Keywords
Diabetes , Cucurbita ,
This article is included in the Plant Science gateway.
Diabetes mellitus (DM) remains a major global health problem and particularly in low to middle income countries both because of the increasing prevalence and as a result of lack of access to affordable and effective treatment. Given the limitations of current pharmacological treatments, such as side effects and poor compliance, there is increasing interest in natural plant-derived alternatives. This systematic review explores the anti-diabetic potential of Cucurbita species, members of the Cucurbitaceae family, which have demonstrated promising hypoglycemic, antioxidant, and insulinotropic activities in preclinical models. Following PRISMA 2020 criteria, a comprehensive search of PubMed, Scopus, and Web of Science identified 16 eligible preclinical studies published between 2016 and 2025 that met strict inclusion criteria. Extracts from Cucurbita maxima, C. moschata, C. ficifolia, and C. pepo were shown to significantly reduce blood glucose concentrations, enhance antioxidant enzyme activity, promote pancreatic β-cell regeneration, and improve lipid profiles in diabetic animal models. These effects were predominantly observed following oral administration, reflecting traditional usage patterns and supporting clinical translatability. The geographically diverse research landscape underscores the global ethnobotanical relevance of Cucurbita species as complementary or alternative treatments for DM. However, the review also highlights gaps in the research, including the need for further pharmacokinetic, toxicological, and clinical studies to validate the efficacy and safety of these botanicals and to inform the development of standardized phytotherapeutic interventions. Overall, Cucurbita plants present a compelling case for further investigation as adjunct or alternative treatments for diabetes mellitus.
Diabetes , Cucurbita ,
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia that persists over time as a consequence of insufficient insulin secretion, improper insulin action, or a combination of both. As such, it accounts for several diseases, including cardiovascular disease, neuropathy, retinopathy, and nephropathy. The international burden of diabetes has been worsening with projections estimating approximately 830 million diabetes cases in 2022, with a disproportionate focus on low- and middle-income countries (World health organization, 2024). Diabetes can be classified as Type 1 diabetes, which is an autoimmune condition that results in the destruction of the pancreatic β-cells, and, Type 2 diabetes, which occurs after a person has developed insulin resistance and β-cell dysfunction (American Diabetes Association, 2023). Currently treatment remains focused on drugs, primarily metformin and insulin, but the adverse effects, expenditure, and adherence problems have increased the attention towards alternative drugs developed from natural resources (Mooradian et al., 2022).
Some of the natural plants investigated for their anti-diabetic properties include the members of the Cucurbitaceae family like, Cucurbita máxima, Cucurbita ficifolia, and Cucurbita moschata. These plants have bioactive compounds such as phenolics, flavonoids, polysaccharides, cucurbitacins which have hypoglycemic, antioxidant and anti-inflammatory activities (Suwannapong et al., 2023). For instance, some preclinical studies showed that extracts of Cucurbita ficifolia activated peroxisome proliferator-activated receptors (PPARs) that control lipid and glucose metabolism resulting in significant reduction of blood glucose levels streptozotocin induced diabetic mice (Rojas et al., 2021). In the same way, some extracts of Cucurbita maxima have been shown to regenerate pancreatic β-cells and augment insulin secretion in alloxan diabetic rats (Mujtaba et al., 2022). This is the reason why these plants should be investigated further for their potential use in diabetes management.
Alongside their hypoglycemic effects, members of the Cucurbita genus demonstrate the ability to counter oxidative stress, which is a diabetes complication, through strong antioxidant activity. For example, a study conducted on the extracts of Cucumis sativus and Cucurbita maxima found that these extracts were successful in reversing the diabetic rats’ liver and pancreas functionality and normalizing the oxidative stress parameters (Suwannapong et al., 2023). Moreover, cucurbitacins, a subclass of triterpenes belonging to the family of Cucurbitaceae plants, have been reported to possess insulin-like activity by increasing glucose utilization and the insulin sensitivity (Bhatti et al., 2021). These multifaceted effects suggest that Cucurbita plants could serve as valuable alternatives to conventional diabetes therapies.
This systematic review intends to scrutinize their mechanisms of action and evaluate therapeutic possibilities and translational avenues by analyzing abundant preclinical studies that verify the anti-diabetic activity of Cucurbita species. By bringing together the findings of recent studies, this review intends to clarify how these plants may be useful in the management of diabetes. Moreover, it points out the insufficiencies of research that need to be conducted to enable the use of these promising natural products in clinical settings.
The review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (Page et al., 2021; Swase et al., 2025). A systematic and comprehensive literature search was conducted on January 17, 2025, across the Web of Science (WoS), Scopus, and PubMed databases. The search strategy utilized the keywords “cucurbita” and “diabetes,” incorporating Boolean operators (AND/OR/NOT), alternative terms, and search modifiers such as quotation marks, parentheses, wildcards, and asterisks (*) (Fasogbon et al., 2023, 2024; Mitaki et al., 2024). The search was restricted to peer-reviewed English-language publications published between 2016 and January 17, 202.
Article selection adhered to the following inclusion and exclusion criteria: preclinical studies involving animal models such as rats, mice, and rabbits, as well as other non-human models demonstrating the role of Cucurbita in diabetes management or prevention. Only studies specifically addressing protective or therapeutic interventions for diabetes mellitus were included. To ensure scientific rigor, only peer-reviewed articles published in English were considered. Additionally, the review focused on studies involving at least one of the following Cucurbita species: C. argyrosperma, C. ficifolia, C. maxima, C. moschata, and C. pepo. The time frame was restricted to publications from 2016 to 2025 to ensure that the findings reflect current research trends. Furthermore, studies were required to report interventions and outcomes in measurable terms, such as biochemical parameters, treatment duration, and statistical significance (e.g., p-values). Conversely, studies were excluded if they did not focus on diabetes therapy or management, including those addressing diabetes insipidus or other unrelated conditions. Clinical trials involving human participants were also excluded. Non-English publications, as well as those investigating Cucurbita species outside the specified five, were not considered. Additionally, review articles, editorials, commentaries, conference abstracts, and book chapters were excluded, as were studies that lacked sufficient methodological details, did not report clear outcome measures, or failed to provide statistical validation of their findings.
The database search results were uploaded to the Rayyan online platform (Johnson & Phillips, 2018). Duplicate records were identified using Rayyan’s duplicate detection feature, followed by manual validation and removal. Angela Mumbua Musyoka and Nancy Bonarere Mitaki independently screened the records in two stages: title and abstract screening, followed by full-text review. Multiple publications originating from the same study were grouped, with the primary publication selected based on its relevance to the research question. Any discrepancies during the screening process were resolved through discussions between Angela Mumbua Musyoka and Nancy Bonareri Mitaki.
The data presented in Table 1 were extracted from each eligible study.
The Scopus search retrieved 88 articles, Web of Science identified 53, and PubMed yielded 29, resulting in a total of 170 articles across the three databases. The search results were screened on the Rayyan platform based on the predefined inclusion and exclusion criteria. During this process, 66 duplicate articles were identified and removed. The remaining 104 records underwent an initial title and abstract screening, leading to the exclusion of 78 articles. A subsequent full-text review eliminated 10 more articles for not meeting the inclusion criteria. Ultimately, 16 articles met the eligibility and quality assessment requirements and were included in the study ( Figure 1).
Results from this systematic review are presented in Tables 1,2 and Figures 1–6 below. Figure 1 illustrates the various plant parts of Cucurbita species that were utilized across the included studies for anti-diabetic research. Parts used include: Seeds, Fruit pulp, Leaves, Flowers, and Juice. This reflects the versatility of Cucurbita plants, indicating that multiple parts contain bioactive compounds with potential therapeutic effects against diabetes. Figure 3 shows the specific species of Cucurbita evaluated in the review, which include: Cucurbita maxima, Cucurbita moschata, Cucurbita pepo, and Cucurbita ficifolia. Cucurbita maxima and Cucurbita moschata were the most frequently studied species, indicating their prominence in traditional medicine and potential pharmacological interest.
| S/No | Plant | Extraction solvent | Phytochemical | Mechanism of action/key findings | Country | References |
|---|---|---|---|---|---|---|
| 1. | Cucurbita maxima | Aqueous | Not Done | Decrease blood sugar levels by stimulating insulin secretion from pancreatic beta cells. | India | Kushawaha et al., 2017 |
| 2. | Cucurbita ficifolia | Aqueous | NMR and GC-MS, p coumaric acid, p- hydroxybenzoic acid, salicin, stigmast-7,2,2-dien-3-ol and stigmast-7-en-3-ol | Exhibits antioxidant, anti-inflammatory and hepatoprotective properties. | Mexico | Garcia et al., 2017 |
| 3. | Cucurbita moschata durch | Seed powder suspended in sodium carboxymethyl cellulose (CMC) 0.5% | Flavonoids, Saponins, Tannins | Decrease blood sugar level by stimulating pancreatic secretion of insulin from existing β-cell. | Indonesia | Marbun & Rumanti, 2019 |
| 4. | Cucurbita maxima | Aqueous | Not Done | Decrease blood sugar level via release of insulin from pancreas β-cell. Decrease AST, ALT and total cholesterol level. | Iraq | Kadhum et al., 2020 |
| 5. | Cucurbita moschata Durch | Water and ethanol | Polysaccharide | Antioxidative properties characterized by increased GPx, SOD & CAT. Decreased MDA and blood sugar level reduction. | China | Yang et al., 2024 |
| 6. | Cucurbita moschata Duchesne & Cucurbita maxima Duchesne | Aqueous & ethanol | Polysaccharide, Total phenol, total flavonoids | pancreatic regeneration (via increased beta-cell number/size) to reduce blood glucose levels and antioxidant mechanisms (DPPH scavenging and ferric ion reduction) to counteract oxidative stress. | Thailand | Suwannapong et al., 2023 |
| 7. | Cucurbita maxima | Methanol | 3,5dihydroxyphenol, genkwanin, Zearalenone estrogenic metabolite, hexamethaylinoic acid, hydroquinine, L-histidin,3 methyl, alpinetin and La-arginine | Antioxidative properties characterized by increased GST, GSH and CAT activities, decreased MDA & NO. Decrease in the blood sugar level. Hypoglycemic effect via improvement of the immunohistochemical expression of insulin in b-cells of islets of Langerhans. | Egypt | Atta et al., 2020 |
| 8. | Cucurbita maxima | Aqueous & ethanol | Flavonoids, Fatty acids phenolic acids | Anti-diabetic effect through reduction blood glucose levels and restore pancreatic β-cells. Reduce the endogenous synthesis of glucose by the liver. | Kenya | Njoki et al., 2024 |
| 9. | Cucurbita pepo | Fruit juice | Not done | Anti-diabetic effect via reduction in blood glucose. | Iran | Sayahi & Shirali, 2018 |
| 10. | Cucurbita maxima | Seed powder | Total Phenolic Total flavonoids, Fatty acids (oleic acid, linoleic acids) | Hypoglycemic, hypolipidemic, and liver protective effects by reducing blood glucose, cholesterol, triglycerides, LDL, and liver enzymes (ALT, AST). | Egypt | Farid et al., 2015 |
| 11. | Cucurbita moschata | Not specific | Carbohydrates, proteins, minerals | Reduced fasting blood glucose level, increased hepatic glycogen, enhance the islet cells proliferation activity. | China | Wang et al., 2017 |
| 12. | Cucurbita maxima | Aqueous | Phenol, Flavonoids, Anthocyanins | Improvement of Lipid Profile: Decreases total cholesterol (TC), LDL, VLDL, and triglycerides (TG). Liver Enzyme Reduction: (ALT), (ALP), (AST) Antioxidant properties: Increases serum insulin, HDL, Superoxide dismutase (SOD), Catalase (CAT), and reduced glutathione (GSH). | Saudi Arabia | Elsadek et al., 2024 |
| 13. | Cucurbita pepo | Aqueous | Polysaccharide | Anti-diabetic activity by decreasing blood glucose level, inhibiting α-glucosidase and α-amylase activities and improving pancreas tissue damage. | Vietnam | Thanh et al., 2021 |
| 14. | Cucurbita moschata Duch | Water and alcohol | Polysaccharide | Reduced fasting blood glucose levels, increase antioxidant enzyme activities i.e CAT, GR, GSH, decreased MDA, stimulating the secretion of endogenous GLP-1, decreasing oxidative damages. | China | Lu et al., 2019 |
| 15. | C. ficifolia bouche | Aqueous | Not done | Hypoglycemic effect. | Mexico | Moya-Hernández et al., 2018 |
| 16. | Cucurbita pepo | Ethanol | Not done | Reduced blood sugar level. | Jordan | Alkofahi et al., 2017 |
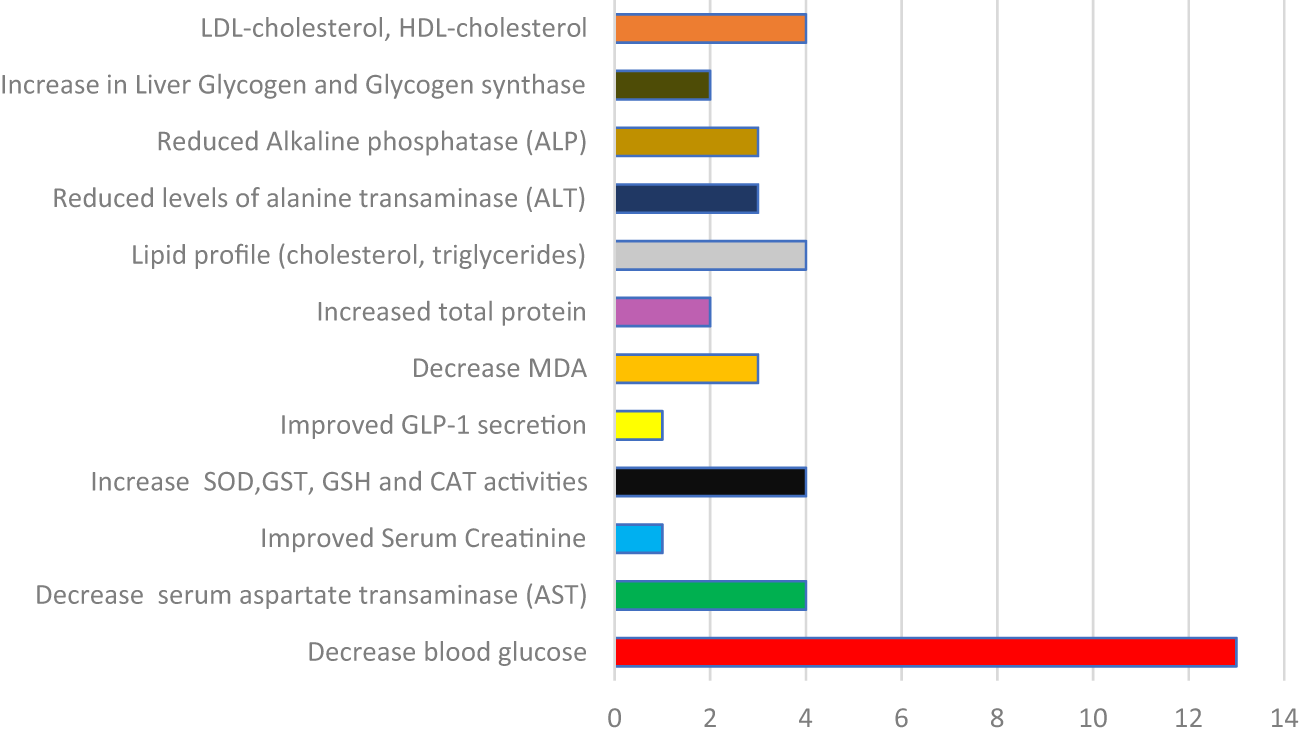
ALT – Alanine Aminotransferase, MDA – Malondialdehyde, SOD – Superoxide Dismutase, GSH – Glutathione, GR – Glutathione Reductase, Alkaline Phosphatase, CAT – Catalase, Glucagon-Like Peptide-1, HDL – High-Density Lipoprotein, LDL – Low-Density Lipoprotein, AST – Aspartate Transaminase.
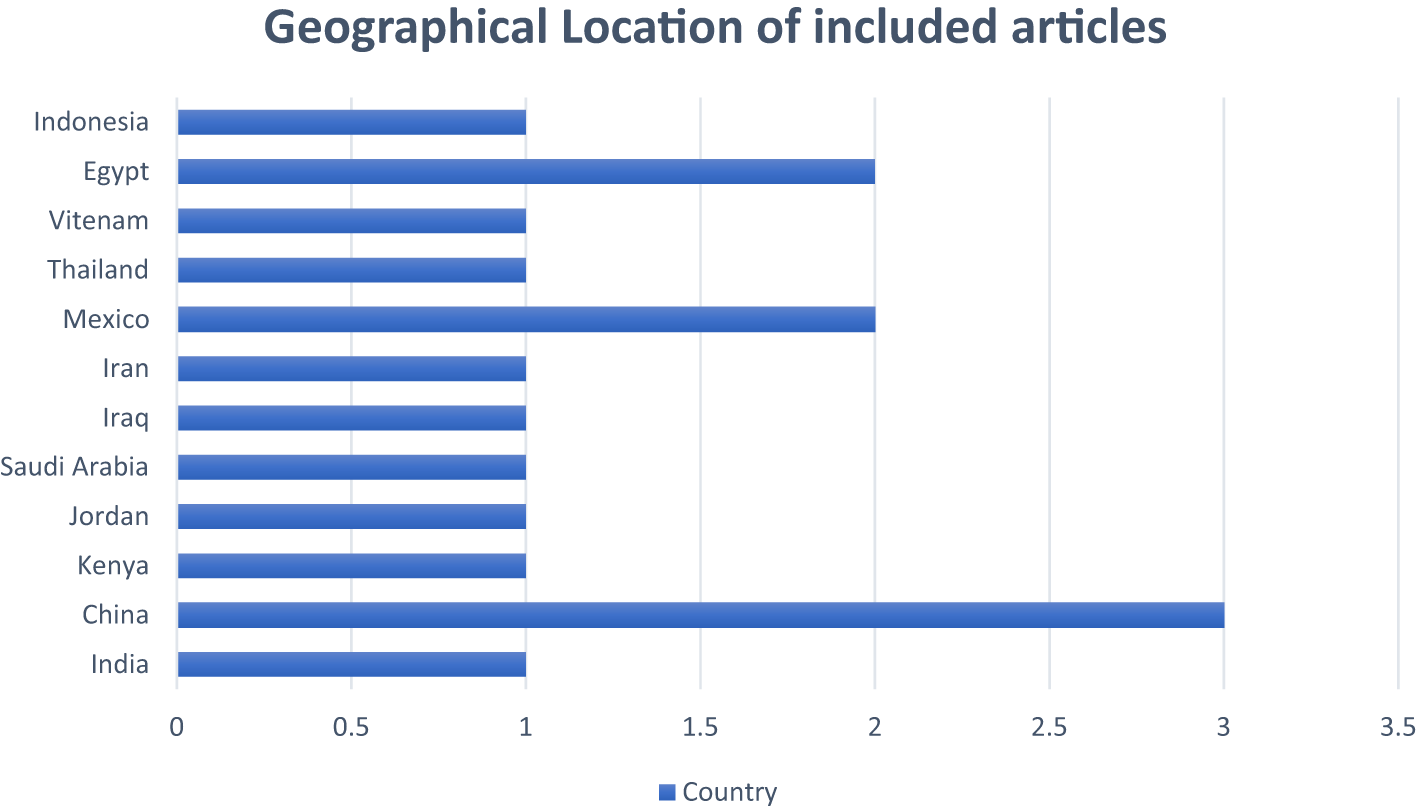
Table 1 reveals that Cucurbita species possess multiple bioactive compounds with diverse therapeutic effects targeting key pathophysiological aspects of diabetes. The hypoglycemic activity is mediated by stimulating insulin secretion, pancreatic regeneration, antioxidant defenses, and improvement in lipid metabolism. The widespread geographical focus of studies underpins the global relevance and ethnobotanical value of Cucurbita plants in managing diabetes. Table 3 summarizes the experimental protocols of Cucurbita species in diabetes management. Most studies used oral administration of aqueous or ethanol extracts over 2–4 weeks, with doses ranging from 50 mg/kg to 1 g/kg. Streptozotocin was the most common diabetes-inducing agent, while glibenclamide was frequently used as the standard control drug. Rats, particularly Wistar and albino strains, were the predominant animal models. The findings confirm the hypoglycemic potential of Cucurbita species, acting through insulin stimulation, antioxidant effects, and pancreatic protection, supporting their ethnomedicinal use in diabetes management. Figure 4 summarizes mechanisms such as: enhancement of antioxidant enzymes (SOD, CAT, GSH, GR), reduction of oxidative stress markers (MDA), insulin secretion and pancreatic regeneration, lipid profile improvement (lowering LDL, triglycerides), liver enzyme reduction (ALT, AST, ALP). A multi-target approach in diabetes management using Cucurbita species enhances therapeutic outcomes by addressing hyperglycemia, oxidative stress, and lipid metabolism.
| S/No | Cucurbita species | Duration of intervention | Dosage | Diabetics induction | Standard drug control | Animal strain | Route of administration | Author/Date |
|---|---|---|---|---|---|---|---|---|
| 1. | Cucurbita maxima | 28 days | 50, 100, 150, 200 and 250 mg/kg | Streptozotocin | Glibenclimide | Albino rats | Orally | Kushawaha et al., 2017 |
| 2. | Cucurbita ficifolia | 30 days | 200 mg/kg | Alloxan | Glibenclimide | Male mice | Orally | Garcia et al., 2017 |
| 3. | Cucurbita Moschata durch | 15 days | 100, 150, and 200 mg/kg | Streptozotocin | Glibenclimide | Male mice | Orally | Marbun & Rumanti, 2019 |
| 4. | Cucurbita maxima | 4 weeks | 300 mg/kg | Alloxan | No standard drug | Female swiss mice | Orally | Kadhum et al., 2020 |
| 5. | Cucurbita Moschata Durch | 7 weeks | 100 & 200 mg/kg | Streptozotocin | Acarbose | Male Mice | Orally | Yang et al., 2024 |
| 6. | Cucurbita moschata Duchesne & Cucurbita maxima Duchesne | 6 weeks | 500 mg/kg | Streptozotocin | Glibenclimide | Male albino Wistar rats | Orally | Suwannapong et al., 2023 |
| 7. | Cucurbita maxima | 28 days | 200 & 400 mg/kg | Alloxan | Glibenclimide | Wistar rats | Orally | Njoki et al., 2024 |
| 8. | Cucurbita maxima | 21 days | 200 & 400 mg/kg | Streptozotocin | Metformin | Sprague Dawley rats (male and Female) | Orally | Atta et al., 2020 |
| 9. | Cucurbita pepo L | 30 days | 500 mg/kg | Streptozotocin | No standard drug | Male Albino rats | Orally | Sayahi & Shirali, 2018 |
| 10. | Cucurbita maxima | 30 days | 3% of pumpkin powder | Alloxan | No standard drug | Male albino rats | Orally | Farid et al., 2015 |
| 11. | Cucurbita moschata | 1 day | 150 mg/kg | Alloxan | Xiaoke pill | Male mice | IP | Wang et al., 2017 |
| 12. | Cucurbita maxima | NS | Cucurbita maxima flowers (CMF-10% and 20%) | Alloxan | No standard drug | Adult male albino rats | orally | Elsadek et al., 2024 |
| 13. | Cucurbita pepo | 7 days | 100 mg/kg/day | Alloxan | Chlorpropamide | Mice | NS | Thanh et al., 2021 |
| 14. | Cucurbita moschata Duch | 3 weeks | 150 mg/kg | Streptozotocin | Acarbose | Adult Male Wistar rats | Orally | Lu et al., 2019 |
| 15. | Cucurbita ficifolia | 6 hour | 500 mg/kg | No-Induction | Glibenclamide | Male Mice | IP | Moya-Hernández et al., 2018 |
| 16. | Cucurbita pepo | 3 hours | 1 g/kg | Alloxan | Glibenclamide | Sprague Dawley rats | Orally | Alkofahi et al., 2017 |
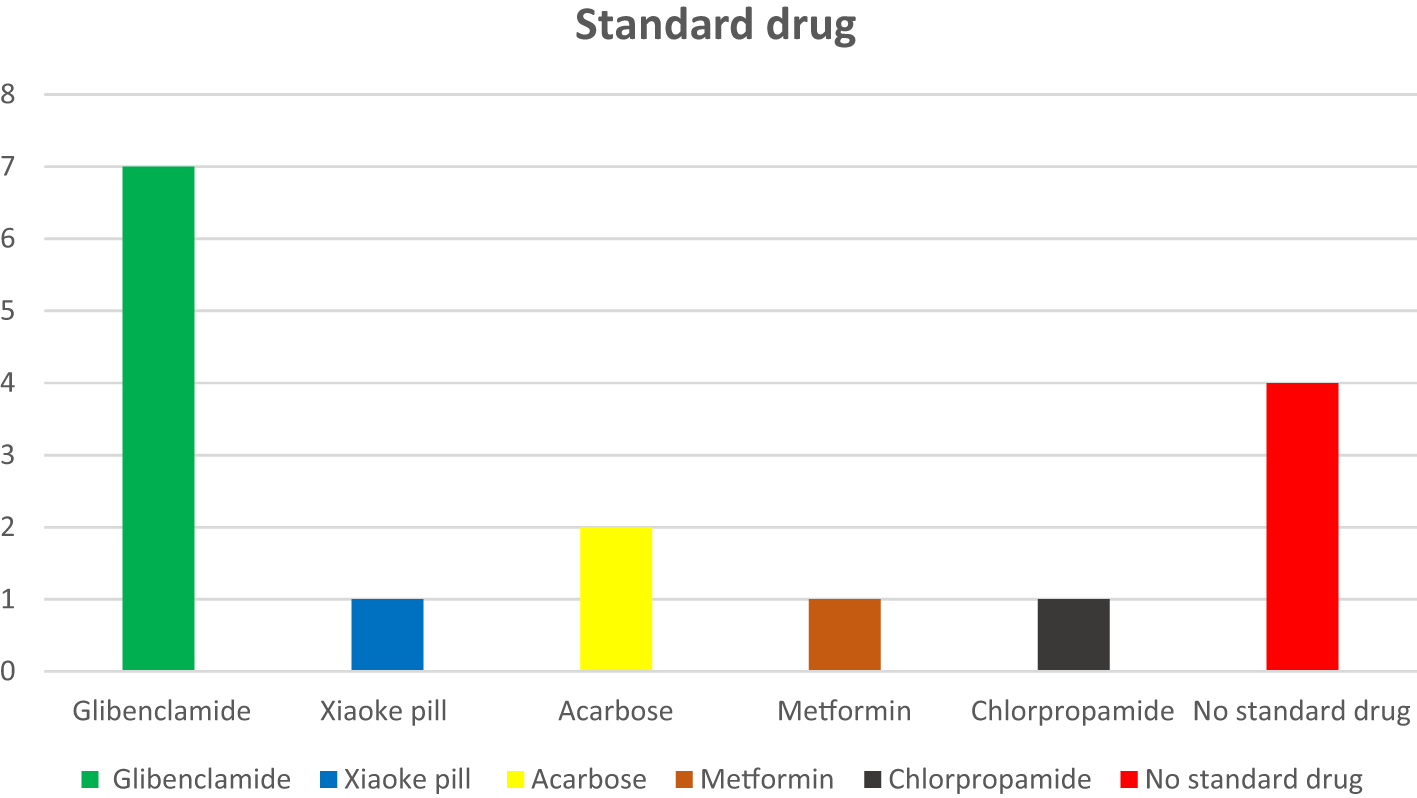
Figure 5 illustrates the route of administration. The majority of the included studies administered Cucurbita extracts orally, reflecting the traditional usage and potential for formulation into oral therapeutics. One study employed intraperitoneal (IP) administration. The oral route’s predominance supports its feasibility in human therapeutic applications. Figure 6 summarizes the geographical spread of studies. This figure illustrates a global distribution of research, with notable contributions from: Asia (India, China, Thailand, Iraq, Indonesia), Africa (Egypt, Kenya), South America (Mexico), the Middle East (Iran, Jordan), and Southeast Asia (Vietnam). The wide geographical distribution indicates the global recognition of Cucurbita species as potential anti-diabetic agents. Figure 7 shows the types and numbers of standard Anti-diabetic drugs used. This depicts the various standard anti-diabetic drugs used in comparison across studies, including: Glibenclamide, Metformin, Acarbose, Chlorpropamide and Xiaoke pill (herbal standard). Most studies used glibenclamide as the standard control drug, enabling a comparative evaluation of Cucurbita’s efficacy against established pharmacological agents.
This systematic review provides robust preclinical evidence that various species and parts of the Cucurbita plant possess significant anti-diabetic properties. These effects are mediated through multiple mechanisms, including hypoglycemic activity, antioxidant defense, pancreatic regeneration, and lipid profile modulation. The studies span a wide geographical range, highlighting global interest. Oral administration remains the predominant route, enhancing the clinical translatability of these findings.
Phytotherapy has continued to expand as a readily available and cost-effective management strategy for different diseases worldwide. In this study, the findings highlighted in Figures 1 and 2 present critical insights into the phytomedical use of Cucurbita species in diabetes management. Our study showed diverse plant parts, including seeds, fruit pulp, leaves, flowers, and juice, were used, underscoring the botanical versatility of the genus Cucurbita (Kushawaha et al., 2017; Yang et al., 2024). Similarly, this broad spectrum of plant parts suggests a widespread distribution of bioactive phytochemicals throughout the plant architecture (George & Brandl, 2021). According to our findings, such compounds may include flavonoids, polyphenols, saponins, alkaloids, and polysaccharides, many of which have been previously reported to exhibit antidiabetic properties through mechanisms like insulin sensitization, β-cell protection (Kadhum et al., 2020), inhibition of carbohydrate-hydrolyzing enzymes, and modulation of oxidative stress (Atta et al., 2020). Additionally, the predominance of Cucurbita maxima and Cucurbita moschata in the reviewed literature reflects their established role in ethnomedicine and greater accessibility in various cultures (Lu et al., 2019; Farid et al., 2015; Agu et al., 2022). This pattern may also be influenced by their favorable agronomic traits, nutritional value, and broader commercial availability, which make them more attractive for preclinical investigation. Importantly, both species have demonstrated diverse pharmacological actions in diabetic models, reinforcing their candidacy for further translational research and potential development into phytotherapeutic agents. Furthermore, these findings support the rationale for exploring multiple Cucurbita species and plant parts in preclinical diabetes research and emphasize the importance of a holistic phytochemical profiling approach. However, comparative studies that directly evaluate the antidiabetic efficacy of different parts and species under standardized conditions remain sparse and are warranted to guide evidence-based selection for clinical translation.
One of the interests in phytotherapy research is to elucidate the molecular mechanisms of action. In this study, the evidence presented in Table 1 and summarized in Table 2 and Figure 6 robustly affirms the multi-targeted antidiabetic mechanisms of Cucurbita species, substantiating their longstanding use in traditional medicine. According to the available data, the convergence of bioactivity across multiple studies reveals a complex pharmacodynamic profile, with extracts from Cucurbita plants mediating therapeutic effects through both pancreatic and extra-pancreatic pathways (Suwannapong et al., 2023). Notably, the stimulation of insulin secretion and pancreatic β-cell regeneration suggests the presence of insulinotropic agents within Cucurbita phytoconstituents (Rorsman and Ashcroft, 2018). This effect is particularly significant in type 1 and late-stage type 2 diabetes models, where β-cell integrity is compromised. Similarly, the improvement in lipid profiles and reduction of hepatic enzymes further indicate systemic metabolic benefits, reinforcing the potential of Cucurbita in managing diabetic dyslipidemia and hepatopathy, which are common comorbidities in poorly controlled diabetes (Atta et al., 2020). Additionally, the upregulation of endogenous antioxidant enzymes (SOD, CAT, GSH, GR) and suppression of lipid peroxidation marker MDA suggest that Cucurbita extracts confer substantial oxidative stress mitigation (Elsadek et al., 2024). Invariably, this is especially relevant given the critical role of oxidative stress in the pathogenesis and progression of diabetes and its complications (Agu et al., 2023a). Again, the multi-target nature of Cucurbita bioactivity, spanning glycemic control, oxidative defense, hepatic protection, and lipid modulation, aligns well with the modern paradigm of polypharmacology in chronic disease management.
From a methodological perspective, as observed in this study, the use of streptozotocin (STZ) to induce diabetes reflects a well-established model for replicating β-cell destruction and mimicking human diabetic pathology (Kushawaha et al., 2017; Yang et al., 2024). The frequent use of glibenclamide as a standard drug control reinforces the credibility of comparative efficacy assessments (Njoki et al., 2024). However, variability in extract preparation, dose ranges (50 mg/kg to 1 g/kg), and treatment duration (2–4 weeks) may introduce heterogeneity, underscoring the need for standardized protocols in future investigations. Furthermore, geographical diversity in the studies extracted further emphasized the trans-cultural relevance of Cucurbita in diabetes management, supporting its candidacy for global ethnopharmacological development (Suwannapong et al., 2023). Therefore, these findings collectively advocate for advancing Cucurbita species into more rigorous pharmacokinetic, toxicological, and eventually clinical evaluations.
In this study, we observed an overwhelming preference by researchers for oral administration of Cucurbita extracts ( Figure 6 and Table 3). This mirrors traditional ethnomedicinal practices and is pharmacologically significant. For instance, oral administration not only ensures ease of delivery and patient compliance but also suggests that the bioactive constituents of Cucurbita are sufficiently stable to survive gastrointestinal conditions and reach systemic circulation in biologically active forms. We maintained that this enhances the plausibility of transitioning these extracts or their active fractions into standardized oral formulations, such as capsules, teas, or nutraceutical supplements (Agu et al., 2023b). Additionally, the solitary use of the intraperitoneal route, while less relevant for human therapy, may have been employed for mechanistic or absorption-focused studies, but does not detract from the overarching translational value demonstrated by the oral route dominance (Al Shoyaib et al., 2019).
Furthermore, as shown in Figure 6, a geographically diverse body of literature from Asia, Africa, South America, the Middle East, and Southeast Asia suggests Cucurbita’s global ethnobotanical prominence and research appeal (World Health Organization, 2024). This broad-based validation points to a rich cross-cultural heritage of therapeutic use. It also suggests ecological and biochemical variability within Cucurbita species that may influence their phytochemical profiles and, consequently, their therapeutic effects. This is a compelling argument for comparative ethnopharmacological studies that examine regional chemovariants and their specific bioactivities. Another interesting observation in Figure 7 was the comparative use of standard antidiabetic agents, most notably glibenclamide, and others like metformin, acarbose, chlorpropamide, and the Xiaoke pill. This multi-standard framework allows for a nuanced benchmarking of Cucurbita’s efficacy across pharmacological classes: sulfonylureas (glibenclamide, chlorpropamide), biguanides (metformin), α-glucosidase inhibitors (acarbose), and polyherbal formulations (Xiaoke pill) (Spengler et al., 1992; Marre et al., 2002; Mujtaba et al., 2022). We suggest that the repeated use of glibenclamide enhances interpretability across studies, as it serves as a well-characterized reference with known insulinotropic effects (Garber et al., 2006). Therefore, Cucurbita extracts exhibit comparable or complementary effects to these standards in many studies, indicating their strong pharmacological promise.
This systematic review has provided compelling preclinical evidence that Cucurbita species possess significant antidiabetic potential through a multi-targeted mechanism involving insulin secretion, pancreatic regeneration, antioxidant enhancement, lipid profile improvement, and hepatic protection. Additionally, the use of various plant parts, most commonly administered orally, and the widespread global investigation across diverse geographic regions underscored the ethnomedicinal relevance and translational feasibility of Cucurbita-based phytotherapies. Furthermore, comparative efficacy against standard antidiabetic drugs further validates their pharmacological promise. Collectively, we propose that these findings support the advancement of Cucurbita species into standardized formulations and clinical studies as complementary or alternative agents in diabetes mellitus management.
All datasets can be accessed in the open science framework (OSF) under:
Repository link: https://osf.io/8y9wm/files/osfstorage
DOI: 10.17605/OSF.IO/8Y9WM (Musyoka et al., 2025).
License: CC0 1.0 Universal
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)