Keywords
KIBRA, WWC1, DDR1-WWC1 complex, DDR1-PRKCZ-WWC1 complex, proteomics, protein complexes, human cognition
This article is included in the Genomics and Genetics gateway.
The WWC1 (KIBRA) gene has been repeatedly implicated in cognitive performance, with carriers of the favorable allele showing enhanced episodic memory, spatial ability, and achievements in chess and STEM fields. However, gene-level associations do not reveal the underlying biological mechanisms. Here, we applied a modern proteomic assay coupled with an AI-based protein complex quantification method to elucidate the protein interaction phenotype involving WWC1 in a Scottish cohort (Viking Genes; N = 200). Although the bulk plasma level of WWC1 was not significantly associated with cognitive g factor, several WWC1-containing protein complexes, including the DDR1-WWC1 and the DDR1-PRKCZ-WWC1 complex scores, demonstrated significant associations with cognitive performance. In particular, one standard deviation increase in the normalized DDR1-WWC1 complex score corresponded to about 14% decrease in cognitive g factor (P = 0.024), even with educational attainment adjusted. These findings validate previous genetic studies and highlight the importance of protein-protein interactions in mediating the cognitive effects of WWC1.
KIBRA, WWC1, DDR1-WWC1 complex, DDR1-PRKCZ-WWC1 complex, proteomics, protein complexes, human cognition
Cognitive abilities such as working memory and spatial learning are fundamental to high-level intellectual pursuits, including chess performance and scientific innovation. The WWC1 gene, also known as KIBRA, has attracted considerable attention after its association with enhanced episodic memory and working memory was reported in previous genome-wide association studies (Ahmetov et al., 2023; Papassotiropoulos et al., 2006). Carriers of the T allele at the rs17070145 polymorphism show superior performance in cognitive tasks, a finding that has been replicated across diverse populations. These cognitive advantages are thought to contribute to the remarkable achievements observed in elite chess players and scientists (Ahmetov et al., 2023).
While the genetic association of WWC1 with cognition is well established, the biological mechanisms underlying these effects remain incompletely understood. WWC1 is a cytosolic scaffold protein that participates in the regulation of the Hippo signaling pathway through interactions with key partners such as LATS1/2 kinases, AMOT proteins, and atypical protein kinase C (aPKC) (Höffken et al., 2021). Notably, the Uniprot database indicates that DDR1 is a homodimeric subunit capable of interacting with WWC1 via a PPxY motif and that DDR1 can form tripartite complexes with WWC1 and PRKCZ, particularly under collagen-regulated conditions (The UniProt Consortium, 2024). WWC1 (KIBRA) was also reported to interact with DDR1 to modulate collagen-induced signaling in breast tissue (Hilton et al., 2008), which is consistent with the formation of a complex. In addition, DDR1 engages in interactions with several other proteins (e.g., SHC1, SRC, MYH9, CDH1, PTPN11, NCK2), highlighting its role as a nexus in signaling pathways that affect cell adhesion, migration, and potentially neural plasticity (The UniProt Consortium, 2024).
Proteins rarely act in isolation; they form complexes defining specific cellular functions. Therefore, characterizing the interaction partners of WWC1 can provide mechanistic insight into how genetic variations in the WWC1 gene might influence cognition. To address this, we combined high-throughput proteomics with an AI-based complex scoring algorithm to quantify protein complexes in plasma samples from the VIKING I cohort, part of Viking Genes. This cohort, derived from a geographically isolated population in the Shetland Isles, offers a unique genetic landscape to study subtle molecular phenotypes. Our study thus aims to bridge the gap between genetic associations and protein interaction networks, thereby validating the contribution of WWC1-involved complexes to human cognitive function.
The Viking Health Study – Shetland (VIKING I) is a geographically defined cohort with participants whose grandparents originate from the Shetland Isles, north of Scotland (Gilbert et al., 2019). Recruitment of 2105 volunteers was conducted between 2013 and 2015. This study selected a subsample of 200 participants, ensuring all four grandparents were from the Shetland Isles and had minimal kinship (max 6% genomic relatedness). All participants gave informed consent, and the study was approved by the Southeast Scotland Research Ethics Committee, NHS Lothian (Date: 23 Oct 2019; Reference: 12/SS/0151). The age range was 19–91 years (mean 52.6, s.e. 16.0) with females comprising 53.5% of the sub-cohort.
Fasting blood samples were collected in EDTA-treated tubes, processed, and frozen at -40°C before long-term storage at -70°C. Aliquots of 500 μl were shipped on dry ice to SomaLogic Inc. (Boulder, Colorado, USA) for analysis using the SomaScan assay version 4.1 (https://somalogic.com/). This assay uses 7,596 aptamers targeting 6,432 unique human proteins, with protein concentrations measured in relative fluorescent units spanning a dynamic range of 10 orders of magnitude (Kuliesius et al., 2024).
Quality control was performed by SomaLogic and further refined in-house. Hybridization controls, median signal normalization, and calibrator samples were used to address variability in aptamer binding. Additional flagging for non-human proteins or unspecific targets resulted in 595 aptamers being excluded. Outlier protein abundance measurements were removed if they fell outside three times the interquartile range, yielding effective sample sizes (median ) for downstream analyses.
The proteomic dataset included measurements for WWC1, DDR1, and PRKCZ. We applied Quantix™ BioSciences AB’s AI-based protein complex quantification algorithm COMFIDENT (COMplex FIngerprint DEconvolutioN Technology) on the SomaScan data to infer protein-protein interaction phenotypes (http://quantix.se/). Using the Reveal 2000 and Reveal 3000 panels of Quantix™ BioSciences, 3,471 protein complex scores were quantified. In particular, the scores for the DDR1-PRKCZ-WWC1, DDR1-WWC1, and PRKCZ-WWC1 complexes were normalized to zero mean and unit variance before subsequent analysis.
The inferred phenotypic scores for the three WWC1-containing complexes were statistically uncorrelated with the bulk WWC1 protein phenotype itself, with all absolute correlation coefficients less than 0.05 ( ). Except for the marginally significant correlation between age and DDR1-WWC1 complex ( , ), no significant association between WWC1 or the three protein complexes with age or sex was detected.
After adjusting for age and sex, bulk plasma WWC1 levels were not significantly associated with cognitive g factor ( ) ( Figure 1a). However, the WWC1-containing protein complexes were associated with cognitive g, with two complexes reaching significance ( ) and one showing marginal associations ( ) ( Figure 1b). Notably, even after adjusting for educational attainment, the DDR1-WWC1 complex remained significantly associated with cognitive g: One standard deviation (SD) change in the normalized DDR1-WWC1 complex score was associated with a 14% SD difference in cognitive g ( ) ( Figure 1c). No significant interaction effects were observed between complex scores and covariates such as age, sex, or educational attainment.
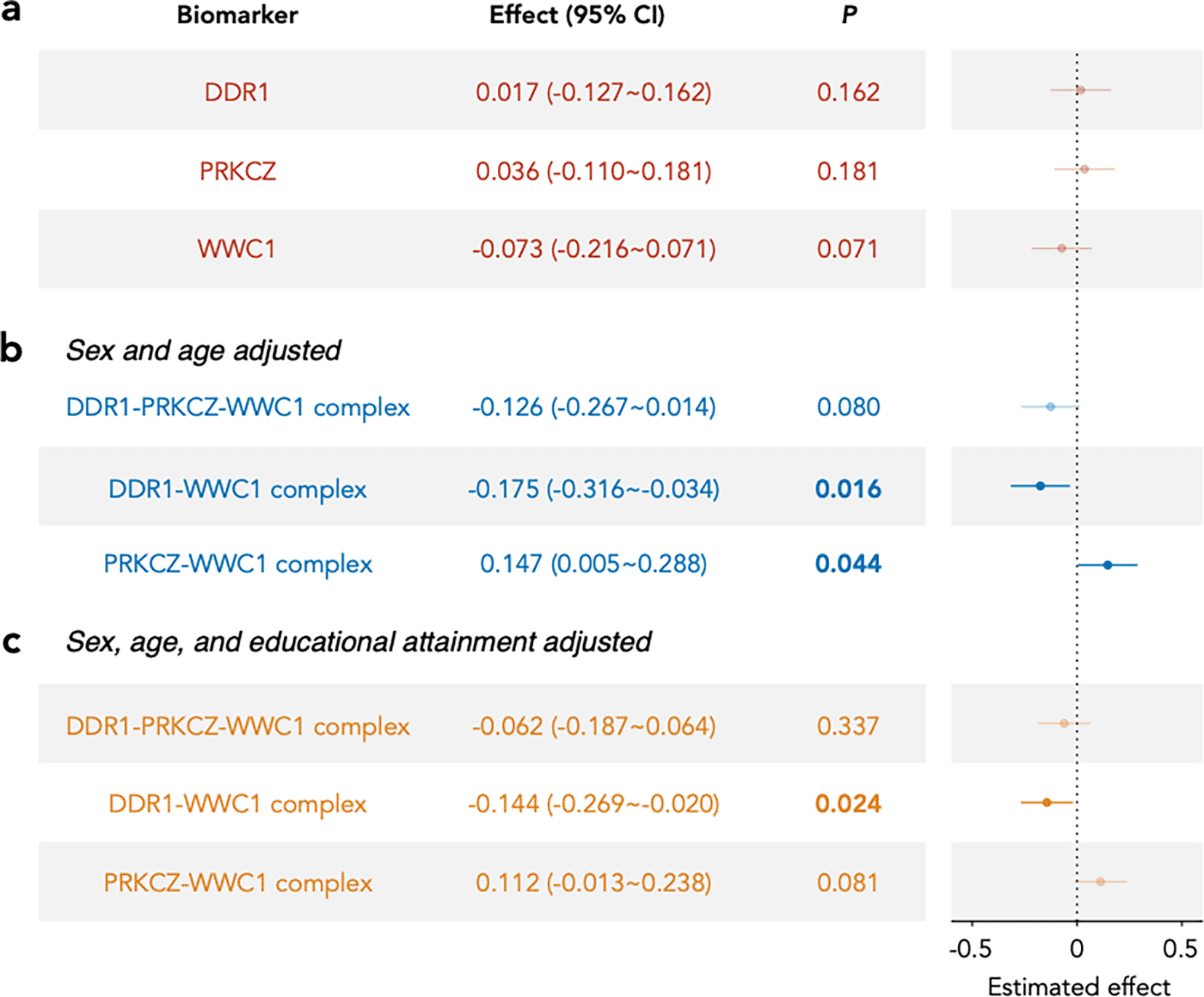
Each panel displays the estimated effect (with 95% confidence intervals) of either individual proteins or inferred protein complexes on the cognitive g factor in the VIKING I cohort, based on linear regression models. The vertical dashed line marks zero effect. The table at left shows the effect estimate (with 95% CI in parentheses) and the associated p-value for each biomarker. P-values less than 0.05 are highlighted in bold. a. Effects of the three assayed protein subunits, with age and sex adjusted as covariates. b. Effects of the three inferred protein complexes, with age and sex adjusted. c. Effects of the three inferred protein complexes, with age, sex, and educational attainment adjusted.
We set out to validate previous genetic findings implicating the WWC1 (KIBRA) gene in cognitive performance by exploring the proteomic architecture of WWC1-containing complexes. While earlier research has robustly linked the KIBRA T allele at rs17070145 with enhanced working memory, spatial ability, and achievements in chess and STEM (Ahmetov et al., 2023; Papassotiropoulos et al., 2006), our work extends these findings by demonstrating that it is not the total plasma level of WWC1, but rather its participation in specific protein complexes, that is associated with cognitive g factor.
These findings align with and extend the current understanding of WWC1 (KIBRA), a known regulator of the Hippo pathway implicated in both cell proliferation and neural plasticity. Indeed, common genetic variants in WWC1 have been strongly associated with human memory performance, suggesting a pivotal role for KIBRA in cognition (Papassotiropoulos et al., 2006). By demonstrating that WWC1 interacted with DDR1 and PRKCZ, we confirm and expand on prior research indicating that KIBRA operates within multiprotein networks to modulate cognitive pathways. In cellular models, KIBRA binds DDR1, and together with PKC (the product of PRKCZ), forms a complex that governs downstream signaling (Hilton et al., 2008). This convergence of our findings with earlier studies underscores the importance of WWC1’s protein-protein interactions in shaping brain-related phenotypes and corroborates existing literature on KIBRA’s role in cognitive function.
Importantly, our protein-protein interaction analyses uncover associations that might be missed by single-protein approaches. Neither DDR1 nor PRKCZ protein has emerged as a strong individual contributor to cognition in previous genomic studies, yet their interaction via the WWC1 protein significantly correlates with cognitive performance. This highlights the value of examining protein complexes or pathways rather than isolated genes when exploring the genetic architecture of complex traits. The DDR1-WWC1 complex, in particular, emerged as a significant predictor of cognitive performance even after controlling for sex, age, and educational attainment. The formation of a DDR1-PRKCZ-WWC1 tripartite complex has been suggested to occur predominantly in the absence of collagen, further supporting a dynamic regulation of WWC1 function (The UniProt Consortium, 2024). Our finding that the DDR1-WWC1 complex score was associated with cognitive g provided an important molecular link between previously observed genetic associations and protein-level interactions.
The significance of protein complexes in mediating cognitive traits is underscored by the fact that individual proteins often exert their function within larger multiprotein assemblies (Höffken et al., 2021). In our analysis, the AI-based algorithm enabled the quantification of thousands of protein complexes from high-throughput proteomic data, illustrating the power of combining modern proteomic techniques with machine learning to unravel complex biological networks.
Our results align with the established role of WWC1 in the regulation of the Hippo pathway, which has been implicated in both cell proliferation and neural plasticity (Höffken et al., 2021). Moreover, the finding that WWC1’s involvement in cognition is mediated by its specific protein-protein interactions resonates with that scaffolding proteins serve as crucial nodes for signal transduction and cellular regulation (Papassotiropoulos et al., 2006).
Several limitations of this study should be noted. The observational design does not confirm causality, and unmeasured confounding or population stratification may influence our findings. Although we attempted to mitigate such factors by adjusting for sex, age, and education, further research is needed to establish a causal link between the complexes and cognition. Additionally, the moderate sample size and the inherent challenges in translating plasma protein levels to central nervous system processes. Nevertheless, the distinct association of the DDR1-WWC1 complex with cognitive performance supports the utility of the protein complex as a complementary biomarker to genetic studies. Although our study provides promising initial evidence linking e.g., the DDR1-WWC1 protein complex with cognitive performance, another limitation is the lack of replication. Future studies in other Scottish cohorts, possibly with larger sample sizes and extended cognitive phenotyping, are planned to validate these associations.
In conclusion, this study not only validates the link between the WWC1 gene and cognitive ability but also highlights the importance of studying its interaction partners rather than merely the bulk protein levels. These provide insights for future research to explore the mechanistic pathways linking protein complexes to cognitive phenotypes and may contribute to the development of novel biomarkers for cognitive performance and related neurological conditions.
All participants gave informed consent, and the study was approved by the Southeast Scotland Research Ethics Committee, NHS Lothian (Date: 23 Oct 2019; Reference: 12/SS/0151). The authors confirm that all necessary patient/participant consent has been obtained in written forms, that the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients, or participants themselves) outside the research group so cannot be used to identify individuals.
The R software used for data analysis is available from: https://www.r-project.org/.
There is neither Research Ethics Committee approval, nor consent from individual participants, to permit open release of the individual-level research data underlying this study. The datasets generated and analyzed during the current study are therefore not publicly available. Instead, the research data and/or DNA samples are available from viking@ed.ac.uk, following approval by the Viking Genes Data Access Committee and in line with the consent given by participants. Each approved project is subject to a data or materials transfer agreement (D/MTA) or commercial contract.
DNA extractions and genotyping were performed at the Edinburgh Clinical Research Facility, University of Edinburgh. We would like to acknowledge the invaluable contributions of the research nurses in Shetland, the administrative team in Edinburgh, and the people of Shetland.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: LTP, memory
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 09 May 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)