ad libitum during acclimatization.
After acclimatisation, the rats were injected intraperitoneally with a mixture of ketamine and xylazine 0.5 mg/ml body weight and ligated with a silk ligature. Rats that are already non-basic, given an injection in the gingival tissue of rats as much as 0.5 mg/ml with P. gingivalis and Streptococcus mutans bacteria until gingivitis occurs for 6 days of observation. after the occurrence of gingivitis, the teeth of the test animal subjects will be brushed routinely every 2x a day with various toothpastes as treatment. The treatment in this study was divided into 3 groups, namely the negative control group (NC) which was rubbed with base toothpaste, the positive control group (PC) was rubbed with commercial toothpaste (Pepsodent mint), and toothpaste mix extract (TPME) was rubbed with toothpaste formulated with a mixture of herbs containing various active compounds. The treatment was carried out for 7 consecutive days.
The Wistar rats were terminated on days 0, 1, 3, 5 and 7. Termination was performed using CO2 gas. Rats are placed in a box that will be filled with CO2. Gradually CO2 will be filled into the box from 30% to 70% of the chamber volume/minute. The CO2 flow was maintained for at least 1 minute to provide an unconscious effect on the test animals. The gingival tissues were collected with fisologic solution for RT-PCR and 50 mL 10% neutral formalin buffer (Sigma Aldrich, HT501128) for haematoxylin eosin (HE), Masson trichome (MT) and IHC.
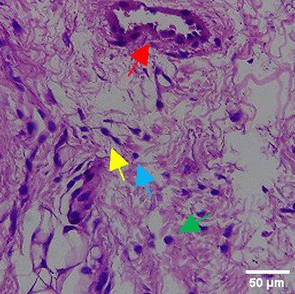
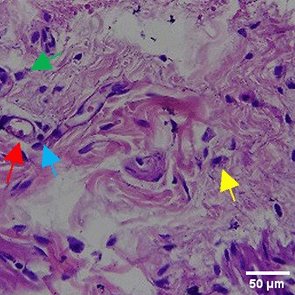
The tissues were fixed in 10% neutral buffered formalin (NBF) solution for 24 hours, then use a solution consisting of 70% alcohol, 80% alcohol, 90% alcohol, anhydrous alcohol, toluene, and paraffin wax for dehydration and classification, gradually over a day. The tissue is sealed with an embedding device filled with liquid paraffin and then refrigerated. Use a microtome with a thickness of ±4-5 microns to slice the cold block. The sections were stained with hematoxylin and eosin (H&E) (BIOSCIENCE, 786-1263) with a volume used of 200-250 mL to fill the slot of the staining rack container for histopathological analysis. The number of new blood vessels formed was counted manually under a binocular microscope with 40x magnification from 5 LPB (large field of view) preparations. The inflammatory reaction was observed through counting the distribution of inflammatory cells such as eosinfils, monocytes, neutrophils, lymphocytes under a binocular microscope with 100x magnification of 5 LPB (large field of view) preparations.35
The Masson Trichome (MT) staining procedure starts with HE results followed by rinsing using distilled water. all reagents used follow the volume of the staining rack container slot of about 200-250 mL. Next, the tissue was stained with Bieblich Scarlet Acid Fuchsin solution (Sigma-Aldrich, HT151) for 10-15 minutes, rinsed again with distilled water. After that, the tissue was stained with phosphomolybdenum-phosphotungstic acid solution (Sigma Aldrich, HT152) for 10-15 minutes, followed by staining using aniline blue solution (Sigma Aldrich, B8563) for 5-10 minutes. The process ended with immersion in 1% acetic acid (Merck, 1000630510) and 95% absolute ethanol (Merck, 1070174000). MT and HE results were examined under a microscope (Primo Star 3, Zeiss). ImageJ software was used to assess fibroblasts, Mononuclear cells, Polymorphonuclear leukocytes, and angiogenesis, and collagen.35
Sample preparation was performed in the same way as the HE method. After rehydration, tissues were incubated with Normal Goat Blocking Buffer (Elabscience, PA6146) for 30 minutes. Samples were then stained with polyclonal antibodies for CD163 (Elabscience, E-AB-70306) and CD86 (Elabscience, E-AB-52526). To visualize the target proteins, samples were incubated with DAB substrate (Elabscience, E-IR-R217D; E-IR-R217E) for 15 seconds in a dark room. Five different areas on each slide were taken as a representation of the overall results. ImageJ software was used to semi-quantify the index of positive cells on IHC slides, which is represented as the percentage of positive area relative to the total area.36,37
Total gingival tissue in rats was minced and following the instructions provided by the manufacturer, tissue RNA was extracted and purified from each mouse test group using the Direct-zol RNA Miniprep Plus Kit (Zymo, R2073). iScript Reverse Transcription Supermix for RT-PCR (Bio-Rad, 170-8841) was used to perform complementary DNA synthesis. To quantitatively assess gene expression, we used the Agilent AriaMx 3000 real-time PCR method.38 The qPCR reaction mixture used was Evagreen Master Mix (Bio-Rad, 1725200). Spectrophotometric measurements of RNA concentration and purity at 260/280 nm are shown in Table 1, and primer sequences (Macrogen) are shown in Table 2.
| Gene | Sekuens Primer (5' - 3') | Product Length (bp) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| IL-6 (Rattus norvegicus) | F: TGATGGATGCTTCCAAACTG | 230 | 53 | >NM_012589.2 |
| R: GAGCATTGGAAGTTGGGGTA | ||||
| IL-1β (Rattus norvegicus) | F: CAACCAACAAGTGATATT | 116 | 55 | >NM_031512.2 |
| R: CTTTCATTACACAGGACAGG | ||||
| b-actin (Rattus norvegicus) | F: TCTACAATGAGCTGCGTGTG | 190 | 58 | >NM_031144.3 |
| R: ATCACAATGCCAGTGGTACG |
Samples were tested in three replicates and analyzed using Statistical Package for Social Sciences software (IBM®, version 20.0). Statistical analysis used Kruskal Wallis (non-parametric ANOVA) and post hoc with p-value <0.05 on quantitative data including membrane thickness, number of new blood vessels, and number of inflammatory cells distribution.
Based on Table 3, there are 34 samples of TPME organoleptic results data. The mean value for the color indicator is 3.58, indicating that the average respondent agrees that TPME has a slightly brownish color close to white which is quite close to commercial toothpaste.
| Organoleptic test | Mean±Standar Deviasi |
|---|---|
| Colors | 3.59 ± 1.54 |
| Taste | 1.91 ± 0.29 |
| Scent | 2.03 ± 0.63 |
| Texture | 2.50 ± 0.56 |
Based on Table 4, frequency distribution regarding the color of TPME, respondents had various responses to the color aspect of this product. A total of 7 people (20.6%) rated the color of the toothpaste as brown, while 1 person (2.9%) stated a light brown color. A total of 4 people (11.8%) chose a slightly brownish color. The most common responses were white, which was chosen by 9 people (26.5%), and cream color, which was the most dominant choice with 13 people (38.2%). Overall, the majority of respondents (38.2%) rated this toothpaste as cream-colored, followed by respondents who rated it as white (26.5%). Meanwhile, the rest were divided between brown, slightly brownish, and light brown colors. These results show that polyherbal toothpastes have a fairly wide variation in color perception among users, with cream and white being the most widely recognized choices.
| Color | Frequency | Percent valid (%) |
|---|---|---|
| Color | 7 | 20.6 |
| Brown | 1 | 2.9 |
| Light brown | 4 | 11.8 |
| Slightly brownish | 9 | 26.5 |
| White | 13 | 38.2 |
| Total | 34 | 100.0 |
Based on Table 5, it can be concluded that respondents who liked the taste of TPME were 31 respondents or 91.2%. While respondents who disliked it were only 3 or 8.8%. The results of the frequency distribution related to the aroma or odor of TPME show that the majority of respondents gave positive responses. A total of 6 people (17.6%) stated that they did not like the aroma of the product, indicating that respondents were less satisfied with the aroma aspect. However, the majority, namely 21 people (61.8%), stated that they liked the aroma of this toothpaste, indicating that the majority of users found the aroma of this product quite pleasant. In addition, there were 7 people (20.6%) who responded that they really liked it, which represents the group with the most positive response to this product. Overall, it was more dominated by respondents who stated that they liked or really liked the aroma of TPME.
| Frequency | Percent valid (%) | |
|---|---|---|
| Toothpaste flavors | ||
| Like | 3 | 8.8 |
| Dislike | 31 | 91.2 |
| Scent | ||
| Dislike | 6 | 17.6 |
| Like | 21 | 61.8 |
| Like very much | 7 | 20.6 |
| Total | 34 | 100 |
Based on the frequency distribution table in Table 6, regarding the texture of toothpaste, it can be interpreted that respondents gave three types of responses regarding the texture of the product. A total of 15 people (44.1%) stated that the texture of this toothpaste was quite hard, indicating that almost half of the respondents felt the hardness in the product. On the other hand, 18 people (52.9%) stated that the texture of this toothpaste was soft, which means that more than half of the respondents felt that this product had a smoother and softer texture. Only 1 respondent said the texture of the toothpaste was hard.
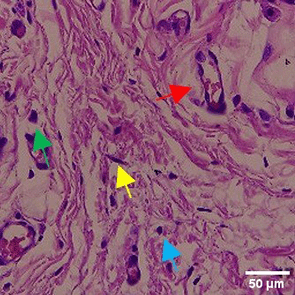
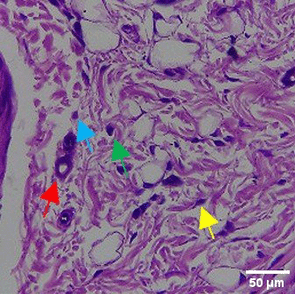
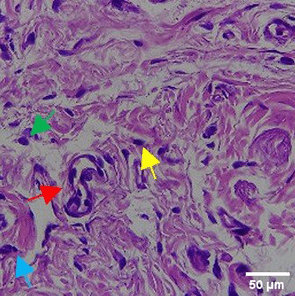
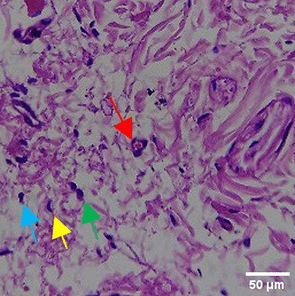
In the observation of the analysis of the number of fibroblasts attached in Figure 1A-C, the number of fibroblasts increased from D-0 to D-5, with a significant increase. After D-5, there was a slight decrease at D-7. This suggests that fibroblast proliferation occurs up to D-5, then followed by stabilization or a slight decrease in cell number. A similar pattern to NC was seen in the PC group, with an increase in fibroblast numbers from D-0 to D-5 and a slight decrease at D-7. However, in general, the number of fibroblasts in PC tended to be higher than NC (p<0.050). Interestingly, the TPME group ( Figure 1C) showed the highest number of fibroblasts compared to the other two groups, especially at D-5 and D-7. A significant increase was seen from D-1 and continued until D-7. This suggests that TPME is highly effective in stimulating fibroblast proliferation, which is a key cell in the synthesis of collagen and other extracellular matrices. Fibroblast densities are visualized in figure Table 7a-o.
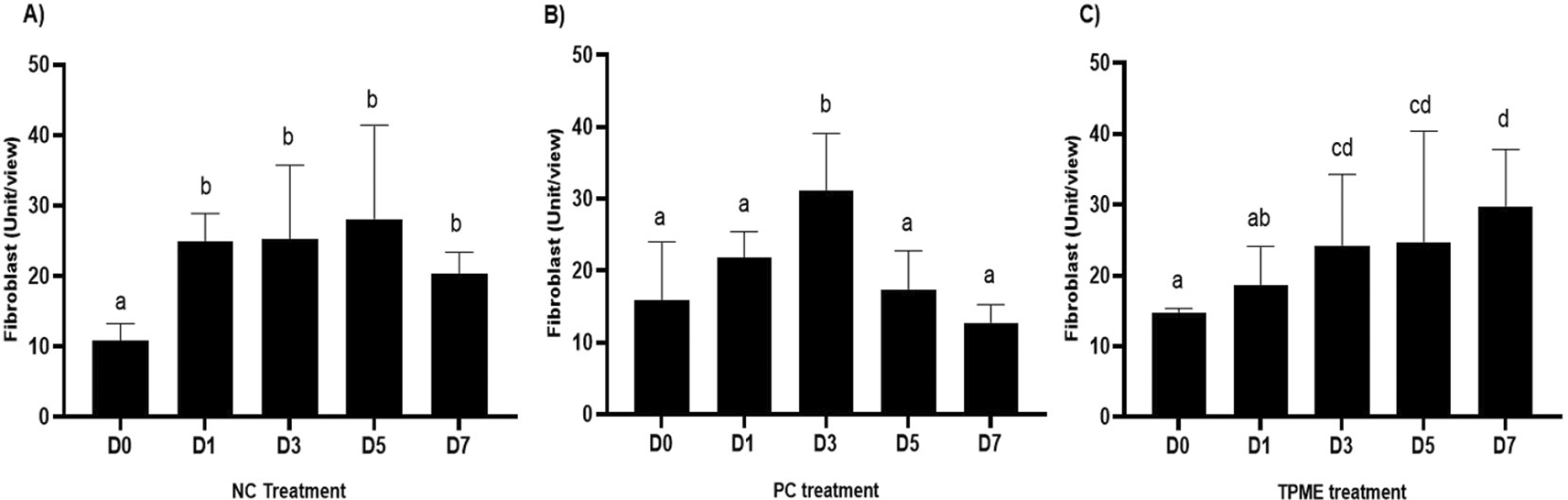
A) Negative control (NC); B) Positive control (PC); C) Totthpaste mix extract (TPME). Data are presented as mean ± standard deviation. For each treatment, the test was performed in five repetitions. NC: Negative Control (Gingivitis rats + toothpaste base 2x/day), PC: Positive Control (Gingivitis rats + commercial toothpaste 2x/day), TPME (Gingivitis rats + toothpaste mix extract 2x/day). Different superscripts (a,b), (a,b), and (a,ab,cd,c) mark significant differences between treatment groups (p<0.05, Tukey HSD test).
| Treatment | Day-0 | Day-1 | Day-3 | Day-5 | Day-7 |
|---|---|---|---|---|---|
| NC | 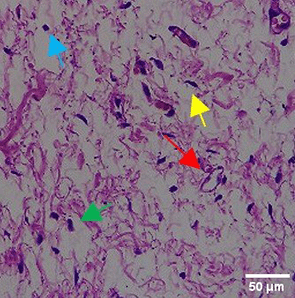
| 
| 
| 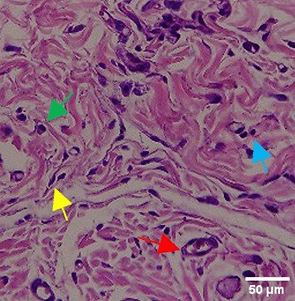
| 
|
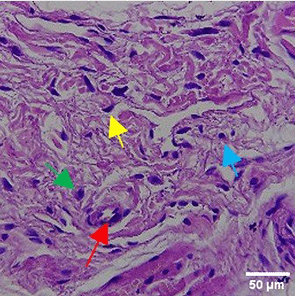
| PC | 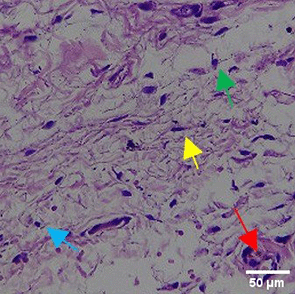
| 
| 
| 
| 
|
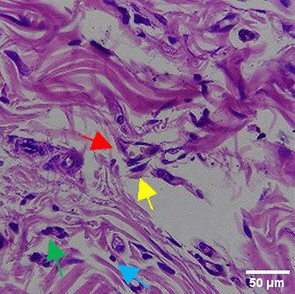
| TPME | 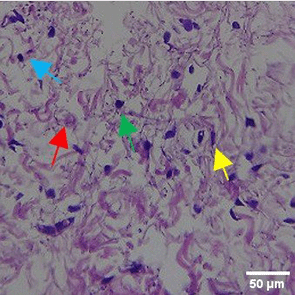
| 
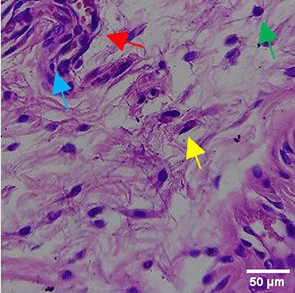
| 
| 
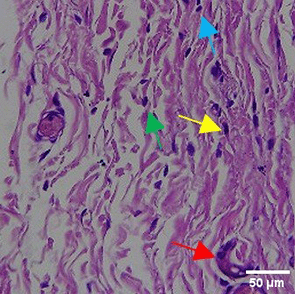
| 
|
All groups were observed for the number of polymorphonuclear cells and monocytes. Based on the results shown in Figure 2, all treatments demonstrated an increase in the number of polymorphonuclear cells and monocytes, indicating an early acute inflammatory response to the toothpaste application on Day 1. The TPME group exhibited a more moderate increase compared to the PC group for both cell types, although still higher than the NC group. Angiogenesis also began to increase in all groups ( Figure 2C). On Day 3, entering the Inflammatory and Modulation phase, differences between the groups became more apparent. In the PC group, the number of monocytes peaked, indicating a more prolonged inflammation. In contrast, in the TPME group ( Figures 2A and 2B), both polymorphonuclear cells and monocytes decreased drastically, indicating effective modulation of the inflammatory response. Angiogenesis in the TPME group peaked on Day 3, suggesting that TPME promotes the formation of new blood vessels earlier than the other groups. In the NC group, the number of inflammatory cells began to decrease, and angiogenesis peaked, indicating a controlled response. On Days 5 and 7, the TPME group showed lower numbers of polymorphonuclear cells and monocytes compared to the NC group, indicating that TPME not only suppresses inflammation but also facilitates the resolution of inflammation and/or tissue healing. The PC group still exhibited higher numbers of inflammatory cells compared to the other groups. Angiogenesis in the TPME group started to decline after peaking, while in the PC group, angiogenesis only peaked on Day 5, indicating a delay in the healing process. On Day 7, all groups showed a decrease in angiogenesis. The TPME treatment demonstrated a more rapid increase in angiogenesis with a gradual decrease in monocytes and polymorphonuclear cells, suggesting that TPME has the potential to be a competitive toothpaste, possibly outperforming other commercial products.
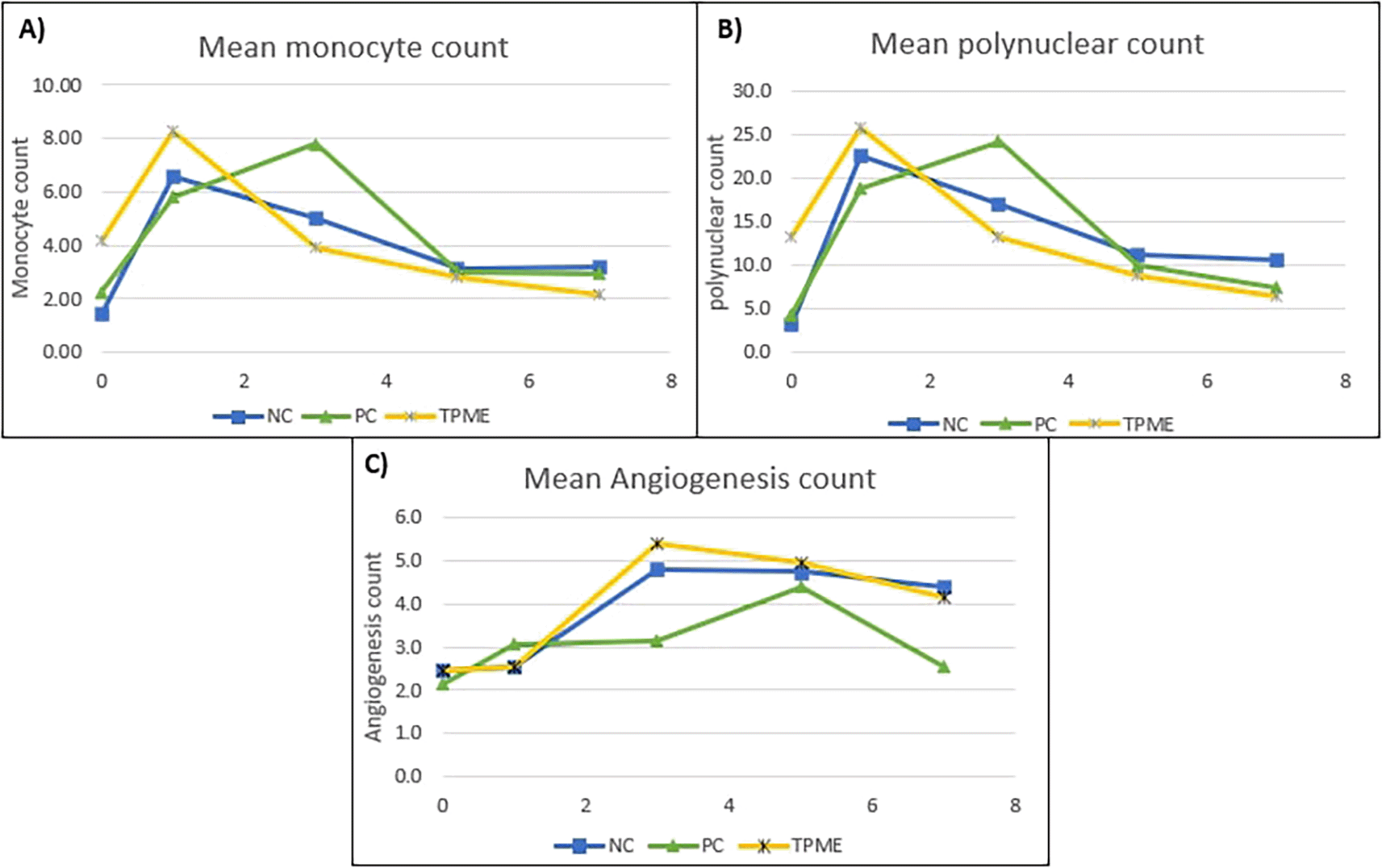
Data are presented as mean. For each treatment, the test was performed in five repetitions. NC: Negative Control (Gingivitis rats + toothpaste base 2x/day), PC: Positive Control (Gingivitis rats + commercial toothpaste 2x/day), TPME (Gingivitis rats + toothpaste mix extract 2x/day).
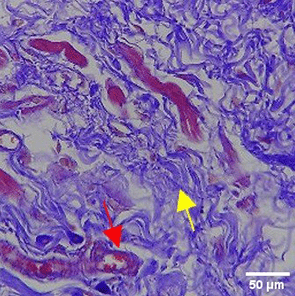
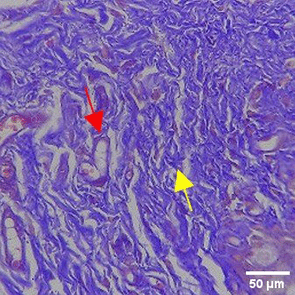
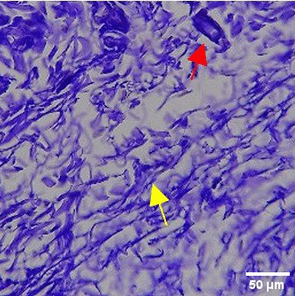
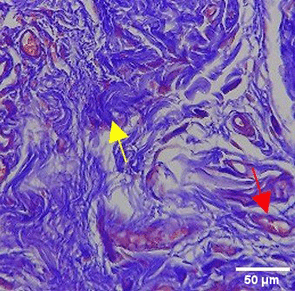
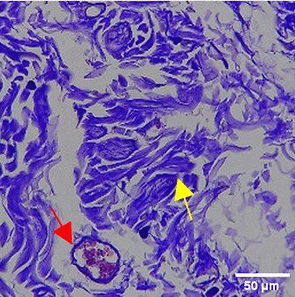
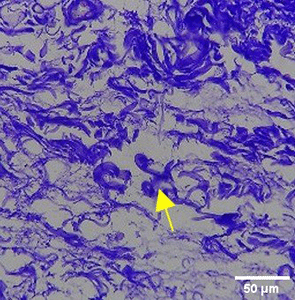
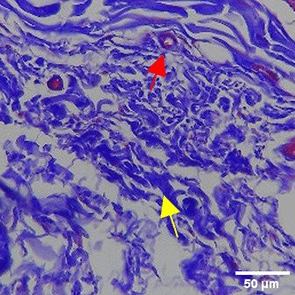
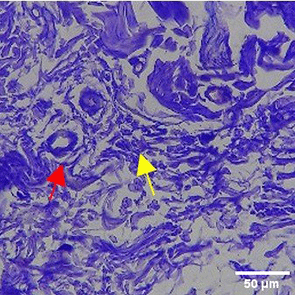
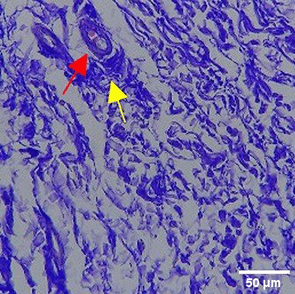
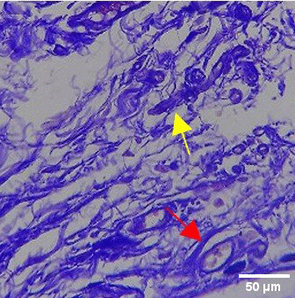
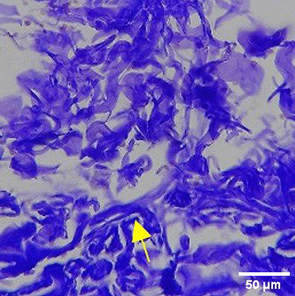
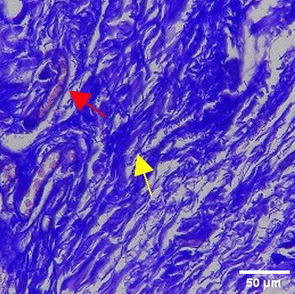
The increase in collagen density also gradually increased from D-0 to D-7 as attached in the Figure 3A-C. A significant increase was seen from D-1 and continued until D-5, with a slight increase again at D-7.This indicates a normal and gradual process of tissue repair. In the PC group, the pattern of collagen density increase was similar to NC, but with a slightly higher increase at some time points, especially at D-5.This indicates that the PC treatment also supports collagen formation, although perhaps by a different mechanism than NC (p<0.050). The TPME group showed the most significant increase in collagen density compared to the other two groups, especially at D-5 and D-7. Collagen densities are visualized in the Figure 3 and figure Table 8 thus indicating that TPME is highly effective in promoting collagen synthesis and deposition, which is important for gingival tissue healing and regeneration. This suggests that TPME is highly effective in stimulating collagen proliferation, which is a key cell in the synthesis of collagen and other extracellular matrices.
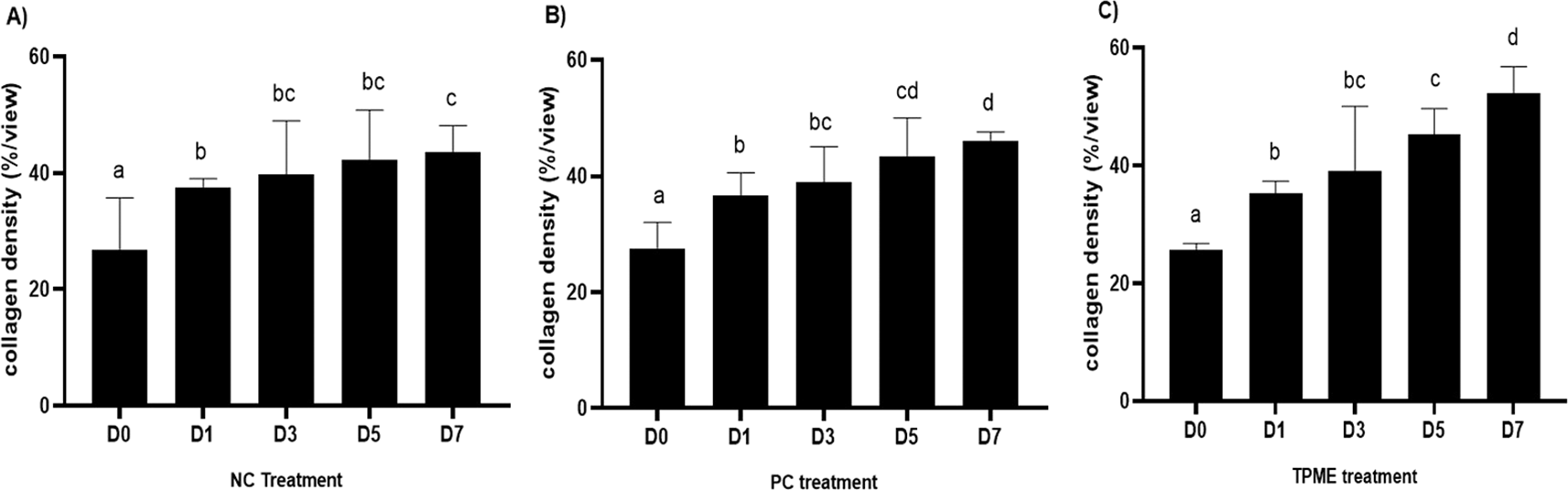
A) Negative control (NC); B) Positive control (PC); C) Toothpaste mix extract (TPME). Data are presented as mean ± standard deviation. For each treatment, the test was performed in five repetitions. NC: Negative Control (Gingivitis rats + toothpaste base 2x/day), PC: Positive Control (Gingivitis rats + commercial toothpaste 2x/day), TPME (Gingivitis rats + toothpaste mix extract 2x/day). Different superscripts (a,b,bc,c), (a,b,bc,cd,d), dan (a,b,bc,c,d) mark significant differences between treatment groups (p<0.05, Tukey HSD test).
| Treatment | Day-0 | Day-1 | Day-3 | Day-5 | Day-7 |
|---|---|---|---|---|---|
| NC | 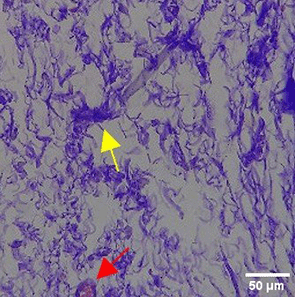
| 
| 
| 
| 
|
| PC | 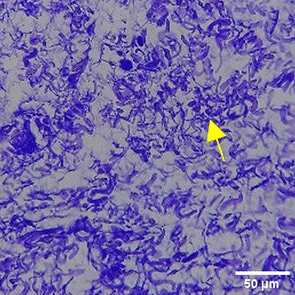
| 
| 
| 
| 
|
| TPME | 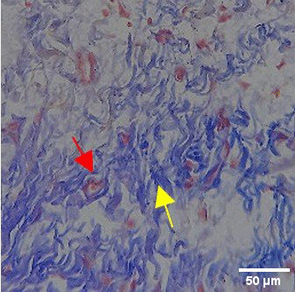
| 
| 
| 
| 
|
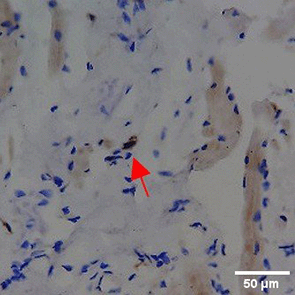
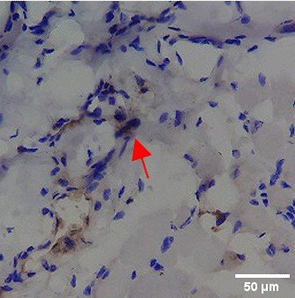
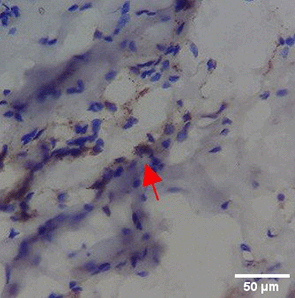
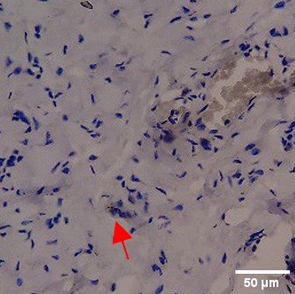
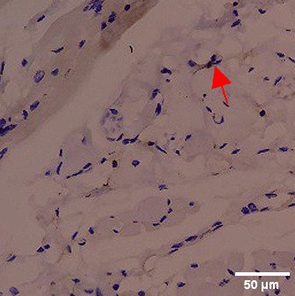
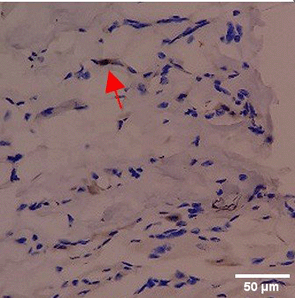
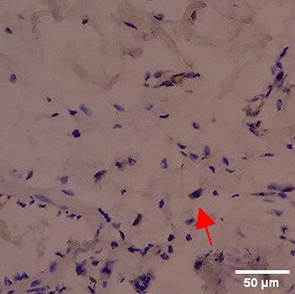
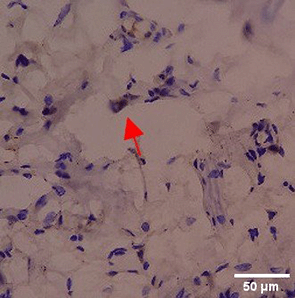
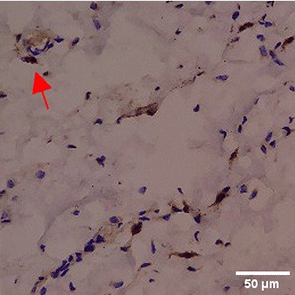
Immunohistochemistry of gingival tissue marker CD86 during the processes of homeostasis, inflammation, proliferation, remodelling, recovery
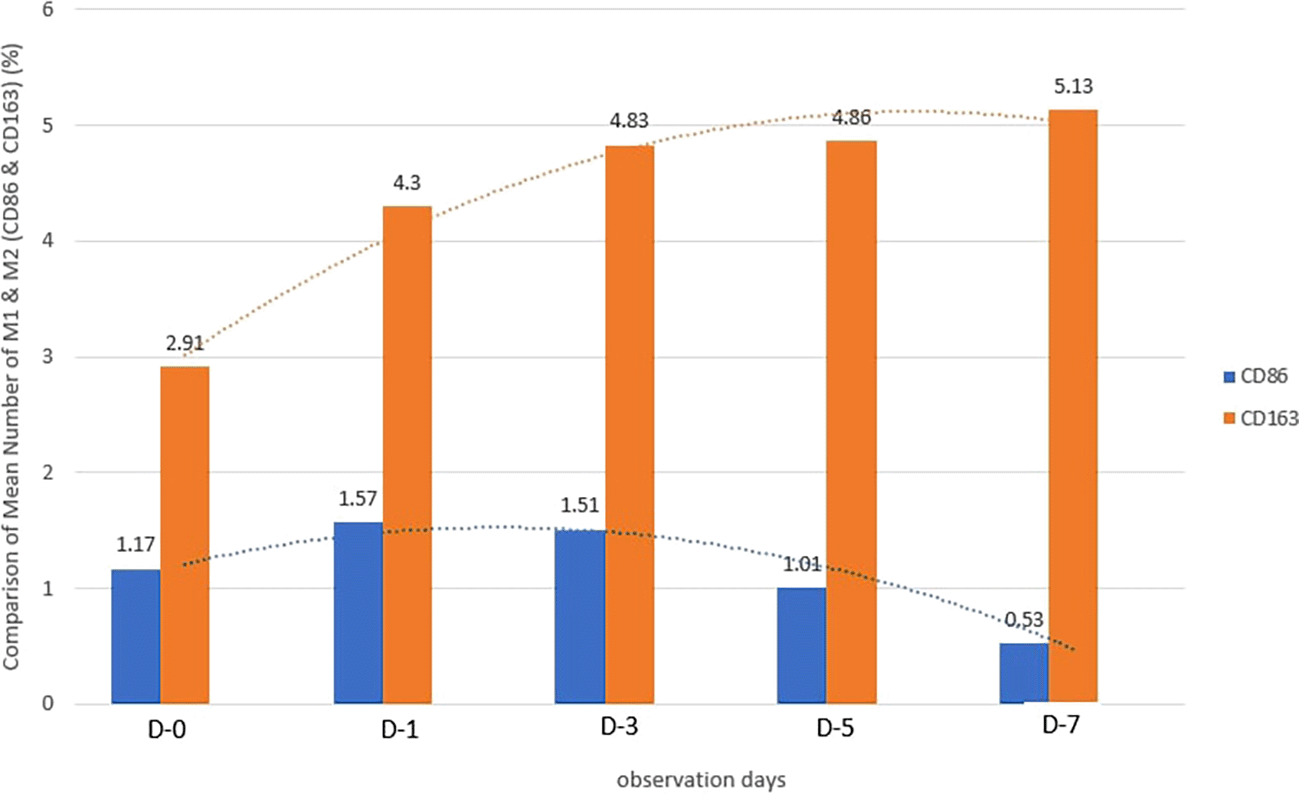
Based on the IHC analysis of the CD86 marker in figure Table 9, it can be seen that the toothpaste mix extract (TPME) showed potential in modulating the immune response in the rat gingivitis model. Although an increase in antigen presenting cell (APC) activation was seen at the start of treatment, TPME then effectively suppressed such activation, as shown by the significant M1 decrease in CD86 expression (Figure 4). These findings support the potential of TPME as an anti-inflammatory and immunomodulatory agent in the treatment of gingivitis.
| Treatment | Day-0 | Day-1 | Day-3 | Day-5 | Day-7 |
|---|---|---|---|---|---|
| NC | 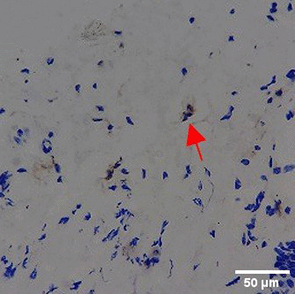
| 
| 
| 
| 
|
| PC | 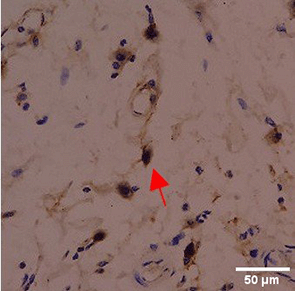
| 
| 
| 
| 
|
| TPME | 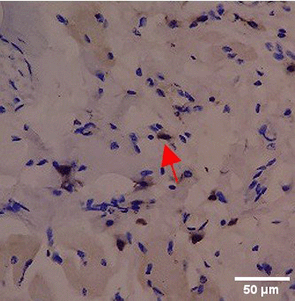
| 
| 
| 
| 
|
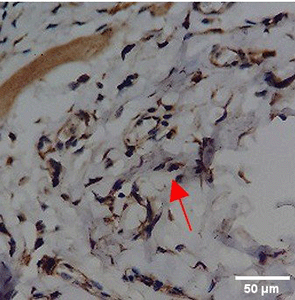
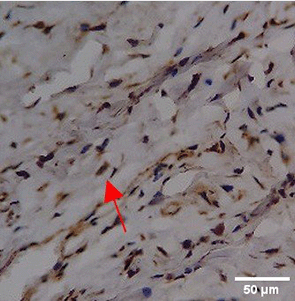
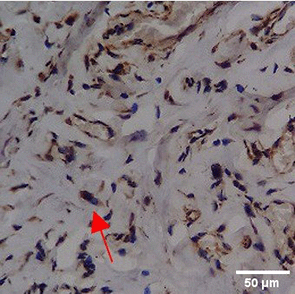
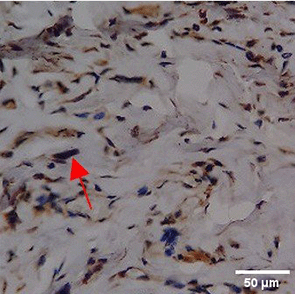
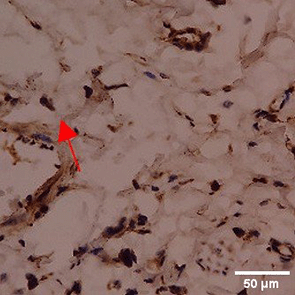
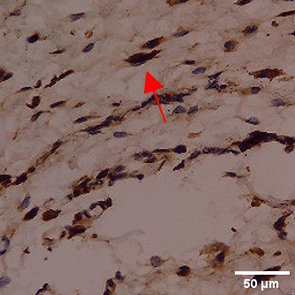
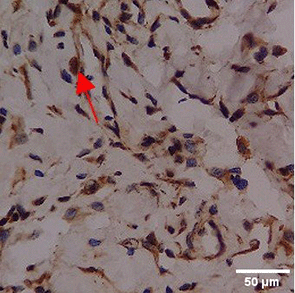
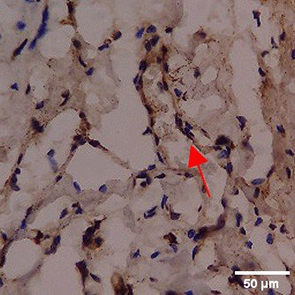
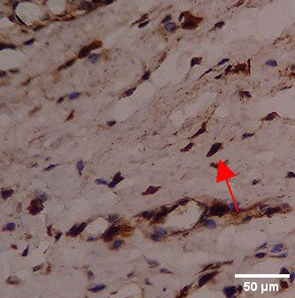
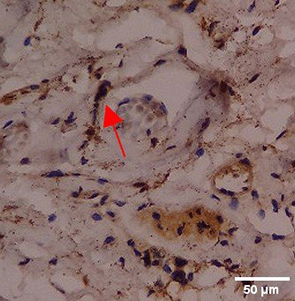
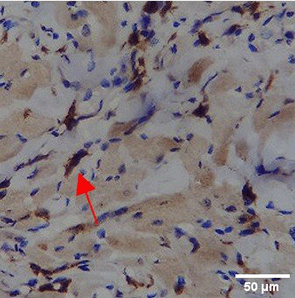
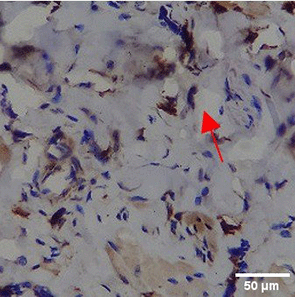
Immunohistochemistry of gingival tissue marker CD163 during the processes of homeostasis, inflammation, proliferation, remodelling, recovery
In the TPME group at D0, CD163 expression was similar to NC (Figure Table 10). However, there was a more rapid and significant increase compared to PC, especially at D3 and D5, and remained high until D7. This suggests that TPME accelerates macrophage polarisation to the M2 (Figure 4) phenotype and increases their activity in inflammation resolution and tissue repair. The sustained increase until D7 indicates a longer effect of TPME in promoting healing.
| Treatment | Day-0 | Day-1 | Day-3 | Day-5 | Day-7 |
|---|---|---|---|---|---|
| NC | 
| 
| 
| 
| 
|
| PC | 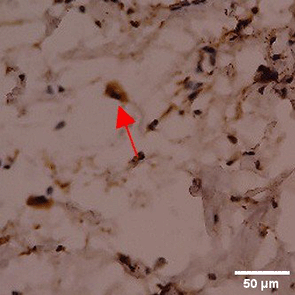
| 
| 
| 
| 
|
| TPME | 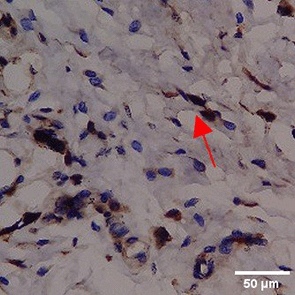
| 
| 
| 
| 
|
IL-1β and IL-6 gene expression towards TPME
Based on the results of the analysis of pro-inflammatory gene expression, specifically IL-1β and IL-6, observations were made through qRT-PCR ( Figures 5 and 6). On day 0 and day 1, no significant differences were observed in the reduction of IL-1β ( Figure 5 and Table 11) or IL-6 ( Figure 6 and Table 12) gene expression across all treatments. However, by day 3, all treatments began to show a decrease in IL-1β and IL-6 gene expression. On days 5 and 7, there was a more pronounced and statistically significant reduction in IL-1β and IL-6 gene expression (p<0.050), with TPME treatment showing the most significant decrease compared to other treatments. These findings suggest that TPME.
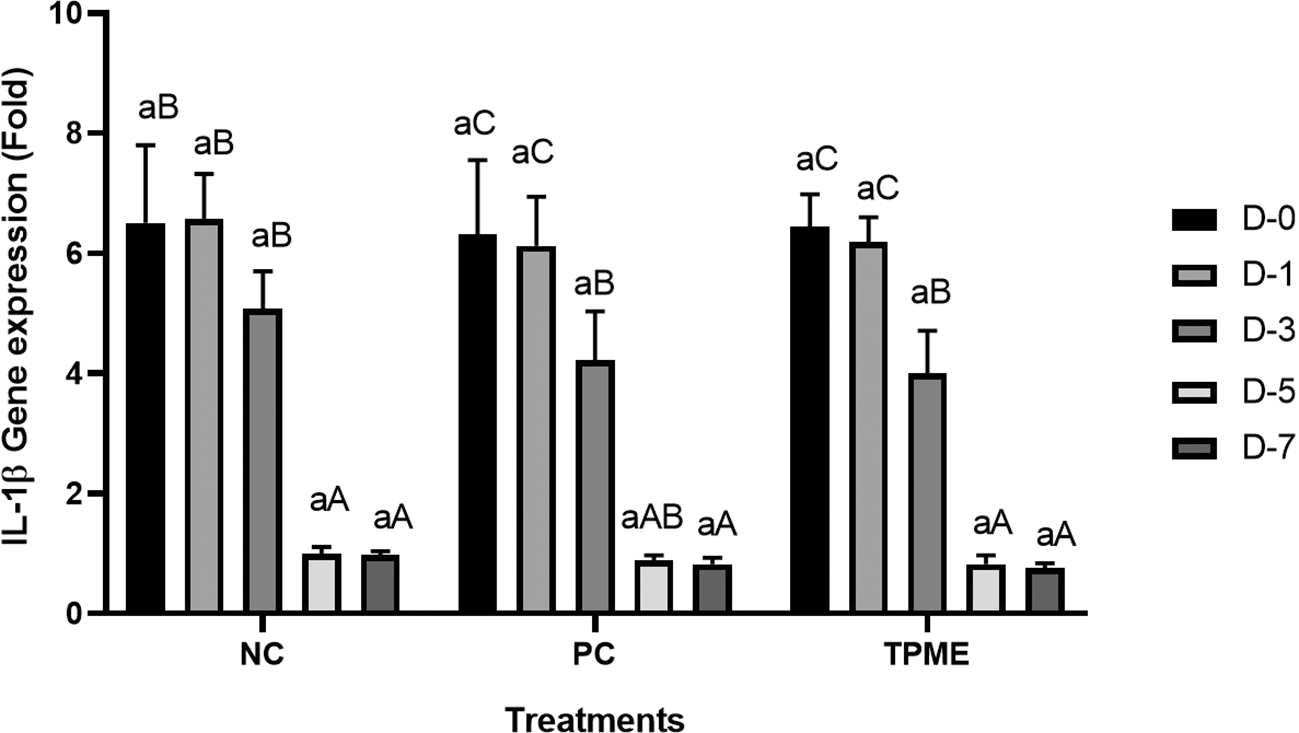
Negative control (NC), Positive control (NC), and toothpaste mixed extract group (TPME). Data presented as mean ± Standard Deviation with three repetitions. Small superscript letters (a) indicate significance between treatments on the same day and large superscript letter differences (A, AB, B, C) indicate significance between days of observation in one treatment group based on Tukey test (p<0.050).
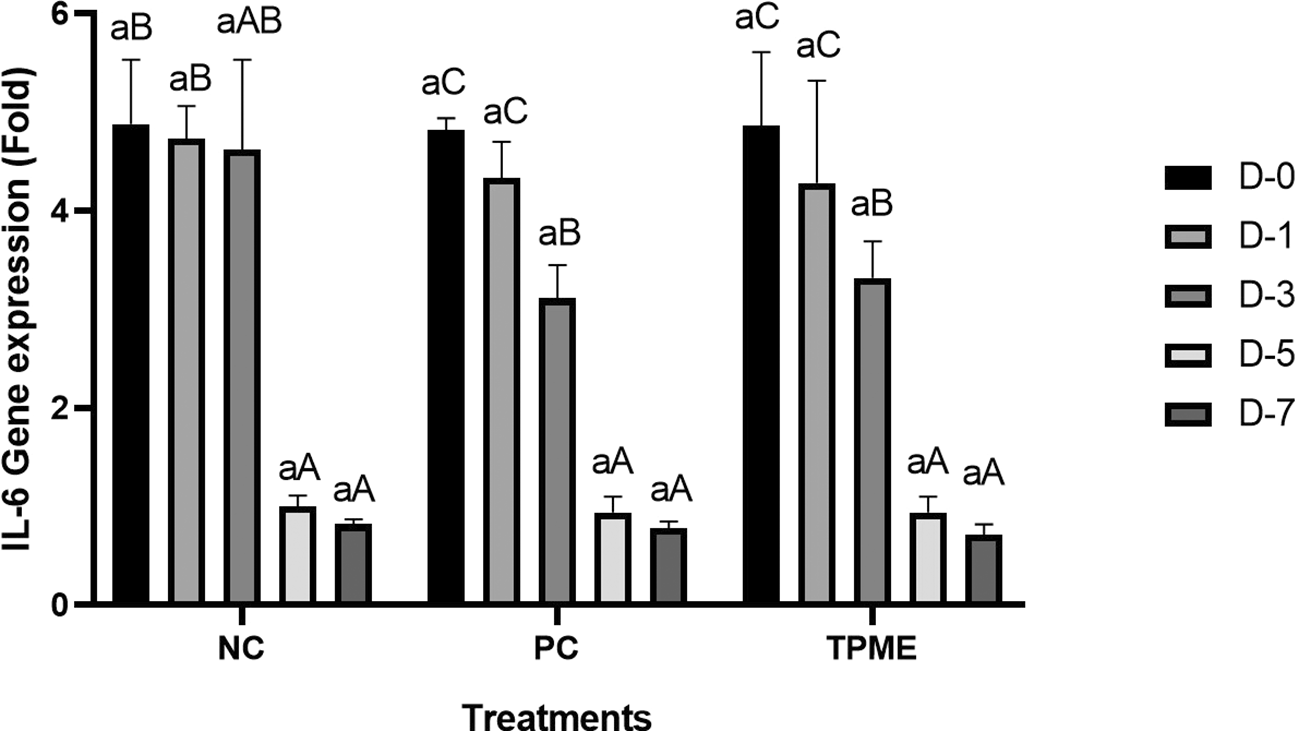
Negative control (NC), Positive control (NC), and toothpaste mixed extract group (TPME). Data presented as mean ± Standard Deviation with three repetitions. Small superscript letters (a) indicate significance between treatments on the same day and large superscript letter differences (A, AB, B, C) indicate significance between days of observation in one treatment group based on Tukey test (p<0.050).
Gingivitis is a common periodontal disease characterized by gingival inflammation caused by the accumulation of plaque containing pathogenic microorganisms. Oral hygiene practices, such as brushing and flossing, are important but sometimes insufficient to control microorganisms, making adjunctive therapies, such as herbal remedies, of interest.39,40 Herbal formulations, such as toothpastes and mouthwashes, provide antibacterial, anti-inflammatory, and antioxidant effects. Incorporating herbs into toothpastes has been shown to support gingival health. Herbal tooth gels offer gentle cleansing and therapeutic properties, and show comparable or better efficacy than conventional toothpastes in controlling plaque and gingival inflammation.40,41 The exploration of herbal toothpaste and mouthwash formulations as adjuncts in the management of gingivitis offers a promising avenue for improving oral health that promotes a more holistic approach to periodontal health.
The quality evaluation of toothpaste through organoleptic testing in this study was conducted considering it as an important evaluation component to ensure consumer satisfaction and product effectiveness ( Tables 1-6). Organoleptic evaluation involves sensory analysis based on appearance, color, aroma, taste, and texture, which are important attributes that influence consumer preference and acceptance.42,43 The importance of organoleptic testing can be seen from its role in herbal and conventional toothpaste formulations. Studies have shown that organoleptic properties, such as taste and aroma, strongly influence user experience and preference.44,45 The acceptance of the results related to the organoleptic test of this study is similar to the study conducted by Wardaniati & Indriastuty44 on herbal toothpaste, which shows that the distinctive aroma and taste of propolis and other herbal extracts are the main factors of consumer acceptance. In this case, the color and taste produced tended to be widely preferred by respondents ( Tables 1-6). Manhajan et al.46 also added that the results of this organoleptic test can be a relevant consideration, especially in the development of toothpaste formulations, where sensory attributes can guide the adjustment of concentrations and combinations of ingredients to optimize performance and consumer appeal.
The results of histopathological analysis of fibroblast numbers in TPME-treated gingivitis studies provided important insights into the cellular dynamics involved in tissue repair and regeneration. The increase in fibroblast numbers observed from day 0 (D-0) to day 5 (D-5) ( Figure 1) indicates a strong proliferative response, which is important for the synthesis of collagen and other extracellular matrix components essential for the healing of gingival tissues affected by inflammation. The slight decrease in fibroblast numbers observed at day 7 (D-7) indicates a stabilization phase after the initial proliferation, which is consistent with the normal tissue remodeling process.8,47 These results also highlight the efficacy of specific, the group treated with herbal toothpaste containing ginger extract (TPME) showed the highest proliferation of fibroblasts, especially at D-5 and D-7. This sustained increase in fibroblast numbers suggests that ginger extract may have unique bioactive properties that promote cell proliferation and support the healing process.48 The mechanism underlying this effect of ginger extract on fibroblast proliferation may be attributed to its anti-inflammatory and antioxidant properties. Ginger has been shown to inhibit pro-inflammatory cytokines and promote the expression of growth factors that are essential for fibroblast activation and collagen synthesis.49,50 This is in line with previous studies reporting similar findings in periodontal disease models, where herbal extracts showed significant improvements in tissue healing and regeneration.51,52
A significant increase in collagen density was also observed in Figure 2C, particularly from day 1 (D-1) to day 5 (D-5), followed by a slight increase on day 7 (D-7), indicating a normal and gradual process of tissue repair. This pattern is consistent with the physiological response of gingival tissue to inflammation and injury, in which collagen plays an important role in restoring structural integrity.53 The presence of the most significant collagen density at day 7 in the TPME group compared to the PC group may be attributed to the effect of certain active ingredients, such as thymoquinone in black cumin contained in the toothpaste mix extract, which promotes fibroblast activity and collagen synthesis.54 These findings suggest that TPME is highly effective in promoting collagen synthesis and deposition, which is essential for gingival tissue healing and regeneration. The increased collagen production in the TPME group may be related to the bioactive compounds contained in the extract combination (such as ginger, black cumin, royal jelly, and cinnamon), which have been shown to stimulate fibroblast proliferation and collagen synthesis.55 Salehinia et al.56 also reported that these herbal extracts have anti-inflammatory properties that can reduce the inflammatory response, thus creating a more conducive environment for fibroblast activity and collagen deposition.56
In addition, the ability of herbal extracts to increase collagen synthesis is in line with findings from another study by Ni et al.57 that demonstrated the effectiveness of various compounds from plants in promoting tissue repair. For example, ascorbic acid (vitamin C) is known to play an important role in collagen synthesis, and its presence in herbal formulations may further enhance the capacity of fibroblasts to produce collagen.57 In addition, Khairnar et al.58 opinion reinforces the finding that modulation of growth factors such as TGF-β by herbal extracts can significantly affect fibroblast behavior and collagen production, thereby contributing to better healing outcomes.58
The increase in collagen and fibroblasts will influence other characteristic properties of the gingival tissue such as blood vessel formation, PMNs, and monocytes. Treatment groups indicated an initial acute inflammatory response to toothpaste application, which was particularly evident on Day 1. This initial inflammatory response is an important component of the body's defense mechanisms, facilitating the recruitment of immune cells to sites of irritation or injury.59 This observation hints that although TPME initiates the inflammatory response, this formulation may also have properties that modulate the intensity of this response, potentially creating a more favorable healing environment.25
As the study progressed to Day 3, differences in inflammatory responses between groups became more apparent. The PC group showed a peak in monocyte counts ( Figure 2A), indicating a sustained inflammatory response, which can be detrimental if prolonged, as chronic inflammation is associated with tissue damage and delayed healing.60 In contrast, the TPME group showed a significant decrease in both PMNs and monocytes ( Figures 2A and 2B), indicating effective modulation of the inflammatory response. These findings are in line with the studies of Coluccia et al.61 and Vajrabhaya et al.62 which showed that certain herbal extracts can promote the resolution of inflammation by enhancing the clearance of inflammatory cells and facilitating tissue repair. The peak angiogenesis (Figure 2C) observed in the TPME group on Day 3 further supports the idea that this toothpaste formulation not only modulates inflammation, but also promotes the formation of new blood vessels, which are critical for providing nutrients and oxygen to the healing tissue.63 The ability of TPME to stimulate angiogenesis earlier than the other groups may contribute to a faster healing process, as sufficient blood supply is essential for tissue regeneration.59
Immunohistochemical observations regarding CD163 and CD86 markers in the gingivitis study provide valuable insights into the inflammatory response and macrophage polarization associated with herbal toothpaste application. all treatments at day 0 showed no significant differences in CD163 or CD86 expression, indicating a lack of modulation in the inflammatory response. This is consistent with previous studies showing minimal changes in macrophage markers in untreated inflammatory conditions.64 Along with the observations attached in Figure 4, the most striking changes were observed in the TPME (toothpaste mix extracts) treatment group on day 7, which showed a significant decrease in CD86 and an increase in CD163 expression (p<0.050). Activated M1 macrophages can express CD86, and produce high levels of ROS and proinflammatory cytokines, such as interleukin-1 and interleukin-6 (IL-1, IL-6), TNF- α, and IFN-γ.65 The positive thing is, on the 5th and 7th days of the study it was found that the average results of M1 macrophages (CD86) tended to be low, this is in accordance with the theory of wound healing that in the late proliferation phase and early remodeling phase there will be a transition of the pro-inflammatory M1 phenotype to anti-inflammatory M2 which will then secrete anti-inflammatory cytokines and growth factors that play a role in the process of tissue repair.66,65 While the average number of M2 macrophages (CD163) in the polyherbal toothpaste treatment group showed a high average number per area compared to the NC group, so it can be seen that the anti-inflammatory content of polyherbal toothpaste is effective in increasing the expression of anti-inflammatory M2 (CD163) macrophages. This finding is in line with previous studies showing that certain herbal extracts can increase macrophage polarization towards a stronger pro-inflammatory state, thus facilitating inflammation resolution.67 The decrease in CD86 and CD163, which are markers typically associated with anti-inflammatory macrophages, suggests that TPME may be effective in modulating macrophage responses, promoting a more favorable environment for tissue healing.68 Furthermore, the ability of TPME to significantly alter the expression of these markers highlights its potential as a therapeutic agent in managing gingival inflammation. Previous studies have shown that effective modulation of macrophage activity is essential to promote tissue repair and regeneration, as macrophages play a dual role in initiating inflammation and facilitating healing.69
The expression analysis of pro-inflammatory genes, particularly IL-1β and IL-6, was observed in this study to determine the inflammatory response associated with herbal toothpaste application. On days 0 and 1, no significant differences in IL-1β (Table 11) or IL-6 (Table 12) gene expression were found between treatments ( Figures 5 and 6), indicating that the initial application of the toothpaste did not induce an immediate inflammatory response. This finding is consistent with previous studies showing a delay in cytokine expression after the introduction of inflammatory stimuli.70 However, by day 3, all treatments began to show a decrease in IL-1β and IL-6 gene expression, indicating that the inflammatory response was modulated over time. The significant decrease in IL-1β and IL-6 in the TPME group compared to the other treatments suggests that TPME containing various herbal extracts has unique anti-inflammatory properties that facilitate resolution of inflammation, which is an important aspect of tissue healing.71 Furthermore, these results suggest that TPME treatment not only reduces the expression of pro-inflammatory cytokines, but may also improve the overall healing process in gingival tissues. This is supported by research showing that modulation of IL-1β and IL-6 is critical for tissue repair and regeneration, as these cytokines play an important role in mediating the inflammatory response and influencing fibroblast activity.72
Initially, the presence of PMNs and monocytes indicates an acute inflammatory response, which is important for fighting pathogens and initiating tissue repair. The increase in these inflammatory cells after TPME application suggests that this toothpaste effectively stimulates the immune response, possibly through the action of bioactive compounds present in the plant extract.73 As observed in previous studies, the recruitment of PMNs and monocytes is essential for the secretion of pro-inflammatory cytokines, such as IL-1β and IL-6, which are known to play a significant role in mediating inflammation and stimulating fibroblast activity.74
The expression of CD86, a marker associated with M1 macrophage activation, and CD163, associated with M2 macrophage polarization, further illustrates the balance between pro-inflammatory and anti-inflammatory responses. In the context of TPME treatment, the decrease in CD163 and increase in CD86 on day 7 indicate a shift toward a pro-inflammatory phenotype, which is essential for effective pathogen clearance and tissue remodeling.75 This modulation of macrophage polarization is critical, as M1 macrophages are known to secrete inflammatory cytokines that promote recruitment of additional immune cells, while M2 macrophages are involved in tissue repair and inflammation resolution.76 The significant reduction in IL-1β and IL-6 gene expression observed in the TPME group suggests that the medicinal plant extract may have anti-inflammatory properties that help downregulate these pro-inflammatory cytokines, thereby facilitating inflammation resolution and supporting healing.77 These findings are consistent with other studies demonstrating the ability of certain herbal compounds to inhibit the expression of inflammatory cytokines in various cell types, including gingival fibroblasts.78 Further visualization of the purposed mechanisms causing gingivitis from various gene interactions and pro-inflammatory markers and herbal blend toothpaste as a solution is in Figure 7.
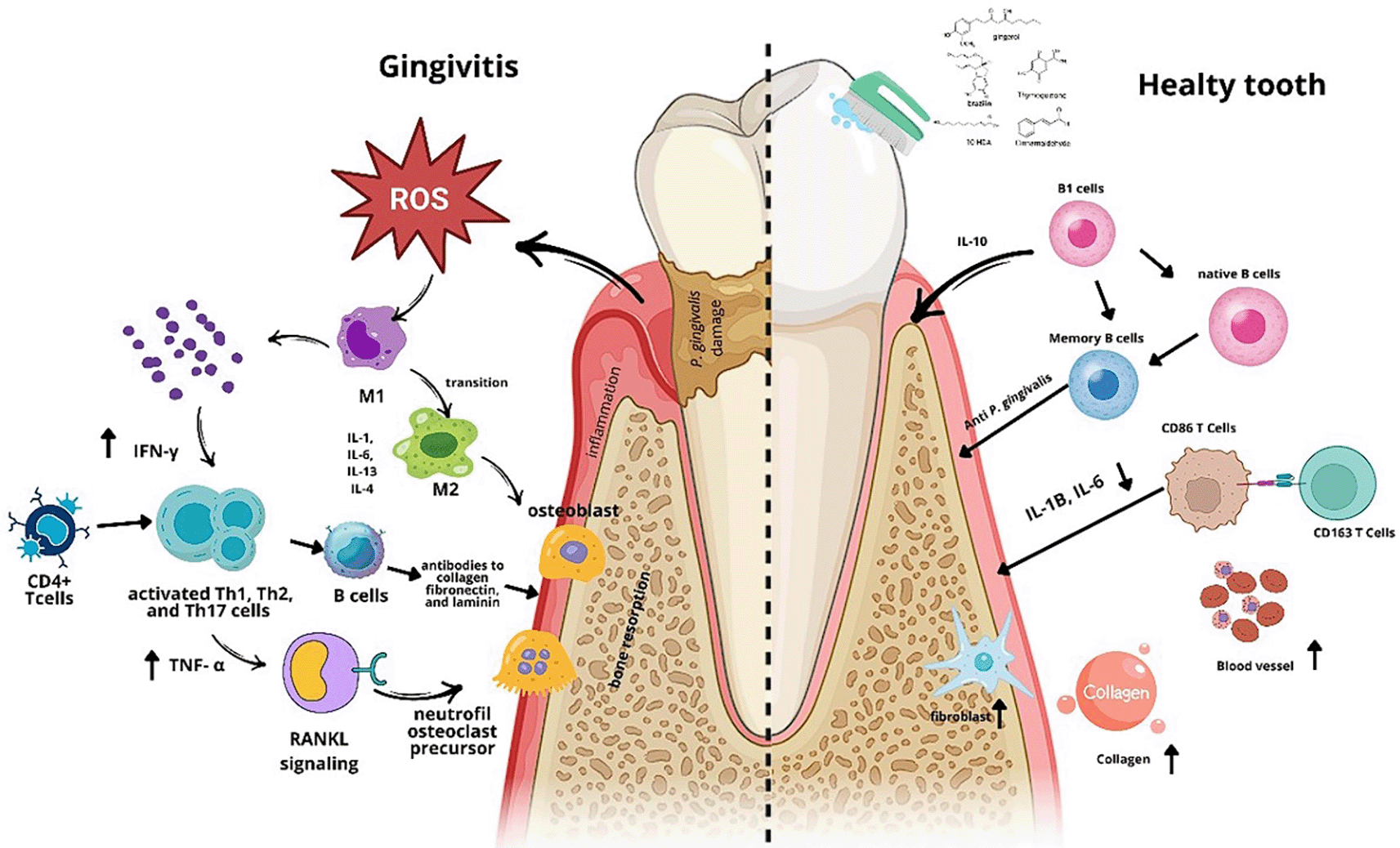
The mechanism of action of TPME in addressing gingival inflammation involves anti-inflammatory effects, immune modulation, and tissue repair. TPME reduces the levels of pro-inflammatory cytokines IL-1β and IL-6, decreases the number of PMNs and monocytes, indicating inflammation resolution and immune response modulation. Additionally, TPME lowers antibody levels, which could potentially cause tissue damage. On the other hand, TPME enhances fibroblast proliferation and collagen production, supporting tissue repair and healing. This combination of effects makes TPME a potential agent for managing gingival inflammation and accelerating periodontal tissue regeneration.
In addition, the increase in fibroblast number and collagen density in response to TPME treatment suggests that modulation of inflammatory cytokines is essential for tissue regeneration. Fibroblasts are responsible for the synthesis of collagen and extracellular matrix components, and their proliferation is often stimulated by pro-inflammatory cytokines such as IL-1β and IL-6.79 However, excessive levels of these cytokines can lead to tissue damage, highlighting the importance of maintaining a balanced inflammatory response for optimal healing.80
Overall, the interactions between fibroblasts, collagen, inflammatory cell counts, macrophage markers, and cytokine expression associated with TPME treatment of gingivitis reflect a complex network of immune responses. The ability of TPME to modulate these parameters suggests its potential as an effective therapeutic agent to manage gingival inflammation and promote tissue repair, highlighting the benefits of herbal extracts in periodontal health. Although this study provides significant insight into the effects of herbal toothpaste mix extracts (TPME), there is a need for further validation through human studies or animal models that more closely resemble human conditions.
The application of TPME twice a day is proven to be able to treat gingivitis which is reflected in an increase in the number of fibroblasts, collagen and angiogenesis, CD163 markers by suppressing the number of PMNs, monocytes, CD86 markers, proinflammatory gene expression IL-1β, and IL-6. So that TPME can be an agent for natural-based toothpaste products that are able to treat gingivitis by brushing your teeth.
The Ethics Commission of the Faculty of Medicine at Maranatha Christian University approved the study procedure through the issuance of ethical clearance number No. 007/KEP//11/2024.
Zenodo: ‘ARRIVE checklist’ for ‘The effect of toothpaste mix extract on angiogenesis and inflammatory cell response in a Wistar rat gingivitis model: Evaluation of IL-1β, IL-6 gene expression, and immunohistochemical markers CD86 and CD163’. https://doi.org/10.5281/zenodo.15062360.81
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This study has received ethical approval from the Health Research Ethics Committee of the Faculty of Medicine, Maranatha Christian University with number 007/KEP/H/2024. The use of experimental animals in this study takes into account three main points of interest in the use of animals, namely (Replacment), the determination of restrictions on the number of animals used (Reduction), and the treatment of test animals that correctly or ethically fulfill the concept of experimental animals that avoid pain (Refinement). In accordance with ethical guidelines, all efforts were made to minimize animal suffering during the course of this experiment. The study was conducted with the approval of the relevant animal ethics committee, and all procedures were carried out under the supervision of qualified personnel. Efforts to ameliorate suffering included the use of appropriate anesthesia and analgesia, as well as close monitoring of animal well-being throughout the study. Additionally, humane endpoints were established, and animals were observed for signs of distress or discomfort, with timely interventions taken when necessary.
This study has received ethical approval from the Health Research Ethics Committee of the Faculty of Medicine, Maranatha Christian University with number 007/KEP/H/2024. The use of experimental animals in this study takes into account three main points of interest in the use of animals, namely (Replacment), the determination of restrictions on the number of animals used (Reduction), and the treatment of test animals that correctly or ethically fulfill the concept of experimental animals that avoid pain (Refinement).
Zenodo: Raw Data for: The Effect of Toothpaste Mix Extract on Angiogenesis and Inflammatory Cell Response in a Wistar Rat Gingivitis Model [Data set]. Zenodo. https://doi.org/10.5281/zenodo.15062159.82
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The data that support the findings of this study are available upon reasonable request from the corresponding author.
The authors would like to thank Universitas Jenderal Achmad Yani and Maranatha Christian University for providing laboratory facilities. Thanks also to Aretha Medika Utama for their invaluable help and support during this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular pathogenesis and diagnostics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology, Nanomedicine, cancer therapy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Apr 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)