Keywords
prevalence, association, IMIDs, IBDs, KAU, Saudi
Inflammatory bowel diseases (IBDs), which includes ulcerative colitis (UC) and Crohn's disease (CD), are chronic immune-mediated inflammatory disorders. While there is a clear link between IBDs and other immune-mediated inflammatory disorders (IMIDs), research into this relationship is limited, particularly in the Middle East. This study sought to determine the predictive factors and prevalence of IMIDs in IBDs patients at King Abdulaziz University Hospital in Saudi Arabia.
A single-center retrospective investigation was carried out on adult patients with confirmed IBDs. Between 2017 and 2022, the study was conducted at King Abdulaziz University Hospital in Jeddah, Saudi Arabia. Eligible patients over the age of 18 with verified IBDs diagnosis were found through the hospital's IBDs registry. Data on demographics, type of IBDs, disease duration, medications, extraintestinal manifestations (EIMs), and laboratory investigations were collected. The prevalence of IMIDs was examined, and multiple logistic regression analysis was done to discover predictors.
A total of 172 patients were randomly selected, with a mean age of 37.33 years; 55.8% were women. Among them, 50.6% had IMIDs. Of the patients with IBDs, 57% had CD and 43% had UC. Patients with IMIDs had a shorter IBDs duration (10.06 years) compared to those without IMIDs (12.23 years, p < 0.05). Male sex was a positive predictor for IMIDs (Odds Ratio [OR]: 2.57), while erythema nodosum (OR: 4.75) and pyoderma gangrenosum (OR: 7.44) were protective.
The study revealed a significant prevalence of IMIDs among patients with IBDs, highlighting the need to recognize predictors for improved clinical management.
prevalence, association, IMIDs, IBDs, KAU, Saudi
Inflammatory bowel disease (IBDs) refers to a set of chronic gastrointestinal inflammatory illnesses. The two most common types of IBD are ulcerative colitis ([UC]) and Crohn's disease ([CD]). CD can affect any part of the gastrointestinal system, but UC is limited to the large intestine (Centers for Disease Control and Prevention, 2023).
Immune-mediated inflammatory disorders ([IMIDs]) occur when the immune system fails to distinguish between normal and foreign cells, resulting in an attack on healthy cells and chronic inflammation, which is a common feature of IMIDs. There are about 80 different IMIDs that affect various body organs (David et al., 2018). Notable IMIDs include rheumatoid arthritis (RA), systemic lupus erythematous (SLE), thyroid disorders, diabetes mellitus (DM), and coeliac disease. IBDs Bar Yehuda et al. (2019) are thought to be caused by an abnormal immune response to gut microbiota in those with genetic predispositions. There is a large overlap between the genetic changes found in several IMIDs (Farh et al., 2015) and those with inflammatory bowel disease (Criswell et al., 2005). That may explain why patients with IBDs are more likely to acquire other IMIDs (Cotsapas et al., 2011; Vyse and Todd, 1996; Chun et al., 2017; Wilson et al., 2016; Halling et al., 2017). Disease classification at the time of diagnosis is critical since it offers important information about the disease's progression. These statistics are critical for providing best medical treatment to patients. The Montreal classification system is used to categories individuals with IBDs based on the severity of the ailment and if it is associated with the development of IMIDs. This classification assesses the extent of disease and severity of symptoms with predictive value (Monstad et al., 2014).
A 2015 study of 17428 patients with IBDs and 17428 non-IBDs cases found that patients with IBDs had a higher prevalence of IMIDs than non-IBDs patients (Wilson et al., 2016). Furthermore, a 2017 study of individuals with 20 distinct IMIDs discovered that they occurred more commonly in those with IBDs.10 Nonetheless, there have been few research examining the relationship between IBD and IMIDs among Middle Eastern and Persian Gulf populations.
Thus, this study aimed to estimate the prevalence of IMIDs in patients with IBDs in Saudi Arabia.
A single-center retrospective analysis was carried out on adult patients with confirmed IBD, regardless of age, country, or gender, who were followed up at King Abdulaziz University Hospital in Jeddah, Saudi Arabia, from 2017 to 2022. The King Abdulaziz University ([KAU]) Inflammatory Bowel Disease Information System ([IBDIS]) registry was used to determine eligible patients. All patients over the age of 18 with a confirmed diagnosis of IBD using standard clinical, endoscopic, and histologic criteria were considered eligible.
The sample size was calculated using a 95% CI and 80% power to detect a difference in the proportion of patients with IMIDs; if patients with IBD had a twofold increased risk of developing an IMID compared with the average risk of 10% in the general population, the minimum sample size of 102 patients was estimated using a sample size calculator algorithm. After estimating the sample size for this study, a random sample of eligible patients was obtained from the registry. A systematic random sample (interval sampling) methodology was used. In this procedure, the investigators pick individuals to be included in the sample based on a systematic rule, with the rule being to include the last patient from every ten patients (Ahmed SK, 2024).
All types of IMIDs searched within the registry using the International Classification of Diseases 10th Revision ([ICD-10]) coding are listed in extended data-Supplementary Table 1. Data collected using a predesigned data extraction sheet included patient demographics, type of IBD ([UC or CD]), duration of illness, medications, extraintestinal manifestations ([EIMs]) of IBD, complications, laboratory investigations including hemoglobin ([Hb]), white cell count ([WBC]), platelet count ([PLT]), C-reactive protein ([CRP]), albumin, aspartate aminotransferase ([AST]), alanine transaminase ([ALT]), total bilirubin, alkaline phosphatase [(ALP]), and outcomes ([for IMIDs]).
The Montreal classification was used to classify patients with UC as follows. Regarding disease course, S0 denotes clinical remission or being asymptomatic, S1 denotes mild UC involving the passage of four or fewer stools/day ([with or without blood]), absence of any systemic illness, and conventional inflammatory markers such as erythrocyte sedimentation rate ([ESR]), S2 denotes moderate UC involving the passage of more than four stools/day, but with minimal UC signs of systemic toxicity, S3 denotes moderate UC involving the passage of more than four stools/day but minimal UC signs of systemic toxicity, S4 denotes severe UC involving the passage of at least six bloody stools/day, a pulse rate of at least 90 beats/minute, a body temperature of at least 37.5°C, hemoglobin of less than 10.5 g/100 ml, and ESR of at least 30 mm/hour.
Regarding the extent of disease, E1 denotes ulcerative proctitis with involvement limited to the rectum ([proximal extent of inflammation is distal to the rectosigmoid junction]), E2 denotes left-sided UC ([distal UC]) reflecting involvement that is limited to the colon and rectum distal to the splenic flexure, and E3 denotes extensive UC ([pancolitis]) with involvement that extends proximal to the splenic flexure. Regarding age at diagnosis, A1 denotes < 16 years, A2 denotes 17 – 40 years, and A3 denotes > 40 years. For CD, the Montreal classification categorizes patients according to location ([L1 = ileal; L2 = colonic; L3 = ileocolonic; L4 = isolated upper disease]), behavior ([B1 = non-stricturing, non-penetrating, B2 = stricturing, B3 = penetrating]), and age at the time of diagnosis ([A1 < 16 years, A2 = 17–40 years, A3 > 40 years]).
This work received ethical permission from the Research Ethics Committee of King Abdulaziz University Hospital in Jeddah, Saudi Arabia [Ethical permission Number: 441-23].
Date of approval: Monday, October 2, 2023.
Data were analyzed using SPSS software version 26. The chi-square test was used for qualitative data expressed as numbers and percentages to assess the association between the variables. Quantitative data are presented as mean and standard deviation ([mean (SD)]), and the Mann-Whitney test was used for non-parametric variables. Multiple logistic regression analysis calculated odds ratios ([OR]) at a 95% confidence interval ([CI]). Statistical significance was defined as a P-value < .05.
Nine hundred and ten patients registered in the IBDIS database were screened for eligibility, and a random sample of 172 patients was selected for this study. The mean age of the patients was 37.33 ([11.03]) years; 55.8% were women, 72.1% had Saudi nationality, 44.2% were married, and 8.7% were smokers. The mean duration of IBD was 11.12 ([6.05]) years. Of the cohort, 57% had CD, and 43% had UC.
Among the patients with UC, 54% had an S2 Montreal classification for severity, and 36.6% had an E3 Montreal classification for the extent of UC. Among the patients with CD, only 5.5% were > 40 years of age at diagnosis, 25.6% had an ileocolonic location, and 32% had a non-stricturing, non-penetrating lesion ([ Table 1]). The mean values of Hg, WBCs, platelet, Albumin, AST, ALT, Bilirubin, and ALP are shown in Table 2. Of the patients with CD ([N = 98]), 64.2% had EIMs; the most common were arthralgia ([26.5%]) and erythema nodosum ([EN]) ([25.5%]). Regarding patients with UC ([N=74]), 63.5% had EIMs; arthralgia ([61.7%]) and EN ([36.1%]) were the most common. Half of the patients with CD had complications, such as osteoporosis ([42.8%]) and fistulas ([36.7%]). Of the patients with UC, 31% developed colon cancer and osteoporosis ([34.7%]).
| Variable | Total | Immune-mediated inflammatory disorders | χ2 | p-value | |
|---|---|---|---|---|---|
| No N (%) | Yes N (%) | ||||
| Mean age (years) | 37.33 ± 11.03 | 36.51 ± 16.55 | 38.14 ± 17.55 | 0.64* | 0.519 |
| Sex | |||||
| Female | 96 (55.8) | 56 (65.9) | 40 (46) | 6.9 | 0.009 |
| Male | 76 (44.2) | 29 (34.1) | 47 (54) | ||
| Nationality | |||||
| Non-Saudi | 48 (27.9) | 28 (32.9) | 20 (23) | 2.11 | 0.146 |
| Saudi | 124 (72.1) | 57 (67.1) | 67 (77) | ||
| Marital status | |||||
| Single | 96 (55.8) | 52 (61.2) | 44 (50.6) | 1.95 | 0.162 |
| Married | 76 (44.2) | 33 (38.8) | 43 (49.4) | ||
| Smoking status | |||||
| Non-smoker | 157 (91.3) | 81 (95.3) | 76 (87.4) | 3.4 | 0.065 |
| Smoker | 15 (8.7) | 4 (4.7) | 11 (12.6) | ||
| Variable | Total N (%) | Immune-mediated inflammatory disorders | χ2 | p-value | |
|---|---|---|---|---|---|
| No N (%) | Yes N (%) | ||||
| Mean disease duration (years) | 11.12 ± 6.05 | 12.23 ± 5.23 | 10.06 ± 6.6 | 3.18* | 0.001 |
| IBD type | |||||
| CD | 98 (57) | 51 (60) | 47 (54) | 0.62 | 0.429 |
| UC | 74 (43) | 34 (40) | 40 (46) | ||
| Ulcerative colitis | |||||
| Montreal classification Severity: (N = 74) | |||||
| S0 | 6 (8.1) | 2 (2.4) | 4 (4.6) | 1.24 | 0.871 |
| S1 | 12 (16.2) | 5 (5.9) | 7 (8) | ||
| S2 | 40 (54) | 19 (22.4) | 21 (24.1) | ||
| S3 | 16 (21.7) | 8 (9.4) | 8 (9.2) | ||
| Extent (UC): (N =74) | |||||
| E1 | 20 (27) | 11 (12.9) | 9 (10.3) | 3.3 | 0.507 |
| E2 | 26 (35.1) | 12 (14.1) | 14 (16.1) | ||
| E3 | 27 (36.6) | 10 (11.8) | 17 (19.5) | ||
| No data | 1 (1.3) | 1 (1.2) | 0 (0.0) | ||
| Age of diagnosis: | |||||
| A1 - below 16 y | 10 (13.5) | 7 (8.2) | 3 (3.4) | ||
| A2 - between 17-40 y | 41 (55.5) | 18 (21.2) | 23 (26.4) | 3.4 | 0.32 |
| A3 - above 40 y | 23 (31 | 9 (10.6 | 14 (16.1) | ||
| Crohn’s disease | |||||
| Age of diagnosis: | |||||
| A1 - <16 | 46 (26.7) | 29 (34.1) | 17 (19.5) | ||
| A2-17-40 | 46 (26.7) | 18 (21.2) | 28 (32.2) | 5.52 | 0.137 |
| A3 - > 40 | 9 (5.2) | 4 (4.7) | 5 (5.7) | ||
| Location: | |||||
| L1 - ileal | 28 (16.3) | 13 (15.3) | 15 (17.2) | ||
| L2 - colonic | 25 (14.5) | 18 (21.2) | 7 (8) | 5.97 | 0.113 |
| L3 - ileocolonic | 44 (25.6) | 20 (23.5) | 24 (27.6) | ||
| Behavior: | |||||
| B1 - non stricturing, non-penetrating | 55 (32) | 27 (31.8) | 28 (32.2) | 1.12 | 0.772 |
| B2 - stricturing | 19 (11) | 11 (12.9) | 8 (9.2) | ||
| B3 - penetrating | 24 (14) | 13 (15.3) | 11 (12.6) | ||
| Perianal diseases | |||||
| No | 72 (73.5) | 40 (47.1) | 32 (36.8) | 1.96 | 0.374 |
| Yes | 26 (26.5) | 11 (12.9) | 15 (17.2) | ||
| Laboratory analysis | |||||
| Hemoglobin | 11.74 ± 3.04 | 11.56 ± 3.42 | 11.92 ± 2.61 | 0.05* | 0.958 |
| WBCs | 7.69 ± 3.93 | 7.11 ± 3.77 | 8.29 ± 4.03 | 2.11* | 0.035 |
| Platelets | 317.71 ± 150.23 | 313.34 ± 163.61 | 322.23 135.83 | 0.82* | 0.41 |
| Albumin | 36.9 ± 10.66 | 34.83 ± 11.79 | 39.04 ± 8.91 | 2.64* | 0.008 |
| AST | 25.4 ± 23.52 | 22.88 ± 18.71 | 28.01± 27.51 | 0.42* | 0.671 |
| ALT | 27.82 ± 34.5 | 28.39 ± 39.48 | 27.22± 28.61 | 0.45* | 0.648 |
| Bilirubin | 16.69 ± 60.46 | 8.3 ± 6.7 | 25.29 ± 85.16 | 0.65* | 0.514 |
| ALP | 115.12 ± 114.29 | 98.73 ± 72.57 | 132.12 ± 144 | 1.13* | 0.357 |
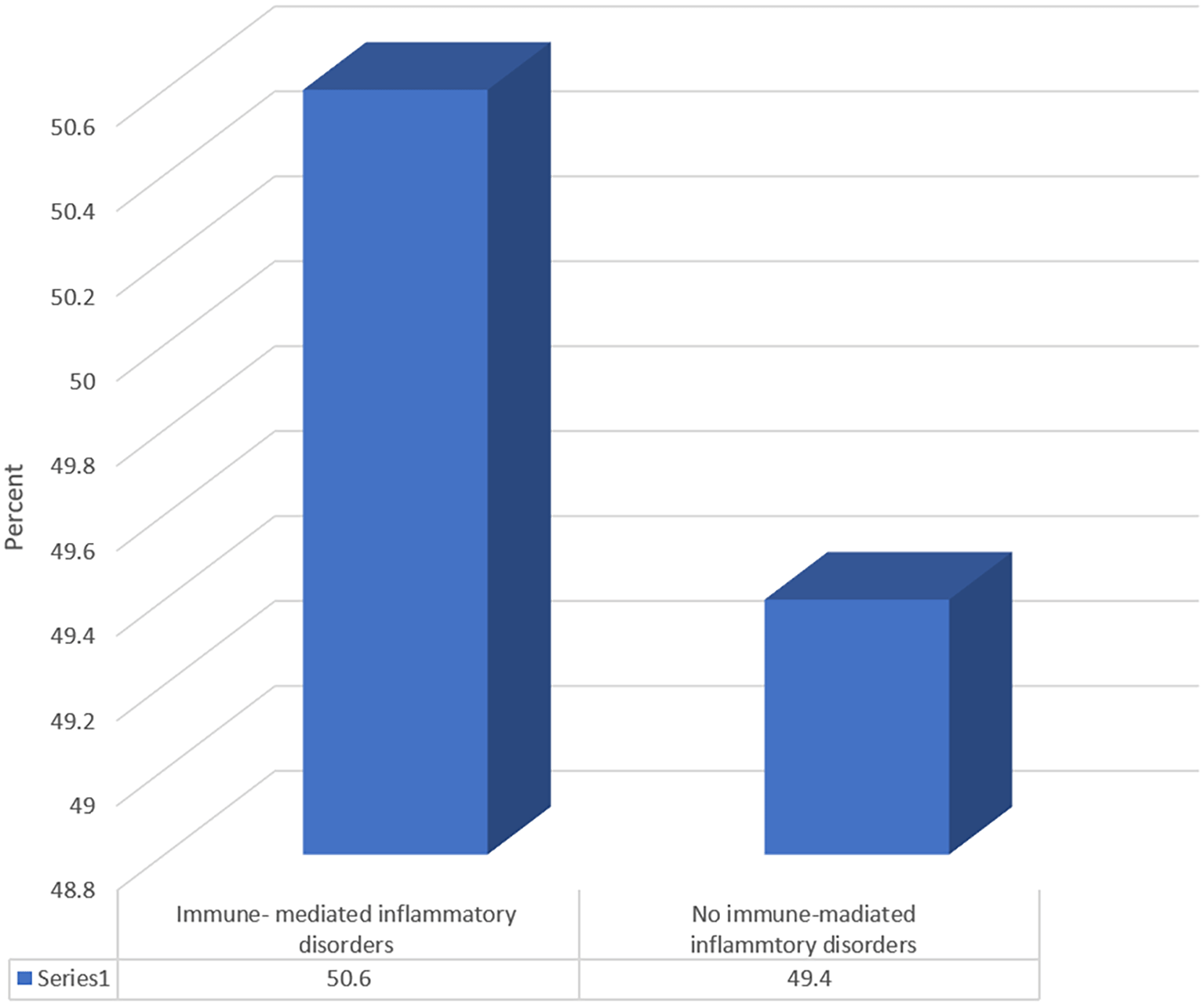
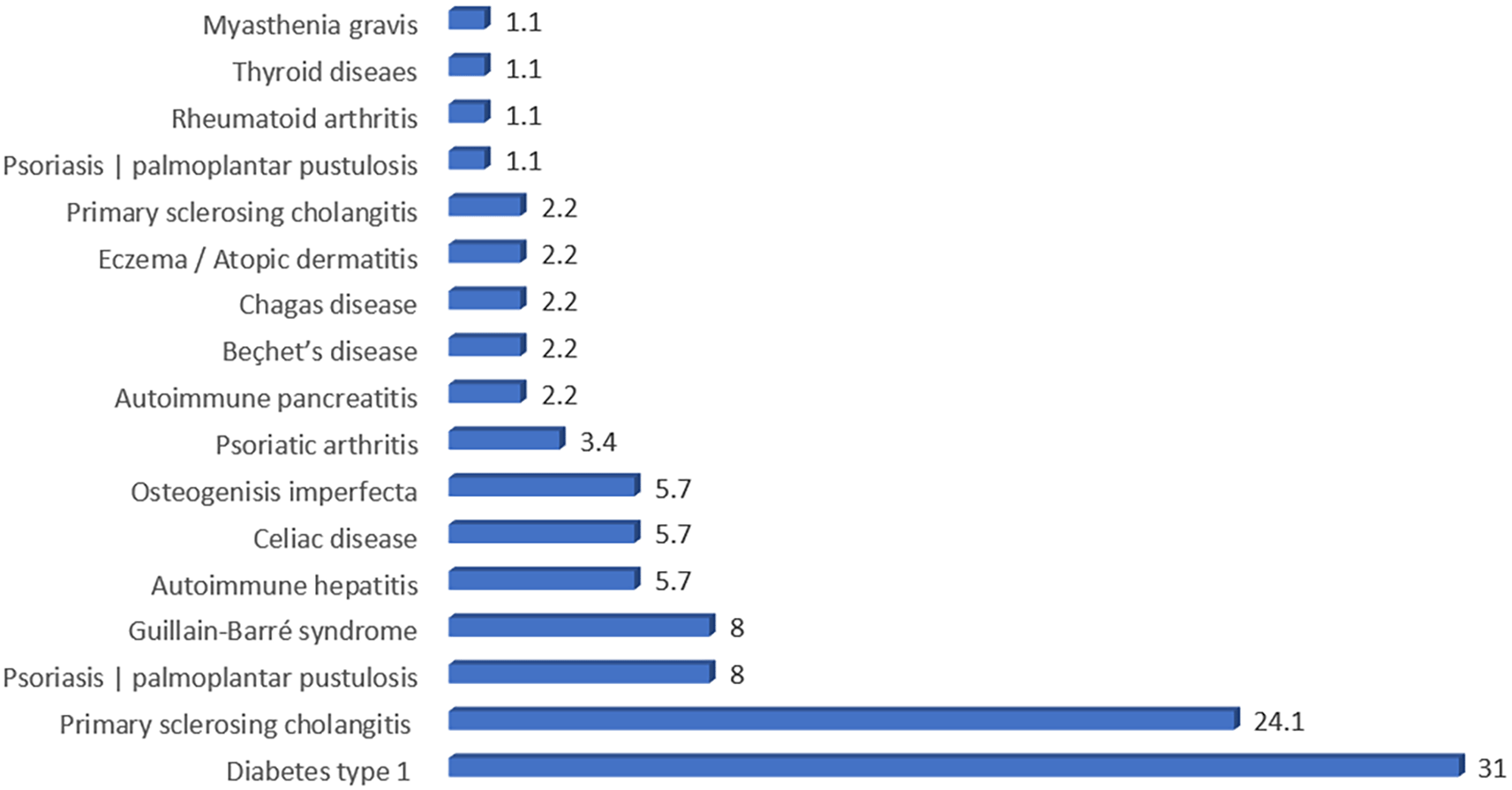
Of the total study cohort ([N = 172]), 87 ([50.6%]) patients had IMIDs other than IBD ([ Figure 1]). Among the patients with IMIDs, the most common disorders were DM type 1 ([31%]) and primary sclerosing cholangitis ([PSC]) ([24.1%]) ([ Figure 2]). The prevalence of IMID was significantly higher among male patients compared to female patients ([54% vs. 46%, respectively]); ([P < .05]) ([ Table 3]). The mean duration of disease was significantly shorter among patients with IMIDs ([10.06 (6.60) vs. 12.23 (5.23) years]) compared to those without IMIDs (P < .05). At the same time, IMID prevalence was significantly higher among patients with higher mean values of WBCs ([8.29 (4.03) vs. 7.11 (3.77)]) and serum albumin ([39.04 (8.91) vs. 34.83 (11.79)]) ([P < .05]). The IMID prevalence was significantly lower among patients with CD with EIMs ([episcleritis, EN, or pyoderma gangrenosum [PG]]) (24.1% vs. 49.4%) ([P < .05]). Among patients with UC, the IMID prevalence was significantly lower among those with EN or aphthous stomatitis (1.1% vs. 18.8%) ([P < .05]). As for IBD complications, the IMID prevalence was substantially lower among patients with CD having fistulas (5.7% 15.3%) ([P < .05]), while the IMID prevalence was significantly higher among patients with UC who developed colon cancer as a complication (8% vs. 1.2%) ([P < .05]) [ Table 3].
N.B.: Thyroid diseases = (Hashimoto’s thyroiditis /chronic lymphocytic thyroiditis/autoimmune thyroiditis).
The prevalence of IMID was significantly lower among patients treated with mesalazine (34.5% vs. 57.6%) ([P < .05]). In contrast, the IMID prevalence was substantially higher among patients who underwent surgery (34.5% vs. 25.9%)([P < .05]) ([ Table 4]).
According to multiple logistic regression analysis, male sex was a risk factor for IMID ([OR: 2.57, 95% CI = 1.08-6.08, P = .031]), while EN ([OR: 4.75, 95% CI = 1.28-6.01, P = .001]), or PG ([OR: 7.44, 95% CI = 1.46-17.95, P = .016]) as an EIM appeared to be significantly protective against developing any form of IMID ([ Table 5]).
IBD, including UC and CD, are chronic IMIDs that cause recurring inflammation of the gastrointestinal tract because of the immune system attacking healthy cells. Shared genetic changes may aid in the coexistence of numerous IMIDs. This study sought to investigate the relationship between IMIDs and IBD. Our findings revealed a substantial relationship between IBD and IMIDs. Specifically, we discovered that roughly half of the patients with IBD ([50.6%]) had multiple IMIDs in addition to IBD ( Figure 1). Concerning specific IMIDs, our retrospective investigation found that T1DM was the most common in our sample, accounting for 31% of cases ([ Figure 2]). This finding is consistent with a prior study that included 47,325 IBD patients and proposed the existence of two possible pathways linking IBD and T1DM. These pathways include potential shared susceptibility genes like PTPN2 and changes in gut microbiota composition (Halling et al., 2017; Bar Yehuda et al., 2019). Additionally, PSC was the second most common IMID found in our retrospective study, accounting for 24.1% ([ Figure 2]). The association between IBD and PSC is well established, with a previous systematic review and meta-analysis conducted in 2021 revealing that patients with UC are 1.7 times more likely to develop PSC than those with CD (Barberio et al., 2021).
These findings should encourage clinicians who manage IBD patients to maintain a high level of clinical awareness and alertness in the face of the possibility of PSC co-occurrence. Careful and regular screening for PSC in this patient population is essential, since early and precise diagnosis is vital for avoiding significant consequences. Interestingly, our current findings indicate a higher likelihood of IMIDs in CD patients. This data is consistent with a recent study conducted in Switzerland, which found a preponderance of IMIDs among CD patients (Wilson et al., 2019). These findings have significant implications for comprehensive management approaches, particularly for educating patients about the increased risk of developing additional IMIDs. Our study investigated the correlation between IMIDs and IBD severity using the Montreal classification. Contrary to previous research indicating heightened autoimmune disease severity in patients with multiple immune-mediated conditions (Wilson et al., 2019), we found no statistically significant association between severe forms of IBD and the development of IMIDs ( Table 2). However, it is crucial to acknowledge that our methodology for assessing IBD severity is relatively rudimentary, and further investigation is needed to validate these preliminary findings.
In our attempt to find predictors of IMID incidence among IBD patients, multiple logistic regression analysis found that male patients were more likely to have other IMIDs, as opposed to earlier research where female patients were more impacted by IMIDs (Gjymishka et al., 2013). However, this disparity may be due to the small sample size from a single center. We also looked at the relationship between IMIDs and laboratory indicators such CRP, ESR, and total leukocyte count ([ Table 2]). The findings indicated no significant associations between CRP levels ([P = .199]), ESR ([P = .321]), total leukocyte count ([P = .234]), and the presence of IMIDs in the study population.
However, it is essential to note that these markers represent only a subset of potential predictors, and further investigation is warranted to explore additional markers or combinations for a more comprehensive assessment of immune-mediated inflammation. While prior research has mostly focused on the increased likelihood of developing IMIDs because of an increasing number of IBD-related symptoms (Wilson et al., 2016), our study reveals a novel discovery on EIMs. We found that combining EN with UC and CD greatly reduced the formation of IMIDs ([P <.001]) ([ Table 3]). This study has significant clinical implications, implying that the existence of EIMs may indicate a more favorable disease course or a lower risk of extra immune-mediated problems in patients with UC and CD.
Further research is needed to clarify the underlying mechanisms and explore potential therapeutic implications. Patients with concomitant IMIDs may have a more aggressive form of IBD that may account for the shorter mean duration of IBD than those without IMIDs. Individuals diagnosed with IMID before IBD exhibit a more aggressive pattern of IBD, according to another study (Akiyama et al., 2022). According to García et al. (2020), the existence of an IMID before IBD is not a reliable predictor of surgery. As a result, the IMID diagnostic date may predict IBD. Before being identified with IBD, people with IMID may require more rigorous treatment and closer monitoring to prevent surgery and improve their quality of life (The 46th Annual Meeting of the European Society, 2020).
Our findings demonstrated substantial relationships between treatment methods and the occurrence of IMIDs in IBD patients. We found a substantial decrease in IMID prevalence among patients treated with mesalazine ([P < .005]), indicating a potential preventive effect against IMID formation. However, additional prospective studies with bigger sample sizes and a controlled study design are required to validate and expand on these findings.
The study's shortcomings include a small and potentially non-representative sample size and the retrospective nature of data collecting from electronic medical records. These issues may limit the findings' generalizability and induce bias due to poor recording, compromising their accuracy. Future research should use larger sample numbers and better data collection methods to improve dependability and develop more strong findings about the relationship between IBD and IMIDs. More research is needed to look into additional indicators or combinations for a more comprehensive assessment of immune-mediated inflammation, as well as to confirm the potential therapeutic applications of EIMs in patients with UC and CD.
This retrospective analysis demonstrates the increased risk of IBD patients having concomitant IMIDs. In our group, 50% of persons with IBD had concomitant IMIDs, with type 1 diabetes and PSC being the most frequent. Several variables have been associated to the onset of IMIDs, including male sex, shorter disease duration, specific laboratory results, and the occurrence of comorbidities. Patients treated with mesalazine, CD patients with EIMs, and UC patients with EN or aphthous stomatitis had considerably lower prevalences. These findings indicate protective factors and areas for further research into managing IMIDs in the setting of IBD.
This work received ethical permission from the Research Ethics Committee of King Abdulaziz University Hospital in Jeddah, Saudi Arabia [Ethical permission Number: 441-23].
Date of approval: Monday, October 2, 2023.
This retrospective study utilized data from patient records for individuals who had previously provided informed consent for the use of their health information in research. Consent was obtained in accordance with ethical guidelines, and the study was conducted in compliance with applicable regulations to ensure the confidentiality and privacy of the participants.
This is a retrospective study and consent was waived by ethical committee for this study.
Figshare: IBD DATA last.xlsx. Doi: https://doi.org/10.6084/m9.figshare.28184474 (Ruckn, Assil (2025a))
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: supplementary table. Doi: https://doi.org/10.6084/m9.figshare.28255574.v1 (Ruckn, Assil (2025b))
This project contains the following extended data:
Supplementary Table represent all types of IMIDs searched within the registry using the International Classification of Diseases 10th Revision ([ICD-10]) coding.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors gratefully acknowledge the cooperation of the administrative staff of the study setting.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Statistics, epidemiology, IBD
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: IBD
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 May 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)