Keywords
: Brassica oleracea var. viridis, molecular docking, AKT1, EGFR, reproductive toxicity, male infertility, bioactive compounds, sperm quality.
Infertility is a global health issue, with male factor infertility contributing to nearly 50% of cases. Dysregulation of Protein Kinase B (PKB/AKT1) and Epidermal Growth Factor Receptor (EGFR) signaling impairs spermatogenesis. Bioactive compounds offer promising alternatives for targeting these pathways. Brassica oleracea var. viridis (collard greens) contains phytochemicals with antioxidant and anti-inflammatory properties, suggesting potential reproductive benefits.
This study evaluates bioactive compounds from B. oleracea var. viridis as AKT1 and EGFR inhibitors through molecular docking and in vivo validation in a cimetidine (Cemet ®)-induced reproductive toxicity model.
Bioactive compounds were identified via Gas Chromatography-Mass Spectrometry and analyzed for physicochemical, pharmacokinetic, and pharmacodynamic properties. Molecular docking assessed binding affinity to AKT1 and EGFR, followed by in vivo validation in cimetidine-exposed Wistar rats. Effects of ethanol extracts and solvent fractions on sperm motility, viability, morphology, count, and agglutination were examined.
Gamma-sitosterol showed the strongest binding affinity to AKT1 (-8.0 kcal/mol) and EGFR (-6.5 kcal/mol), comparable to co-crystallized ligands. Computational analysis indicated high Gastro-intestinal absorption and low toxicity for most compounds. In vivo, B. oleracea extracts significantly improved sperm motility, viability, and count, mitigating cimetidine-induced toxicity. Ethanol Leaf Extract of Brassica Oleracea (ELEBO), AFBO (Aqueous fractions of brassica Oleracea) and BFBO (n- Butanol fractions of brassica Oleracea) fractions had the most pronounced protective effects, reducing sperm abnormalities and agglutination.
B. oleracea var. viridis bioactive compounds show spermatoprotective effects, likely via AKT1 and EGFR inhibition. These findings support further research into B. oleracea derivatives for male reproductive health applications.
: Brassica oleracea var. viridis, molecular docking, AKT1, EGFR, reproductive toxicity, male infertility, bioactive compounds, sperm quality.
Infertility is a global health concern affecting millions of individuals and couples of reproductive ages. It is clinically defined as the inability to achieve pregnancy after 12 months or more of regular, unprotected sexual intercourse.1 Infertility can be classified as primary, where a couple has never conceived, or secondary, where conception has occurred previously but is now unsuccessful.2 The condition has profound medical, psychological, and socio-economic implications, particularly in societies where childbearing is deeply valued.3
According to a 2023 report by the World Health Organization (WHO), approximately 17.5% of adults worldwide, 1 in 6 experience infertility at some point in their lives. In Africa, infertility rates exhibit notable regional variations. A systematic review highlighted that primary infertility is more prevalent in North Africa, accounting for approximately 70.56% of cases, while secondary infertility is most common in East Africa.4 Globally; the prevalence of infertility varies across regions. The WHO report notes that lifetime prevalence rates are 17.8% in high-income countries and 16.5% in low- and middle-income countries.4 Both male and female factors contribute to infertility. Male infertility is primarily associated with sperm abnormalities, including low sperm count, poor motility, and DNA fragmentation. Environmental factors, lifestyle choices, genetic predispositions, infections, and underlying medical conditions can further exacerbate infertility risks in both sexes.5 Advancements in reproductive medicine have led to various diagnostic and therapeutic options for managing infertility, including assisted reproductive technologies (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI).6 However, despite these advancements, the underlying causes of infertility remain incompletely understood in many cases, necessitating further research into genetic, molecular, and environmental influences. Understanding the biological mechanisms of infertility is crucial for developing effective treatments and improving reproductive health outcomes worldwide.
Spermatogenesis is a complex and highly regulated process that involves the proliferation, differentiation, and maturation of male germ cells.7 As shown in Figure 1 below Several signaling pathways, including the AKT1 and epidermal growth factor receptor (EGFR) pathways play critical roles in the regulation of this process.8 Dysregulation of these pathways has been associated with male infertility due to impaired spermatogenic function. Therefore, targeting AKT1 and EGFR with natural bioactive compounds may provide a novel therapeutic approach for male reproductive disorders.8 AKT1, a serine/threonine kinase, is a key component of the phosphoinositide 3-kinase (PI3K)/AKT pathway, which regulates cell survival and apoptosis.9 It is essential in maintaining cellular homeostasis and is particularly significant in reproductive health, as it influences spermatogenesis by promoting germ cell survival and proliferation.10 However, aberrant activation of AKT1 has been linked to infertility, testicular dysfunction, and tumorigenesis.11 EGFR, a transmembrane receptor tyrosine kinase, is another critical regulator of cellular growth and differentiation.11 It binds to epidermal growth factors, triggering downstream signaling cascades that modulate cell proliferation and survival.12 In the male reproductive system, EGFR is involved in spermatogonia cell differentiation and the maintenance of Sertoli cell function.12 However, excessive EGFR activation can lead to pathological conditions, including testicular cancers and impaired spermatogenesis.13
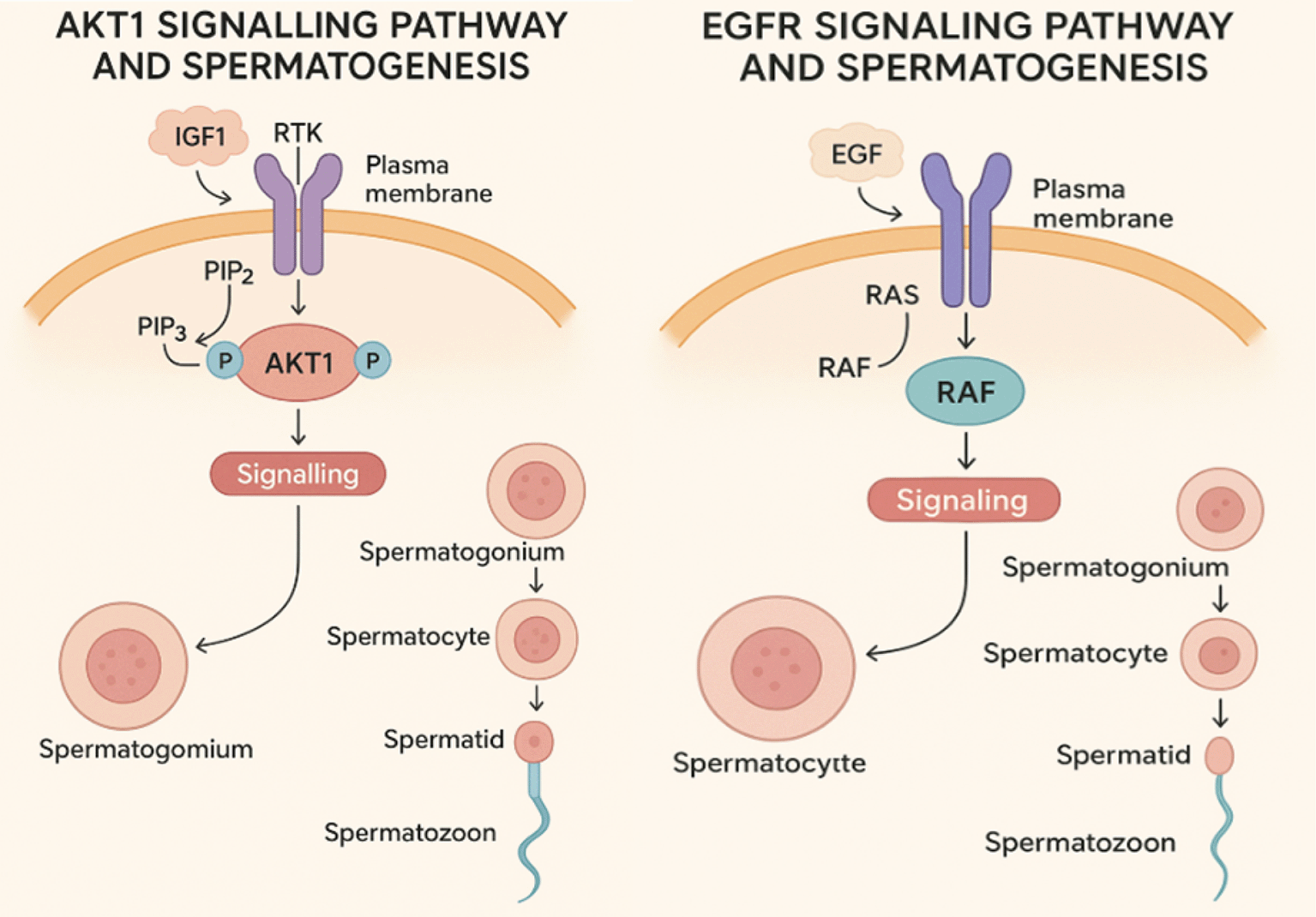
IGF1- (Insulin-like Growth Factor 1), RTK- Receptor Tyrosine Kinase, PIP2- Phosphatidylinositol 4,5-bisphosphate, PIP3- Phosphatidylinositol (3,4,5)-trisphosphate,
RAS- Rat Sarcoma, RAF- Rapidly Accelerated Fibro sarcoma, EGF- Epidermal Growth Factor.
Brassica oleracea var. viridis, a member of the cruciferous vegetable family, is known for its rich phytochemical composition, including flavonoids, glucosinolates, and phenolic compounds.14 These bioactive constituents have been reported to exhibit antioxidant, anti-inflammatory, and anti-carcinogenic properties, as shown in Figure 2.14 Recent studies suggest that certain phytochemicals in Brassica species may interfere with key molecular targets involved in cellular signaling pathways, making them potential candidates for modulating spermatogenesis.15 Given their pivotal roles, AKT1 and EGFR are attractive targets for therapeutic interventions in conditions associated with reproductive dysfunctions and malignancies.16 Recent research has focused on identifying natural and synthetic inhibitors of these pathways to regulate their activity and restore normal cellular function.17

In this study, we explore the potential of bioactive compounds from Brassica oleracea var. vividus as inhibitors of AKT1 and EGFR using an integrated in silico and in vitro approach. Computational docking was employed to predict the binding affinity of selected compounds against AKT1 and EGFR, while experimental validation was conducted to assess their biological effects on spermatogenic cells. The findings of this study may provide valuable insights into the role of natural bioactive compounds in male reproductive health and contribute to the development of alternative therapeutic strategies for male infertility.
Crystal structures of the target proteins, AKT1 and EGFR, were sourced from the Protein Data Bank18 (PDB IDs: 3cqu and 2itx respectively). Initial preparation involved: removal of all non-protein components such as water, co-crystallized ligands, and heteroatoms, optimization by adding missing hydrogen atoms and charges, using the UCSF Chimera software suite.19
The potential inhibitory compounds used in the study are bioactive compounds in Brassica oleracea var. viridis (collard greens), identified by gas chromatography/mass spectrometry (GC/MS). The compounds included: Gamma-Sitosterol, 7,10,13-Hexadecatrienoic acid, (Z,Z,Z)-Phytol, 9,12,15 Octadecatrienoic acid, (Z,Z,Z)-, 9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z), 2,2,4,4-Tetramethyl-6-(2-methylbutanoyl)cyclohexane-1,3,5-trione, Pentadecanoic acid, 1-(cyclopropylcarbonyl)piperidin-3-amine, 6-Isobutyryl-2,2,4,4-tetramethylcyclohexane-1,3,5-trione, Phenol, 4-ethenyl-2,6-dimethoxy-, Hexatriacontane, Dichloroacetic acid, tridec-2-ynyl ester, Bacteriochlorophyll-c-stearyl.
The physicochemical properties including molecular weight, lipophilicity (logP), and solubility (logS) of the compounds were compared with those of the co-crystalized ligands (CQU-N-[2-(5-methyl-4H-1,2,4-triazol-3-yl)phenyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine, and ANP-phosphoaminophosphonic acid-adenylate ester, respectively), using SwissADME (http://www.swissadme.ch/).20 Their pharmacokinetics and drug-likeness properties, such as gastrointestinal absorption, blood-brain barrier permeability, and interaction with cytochrome P450 enzymes, were also predicted using ADMETlab 3.0 (https://admetlab3.scbdd.com/).20
The compounds were subjected to molecular docking using the AutoDock Vina algorithm within PyRx software21 determining binding interactions between each ligand and the active sites of 3cqu and 2itx receptors. The XYZ-coordinates of the interaction sites of the proteins were set at: 7.30, -2.65, 18.56 (for 3cqu), and -51.74, -0.86, -27.97 (for 2itx). PyRx computational scoring is based on the binding affinities guided compound prioritization. Post-docking analyses utilized Discovery Studio Visualizer to identify critical binding interactions like hydrogen bonds and hydrophobic contacts.22
Fresh leaves of Brassica oleracea var. viridis (collard greens) were collected from Bwejuragye in Ishaka Town, Busheyin Local Government Area of Western Uganda and brought to the University of Mbarara’s herbarium unit for correct identification and verification. The leaves were identified and authenticated by Dr. Olet Eunice of the Department of Botany, Faculty of Biological Science, Mbarara University of Science and Technology, Uganda. The plant was deposited in the herbarium of the Department of Botany, Mbarara University of Science and Technology, with voucher number (IOE-24-001).
Cimetidine tablets (400 mg) used in this study was purchased from Cosmos Ltd, located at Rangwe Road, off Lunga Lunga Road, Nairobi, Kenya. For the extraction and fractionation process, a total of 5.5 liters of 99% ethanol and 400 mL each of n-hexane and n-butanol were used. These solvents were purchased from God’s Grace Biomed Supply Ltd, located at Arua Park Police Post, Ben Kiwanuka Road, Kampala, Uganda. The chemical-grade solvents were sourced from established manufacturers and used without further purification. Specifically, the n-hexane was obtained from Sigma-Aldrich (Cat. No. 296090), n-butanol from Merck (Cat. No. 101990), and 99% ethanol from Sigma-Aldrich (Cat. No. 24103). All reagents used in the study were of analytical grade and handled according to standard laboratory procedures.
Fresh leaves of Brassica oleracea var. viridis (collard greens) were cleaned with water to get rid of dirt and sand, then dried out and ground into powder using a table grinder. The powdered leaves were then stored in airtight containers in a dry environment. The Ethanol Extraction was done using a modified version of23 technique, the ethanol extract solvent was removed by a rotator evaporator followed by drying in an oven with a temperature of not greater than 40°C, and the aqueous part was removed by lyophilization under reduced pressure. Then, the remaining solvent free extract was kept alone in a refrigerator until required for use, 5g of the Ethanol extract was subjected to GC-MS (Gas Chromatography-Mass spectrometric) to identify and quantify the Bioactive components, the bioactive compounds were identified by comparing their mass spectra to those in the NIST20 library.
For fractionation,24 techniques was followed, 100 g ethanol extract was suspended in a separatory funnel with 400 ml of distilled water. Then, the suspension was shaken by adding 400 ml volume of n-hexane. Then, the n-hexane layer so formed was poured into a beaker and labeled as “n-hexane fraction.” The aqueous remainder was again mixed with same quantity of n-butanol shaken similarly, and the n- Butanol layer obtained was decanted to a second beaker and labeled as “n-butanol fraction” likewise. The remaining aqueous residue was lyophilized to obtain pure aqueous fraction, placed in a third beaker and labeled as “aqueous fraction,” and the n-hexane and n-butanol were allowed to concentrate in an oven under a temperature set at 40°C Finally, 15 g hexane and 9 g of n-butanol fractions and 120 g being aqueous fraction. All fractions were put in an amber bottle and stored in a fridge till they were going to be used for the experiment.
An acute oral toxicity study was conducted in compliance with OECD Guideline 425 (Up-and-Down Procedure). A young adult male Wistar rat, weighing 200g, was selected for the limit test and acclimatized under standard laboratory conditions with ad libitum access to water and a standard pellet diet. Prior to dosing, the animal was fasted for 3-4 hours, with water available throughout the fasting period. The rat received a single oral dose of the test substance at the limit dose of 2000 mg/kg body weight, administered via oral gavage using an appropriate cannula. The corresponding dosing volume was 2 mL/kg body weight. Post-administration, the animal was closely observed for clinical signs of toxicity during the first 30 minutes, periodically for the next 24 hours, and subsequently on a daily basis for 14 days. Observations included assessments of behavioral changes, clinical signs of toxicity, alterations in body weight, and mortality. No signs of toxicity or mortality were observed throughout the 14-day observation period.
Following the initial test, and in accordance with25, an additional four Wistar rats were enrolled and subjected to the same experimental conditions. For the in vivo study of the test substance (ethanol extract and its solvent fractions), an intermediate dose of 200 mg/kg body weight was employed. All animals were monitored similarly for any adverse effects.
For this study, thirty-five (35) adult male Wistar rats, aged 8–10 weeks and weighing between 180–220 g, were utilized. The animals were procured from the Animal House of Kampala International University. Verbal consent was obtained for the procurement of Wistar rats because the study was conducted within Kampala International University, where the animal house staffs are familiar with the research protocols and ethical standards. As the research had ethical approval and involved no external transfer or commercial transaction, verbal consent was considered appropriate and consistent with institutional practice. Ethical approval for the study was granted by the Research Ethics Committee (REC) of Kampala International University, Western Campus (REC No. KIU-2024-389), and further approved by the Uganda National Council for Science and Technology (UNCST) under registration number HSF5192ES. The experimental design is summarized in Table 1.
A stock solution was prepared by dissolving 5.2 g of cimetidine in 100 mL of distilled water. From this stock, a dose of 0.3 mL/kg body weight was administered orally to 200 g Wistar rats using an oral cannula. The dosing regimen for cimetidine administration was adapted from.26 Considering that each cimetidine tablet contains 400 mg, the stock solution was prepared by dissolving eight (8) tablets in 100 mL of distilled water, resulting in a final concentration suitable for delivering 0.1 mL of solution per dose.
Cimetidine was administered orally
All extract was administered orally via oral cannula.
One-way analysis of variance (ANOVA) was used for data analysis with the aid of Graph Pad software version 8. Comparison was done using Tukey test. Results were expressed as mean ± S.E.M. p < 0.05 was taken as accepted level of significant difference.
At the end of the eight-week treatment period, all experimental animals were anesthetized with a combination of ketamine (80 mg/kg) and xylazine (10 mg/kg). The anesthetic agents were freshly prepared in sterile saline and administered intramuscularly using a 1 mL insulin syringe fitted with a 26-gauge needle. This approach ensured rapid absorption and effective induction of anesthesia, thereby facilitating humane handling and tissue collection in accordance with standard laboratory animal care and ethical guidelines. After confirming full anesthesia, the animals were humanely sacrificed by exsanguination via cardiac puncture, a method that ensured minimal pain and distress while allowing immediate collection of blood and tissues for analysis. Sperm parameters were assessed by extracting spermatozoa from the caudal epididymis and examining them microscopically.
The testis was carefully excised through pelvic incision by carefully trimming off the surrounding fats. The caudal epididymis were removed and minced in 0.9 mls of normal saline, a suspension was obtained and the following sperm parameters were measured.
On a microscope slide, two drops of the suspension were applied before being covered by a cover slip. Under the microscope, the slide was analyzed and graded on a scale of 100 with an x40 objective and low light.28
The method of doing a sperm viability investigation (% of live spermatozoa) using the Eosin/Nigrosin stain.27 Two drops of the solution and two drops of the stain were put on a microscope slide. The sperm cells were counted under an x40 microscope and an average value for each was documented from which % viability (live-dead ratio).28
The sperm smears on microscope slides were air dried before being stained with two drops of Walls and Ewas dye to ascertain the sperm morphology. An oil immersion microscope and an x100 objective were used to examine the slides. There was a count of abnormal sperm cells.28 The overall number of anomalies, including head deformities (headless-tail, tailess-head), mid-piece deformities (bent mid-piece, curved mid-piece), and tail deformities (looped tail, curved tail), was compared to the average morphology, and the results were expressed as a percentage.
The enhanced Neubauer haemocytometer was used to do the sperm count under a microscope. The suspension will be diluted in a 1:19 ratio with sodium bicarbonate and formalin (1 in 20). The enhanced Neubauer haemocytometer chamber was filled with sperm that has been thoroughly diluted, and the sperm cells was then be counted in the chamber’s 2 square millimeter area. The number of detected cells and hemocytometer dimensions will be used to calculate the sperm concentration, which will then be multiplied by the dilution factor (0.98 ml). The amount of sperm expressed in millions per milliliter.28
A drop of diluted semen was applied to a clear slide, fixed in ethanol, and stained with the Leishman stain to determine the degree of agglutination. The slide was subsequently be examined at 450 to gauge the frequency of sperm agglutinations.29
The bioavailability and efficacy of bioactive compounds are significantly influenced by their physicochemical properties, pharmacokinetics, and pharmacodynamics. Key factors such as Molecular weight (MW), Molecular Formula (MF), lipophilicity (log P), Angstrom Squared (Å2), Hydrogen bond donors (HBD), Hydrogen bond acceptors (HBA), SC (Surface Complexity or Structural Complexity), Lipinski and Logarithm of Solubility (log S) play crucial roles in determining the drug-likeness of a compound, NA- means not applicable. These properties affect absorption, distribution, metabolism, and excretion (ADME) characteristics, which are essential for evaluating their potential as therapeutic agents.
In this study, bioactive compounds identified in Brassica oleracea var. viridis (collard greens) were analyzed as shown in the chromatograph in Figure 3 and their Physicochemical Properties, drug-likeness, Lipophilicity, and Solubility of the Compounds profiles, as shown in Table 2. The lipophilicity and solubility of these compounds were evaluated to determine their potential oral bioavailability and metabolic stability. Further, Table 3 presents the pharmacokinetic parameters, including gastrointestinal absorption, blood-brain barrier permeability, P-glycoprotein (P-gp) interactions, and cytochrome P450 enzyme inhibition, which provide insights into the systemic bioavailability and potential drug-interactions of these compounds as showing the chromatograph of ethanol extract of Brassica oleracea var. viridis. The results indicate that certain compounds exhibit favorable ADME properties, making them promising candidates for further molecular docking and bioactivity screening. Compounds with high GI absorption and drug-likeness scores were prioritized for in silico and in vitro validation.
| S/N | Compound | Structure | MF | MW(g/mol) | W Log P | (Å2) | H BD | H BA | RB | Log S | SC | Lipinski |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 1-(cyclopropylcarbonyl)piperidin-3-amine | 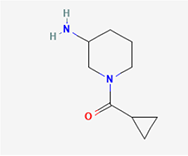
| C9H16N2O | 168.24 | -0.10 | 46.33 | 1 | 2 | 2 | -0.62 | Very soluble | 0 |
| 2. | 6-Isobutyryl-2,2,4,4-tetramethylcyclohexane-1,3,5-trione | 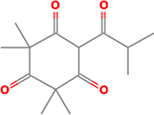
| C14H20O4 | 252.31 | 1.60 | 68.28 | 0 | 4 | 2 | -2.97 | Soluble | 0 |
| 3. | Phenol, 4-ethenyl-2,6-dimethoxy- | 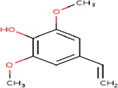
| C10H12O3 | 180.2 | 1.94 | 38.69 | 1 | 3 | 3 | -2.54 | Soluble | 0 |
| 4. | 2,2,4,4-Tetramethyl-6-(2-methylbutanoyl)cyclohexane-1,3,5-trione | 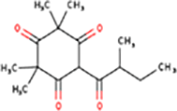
| C15H22O4 | 266.33 | 1.99 | 68.28 | 0 | 4 | 3 | -3.21 | Soluble | 0 |
| 5. | Bacteriochlorophyll-c-stearyl | 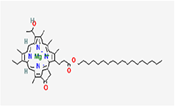
| C52H72MgN4O4-2 | 841.5 | 10.52 | 89.39 | 1 | 8 | 23 | NA | NA | NA |
| 6. | (7Z,10Z,13Z)-hexadeca-7,10,13-trienoic acid | 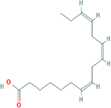
| C16H26O2 | 250.38 | 4.88 | 37.30 | 1 | 2 | 11 | NA | NA | NA |
| 7. | Pentadecanoic acid | 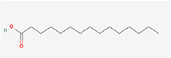
| C15H30O2 | 242.4 | 5.16 | 37.30 | 1 | 2 | 13 | -4.66 | Moderately soluble | 0 |
| 8. | Phytol | 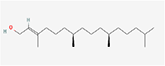
| C20H40O | 296.5 | 6.36 | 20.23 | 1 | 1 | 3 | -5.98 | Moderately soluble | 1 |
| 9. | 9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | 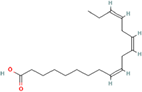
| C18H30O2 | 278.4 | 5.66 | 37.30 | 1 | 2 | 13 | NA | NA | NA |
| 10. | Dichloroacetic acid, tridec-2-ynyl ester | 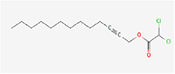
| C15H24Cl2O2 | 307.3 | 4.95 | 26.30 | 0 | 2 | 11 | -0.53 | Moderately soluble | 1 |
| 11. | 2,3-dihydroxypropyl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate | 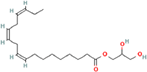
| C21H36O4 | 352.5 | 4.47 | 66.76 | 2 | 4 | 17 | NA | NA | NA |
| 12. | Hexatriacontane | 
| C36H74 | 506 | 14.29 | 0.00 | 0 | 0 | 33 | -12.85 | Insoluble | 2 |
| 13. | gamma-Sitosterol | 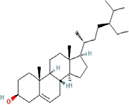
| C29H50O | 414.7 | 8.02 | 20.23 | 1 | 1 | 6 | -7.90 | Poorly Insoluble | 1 |
The interaction between a ligand and its target receptor is a crucial determinant of its biological activity. Molecular docking was employed to evaluate the binding affinity of the identified compounds from Brassica oleracea var. viridis against AKT1 and EGFR receptors, two key signaling molecules involved in spermatogenesis and male reproductive health.
As presented in Table 4, gamma-sitosterol (light blue) demonstrated the highest binding affinity for both AKT1 and EGFR receptors, with values comparable to those of the co-crystallized ligands (Yellow). Other notable compounds, including 7,10,13-hexadecatrienoic acid and phytol, exhibited moderate binding affinities, suggesting potential inhibitory effects on these molecular targets. The co-crystalized ligands are: N-[2-(5-methyl-4H-1,2,4-triazol-3-yl)phenyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine, and phosphoaminophosphonic acid-adenylate ester, for AKT1 and EGFR respectively.
Figures 4–9 illustrate the surface, 3D, and 2D interaction profiles of AKT1 and EGFR with their co-crystallized ligands and gamma-sitosterol, respectively. These visual representations highlight the key hydrogen bonding interactions and hydrophobic contacts that contribute to the binding stability of gamma-sitosterol within the active site of these proteins. The findings suggest that gamma-sitosterol, among other compounds, may serve as a potential natural inhibitor of AKT1 and EGFR, warranting further biological validation.
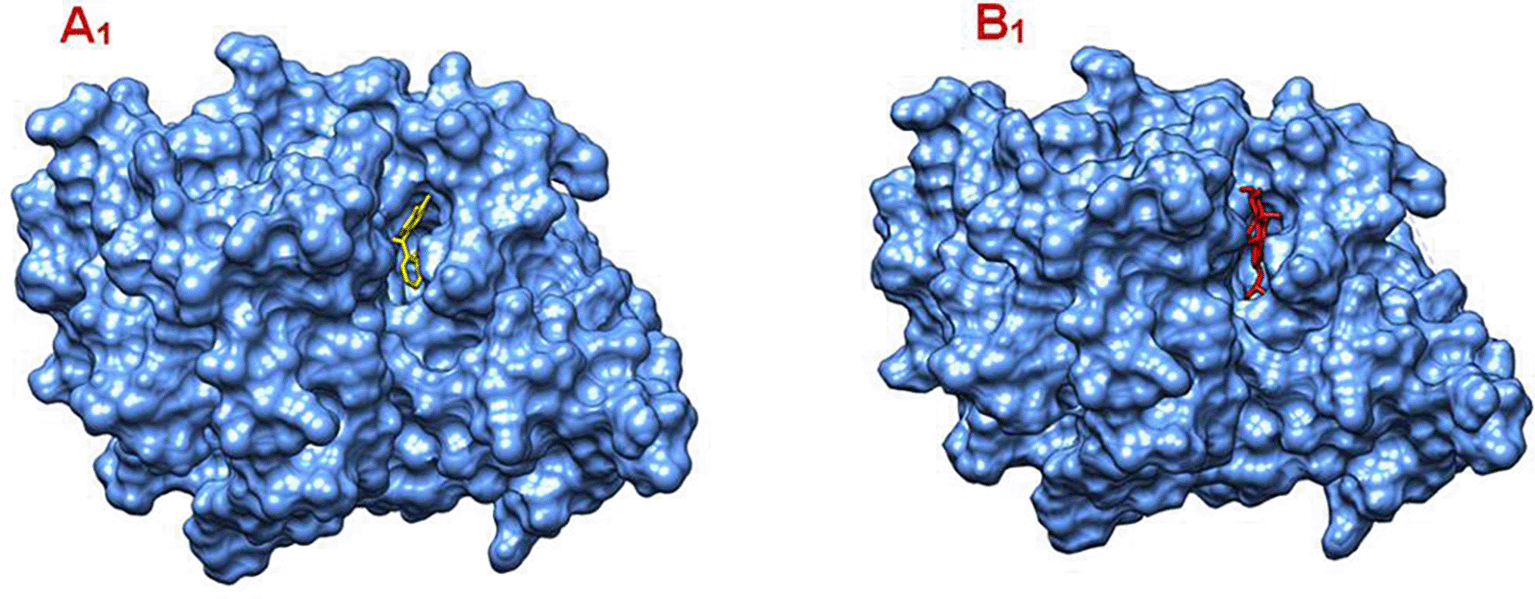
A1: Surface Interaction of AKT1 and its co-crystalized ligand, B1: Surface Interaction of AKT1 and Gamma-Sitosterol.
Panel A1 – Co-crystallized Ligand: The ligand shown in yellow is the co-crystallized inhibitor: N-[2-(5-methyl-4H-1,2,4-triazol-3-yl)phenyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine. It is bound within a deep binding pocket of AKT1, indicating a well-fitted interaction, This ligand also exhibited the strongest binding affinity in the study: −9.3 kcal/mol.
Panel B1 – Gamma-Sitosterol: The ligand shown in red is Gamma-Sitosterol; a plant-derived sterol. It is also bound in a similar region of the protein surface, likely overlapping with or close to the co-crystallized binding site. Its binding affinity was −8.0 kcal/mol, which is slightly weaker than the co-crystallized ligand but still indicates a strong interaction.
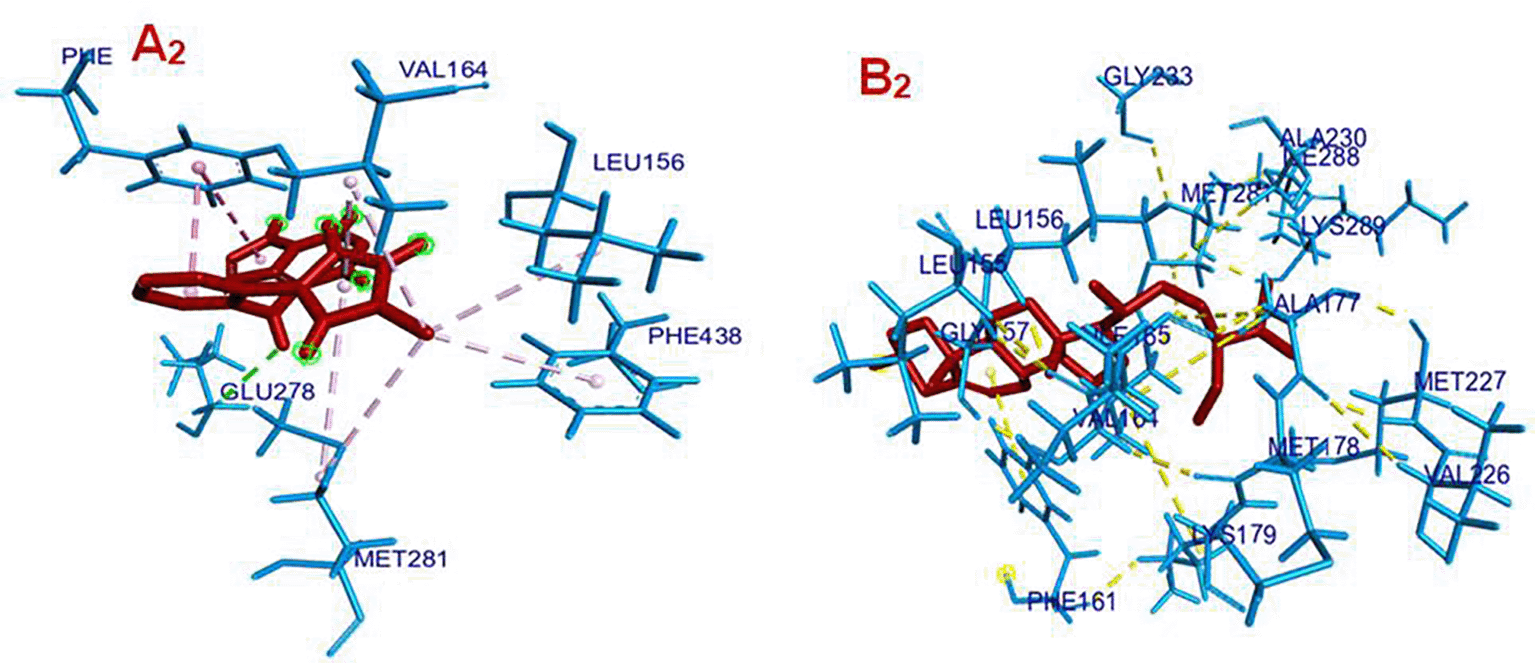
Panel A2 – Co-crystallized Ligand with AKT1 (Binding Affinity: -9.3 kcal/mol).
The co-crystallized ligand (in red) is embedded in the AKT1 binding pocket, forming multiple hydrophobic and polar interactions. Key interacting residues include: PHE438, LEU156, VAL164, GLU278, MET281, Magenta dashed lines represent hydrophobic or polar contacts, suggesting strong anchoring of the ligand. The ligand is surrounded by a well-packed environment, contributing to its high binding affinity. The green-highlighted atoms likely indicate interaction hotspots or pharmacophore features (such as H-bond donors/acceptors).
Panel B2 – Gamma-Sitosterol with AKT1 (Binding Affinity: -8.0 kcal/mol): Gamma-sitosterol (in red) is shown occupying the AKT1 active site, with an elongated conformation. It interacts primarily via hydrophobic contacts (shown as yellow dashed lines) with several residues: LEU156, LEU155, GLY157, PHE161, ALA177, MET178, VAL226, MET227, MET281, LYS289. The interaction network is less polar and more hydrophobic compared to the co-crystallized ligand. The binding pose suggests a stable but less specific fit, consistent with its slightly lower binding affinity.
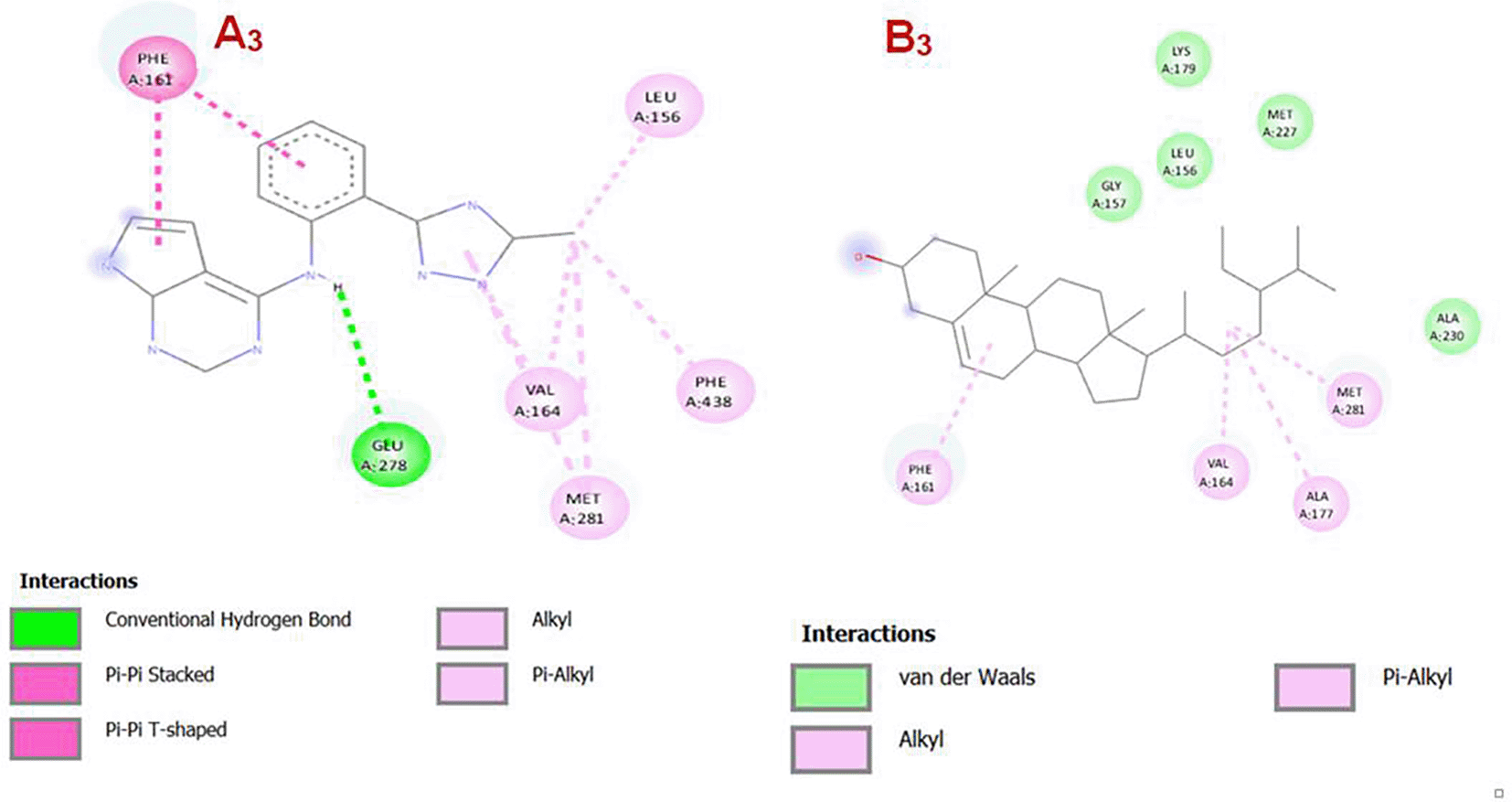
Panel A3 – AKT1 with co-crystallized ligand: The co-crystallized ligand forms multiple specific interactions with AKT1. Key interactions include: Conventional hydrogen bond with GLU A:278 (green dashed line), Pi-Pi stacked interaction with PHE A:161, Pi-Pi T-shaped interactions and Pi-alkyl interactions with residues like VAL A:164, MET A:281, and PHE A:438. Additional alkyl interactions are noted with LEU A:156 and others. These interactions suggest a strong and specific binding mode involving both polar and hydrophobic residues.
Panel B3 (right) – AKT1 with Gamma-Sitosterol: Gamma-sitosterol exhibits fewer specific interactions, It primarily engages in hydrophobic interactions. Alkyl and Pi-alkyl interactions with residues such as VAL A:164, MET A:281, and ALA A:177. Van der Waals interactions (green-highlighted residues) with LEU A:156, GLY A:157, LYS A:179, and ALA A:230. one Pi interaction with PHE A:161 is observed, indicating less aromatic stacking. Also the interactions suggest a similar binding mode compared to the co-crystal ligand.
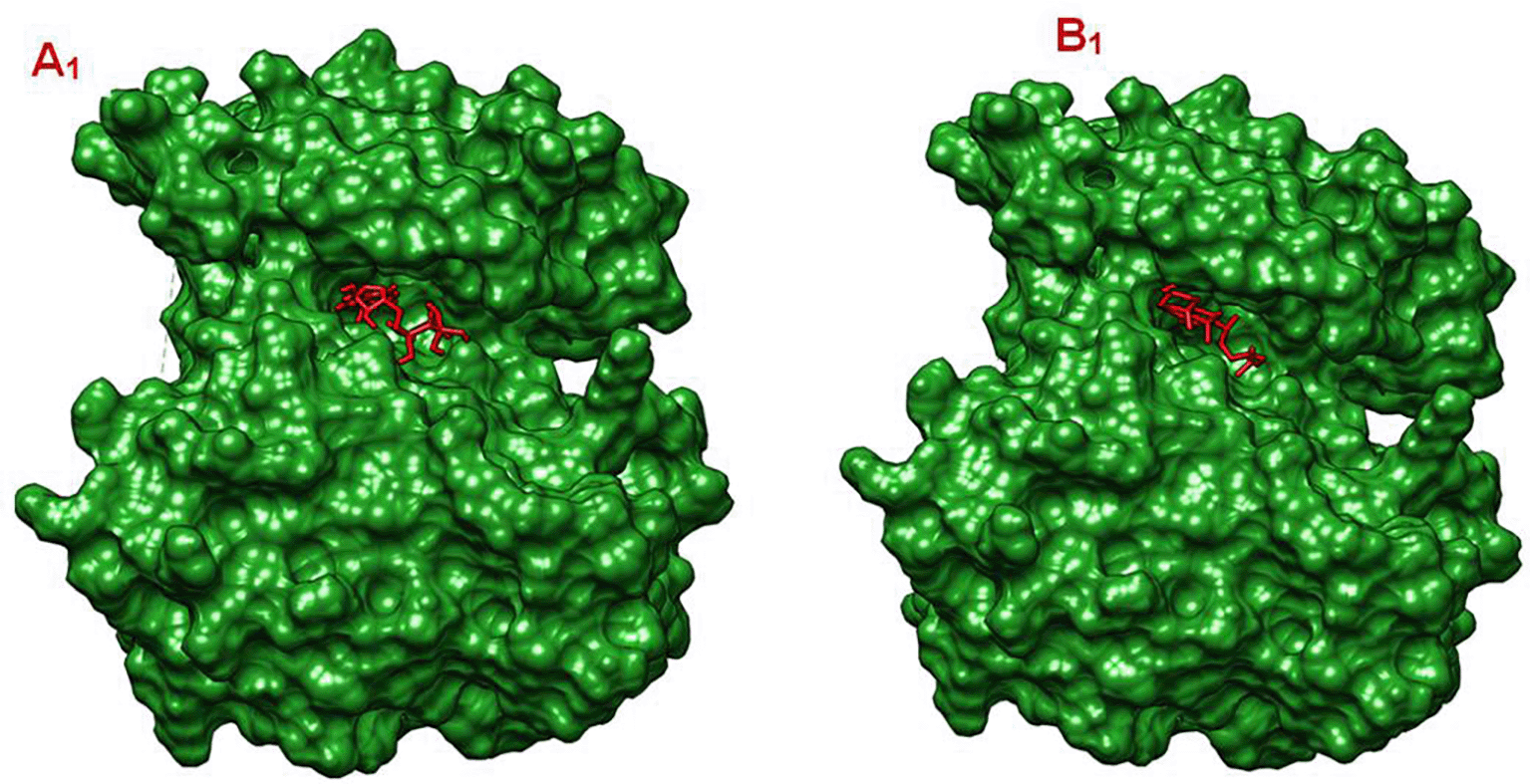
A1: Surface Interaction of EGFR and its co-crystalized ligand, B1: Surface Interaction of EGFR and Gamma-Sitosterol.
Panel A1 – EGFR with Co-Crystallized Ligand: The co-crystallized ligand is shown in red bound within a surface pocket of the EGFR protein (green surface). The ligand is deeply buried in a well-defined binding pocket, suggesting a precise fit and stable interaction. Binding Affinity: −6.5 kcal/mol, indicating moderate binding strength.
Panel B1 – EGFR with Gamma-Sitosterol : Gamma-sitosterol (also in red) is shown occupying a similar binding site within EGFR. The fit appears slightly less buried or structured compared to the co-crystallized ligand but still shows substantial surface interaction. Binding Affinity: −7.8 kcal/mol, which is stronger than the co-crystallized ligand, suggesting gamma-sitosterol may have a better overall binding potential in terms of energy. Gamma-sitosterol exhibits a stronger binding affinity than the co-crystallized ligand (−7.8 vs. −6.5 kcal/mol), implying it may form more energetically favorable interactions despite lacking specific hydrogen bonds or Pi-stacking interactions.
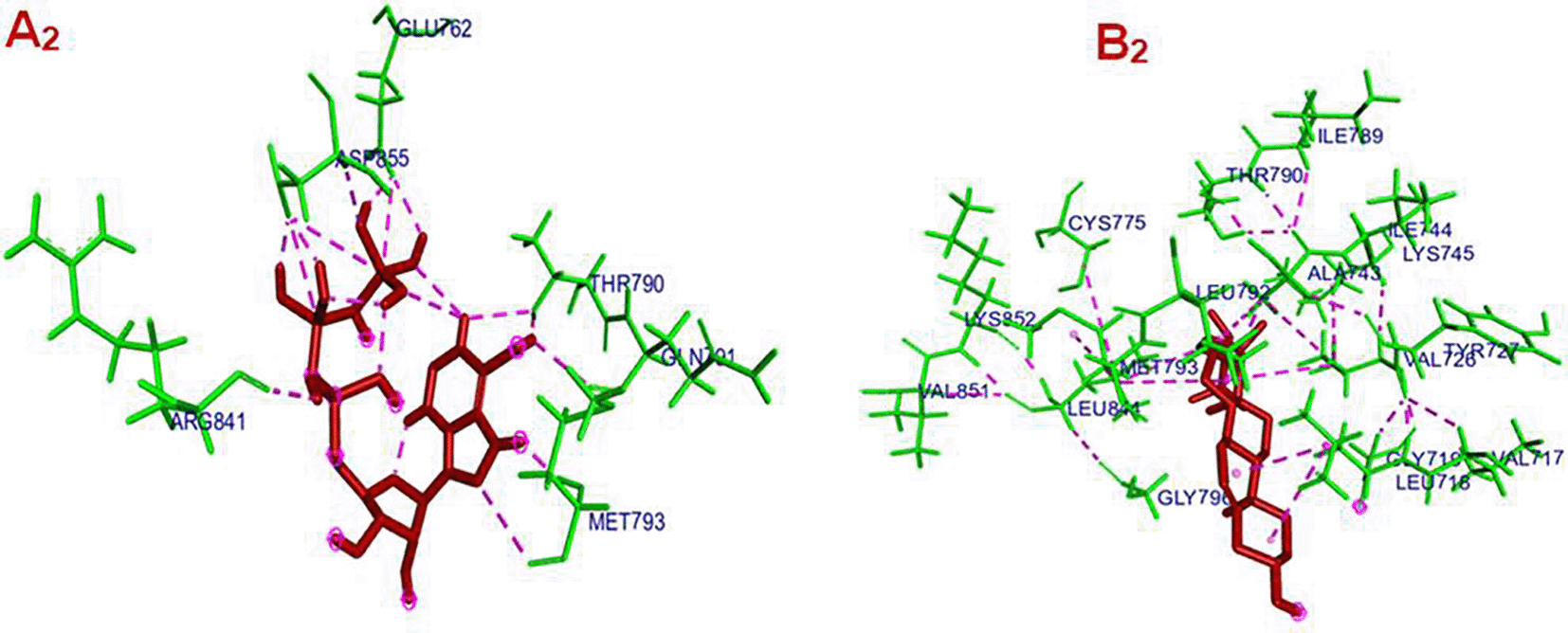
Panel A2: Shows the interaction between EGFR and its co-crystallized ligand. The ligand is represented in dark red, and the EGFR residues are shown in green. Several interactions (likely hydrogen bonds, hydrophobic contacts, or π-stacking) are depicted as dashed pink lines, connecting the ligand to various residues in the binding pocket. Key interacting residues include: ARG841, GLU762, ASP855, THR790, GLN791, MET793, among others. These interactions suggest a strong binding affinity and specificity of the native ligand within the EGFR active site.
Panel B2: Depicts the interaction between EGFR and Gamma-Sitosterol , a phytosterol compound. Gamma-Sitosterol is also represented in dark red, with interacting EGFR residues again in green. Similar pink dashed lines represent the molecular interactions between the compound and the protein. Interacting residues in this complex include: LEU792, MET793, GLY796, LEU718, VAL726, LYS745, CYS775, and others. The interaction pattern is more distributed across the binding pocket, reflecting the different structure and possibly different binding mode of Gamma-Sitosterol compared to the co-crystallized ligand. Gamma-Sitosterol shows a broader interaction profile, which might affect binding strength or functional modulation differently.
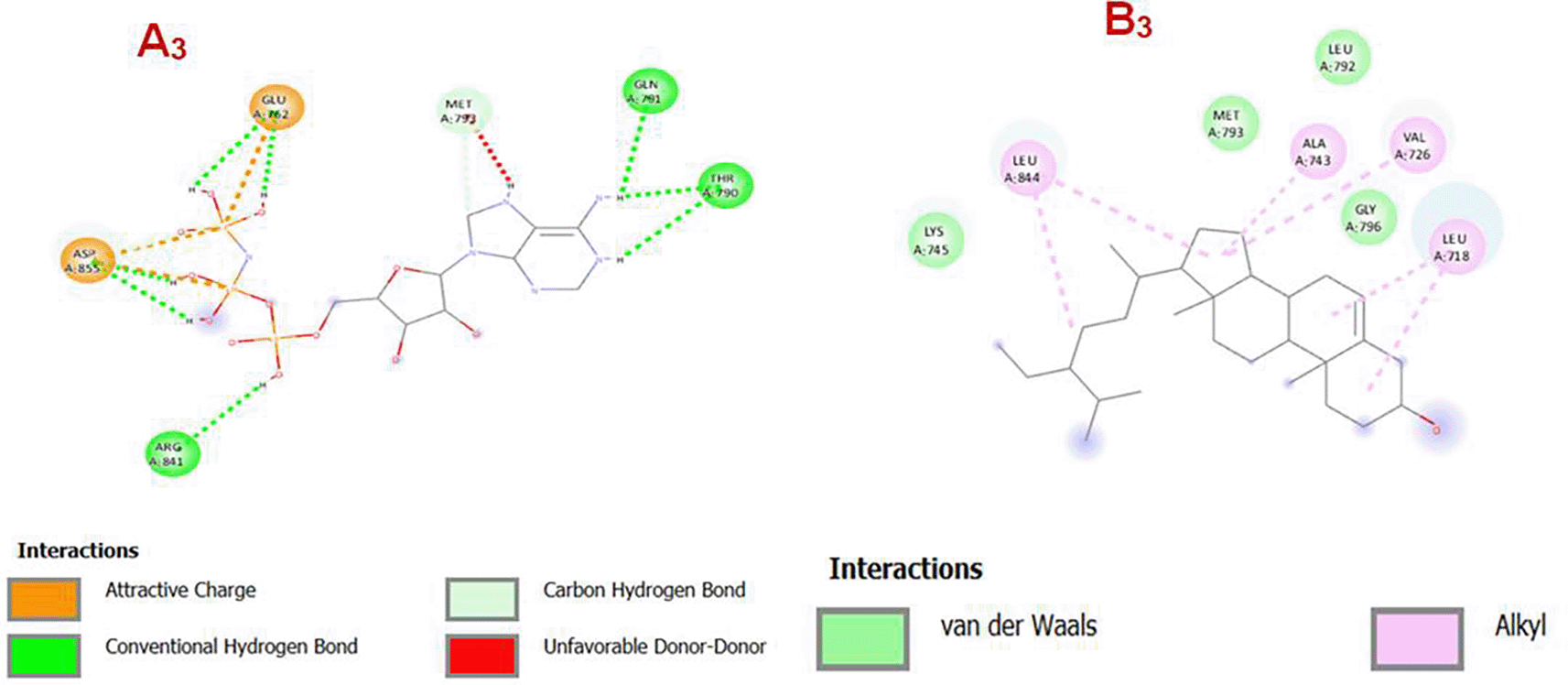
Panel A3: EGFR and its Co-Crystallized Ligand, The ligand is involved in multiple specific interactions with EGFR residues. Conventional hydrogen bonds (green lines) are observed with residues like ARG841, GLU762, GLN791, THR790, ASP855. Attractive charge interactions (orange lines) are evident between the ligand and ASP855, GLU762, indicating electrostatic attractions. Carbon hydrogen bonds (light green shaded areas) are noted, enhancing ligand affinity. A red dashed line indicates an unfavorable donor-donor interaction with MET793, potentially decreasing binding stability.
Panel B3: EGFR and Gamma-Sitosterol, The interaction profile is dominated by hydrophobic and van der Waals interactions, characteristic of steroid-based compounds like Gamma-Sitosterol. Alkyl interactions (pink dashed lines and shaded areas) are present with residues such as LEU844, LEU792, ALA743, VAL726, LEU718, suggesting binding in a hydrophobic pocket. Van der Waals interactions (green shaded circles) are spread throughout the ligand-receptor interface, including residues like MET793, GLY796, LYS745. The absence of hydrogen bonds or charged interactions implies that Gamma-Sitosterol binds primarily via hydrophobic contacts. The co-crystallized ligand exhibits a rich and diverse set of interactions, including hydrogen bonding and electrostatic interactions, which are typically associated with strong and specific binding. In contrast, Gamma-Sitosterol relies heavily on hydrophobic interactions (alkyl and van der Waals), which might lead to lower binding specificity but potentially favorable affinity in hydrophobic pockets.
Male reproductive toxicity induced by cimetidine, a histamine H2 receptor antagonist drug, commonly used to treat conditions like acid reflux and peptic ulcers, has been widely reported to cause spermatogenic disruption, reduced sperm count, motility, and viability. To assess the potential protective effects of Brassica oleracea var. viridis ethanol extracts and its fractions, sperm motility, viability, morphology, total sperm count, and agglutination were evaluated in Wistar rats exposed to cimetidine.
The results, depicted in Figures 10–14 below shows that treatment with ethanol extracts and solvent fractions of Brassica oleracea var. viridis significantly improved sperm motility, viability, and count, compared to the cimetidine-treated group. Notably, Ethanol fractions (AFBO+CTD), (ELEBO+CTD) (BFBO+CTD)(HFBO+CTD) and ethanol (ELEBO) demonstrated the most pronounced improvements in sperm health parameters when compared with the cimetidine group (toxic group), suggesting a protective or restorative effect against cimetidine-induced reproductive toxicity.
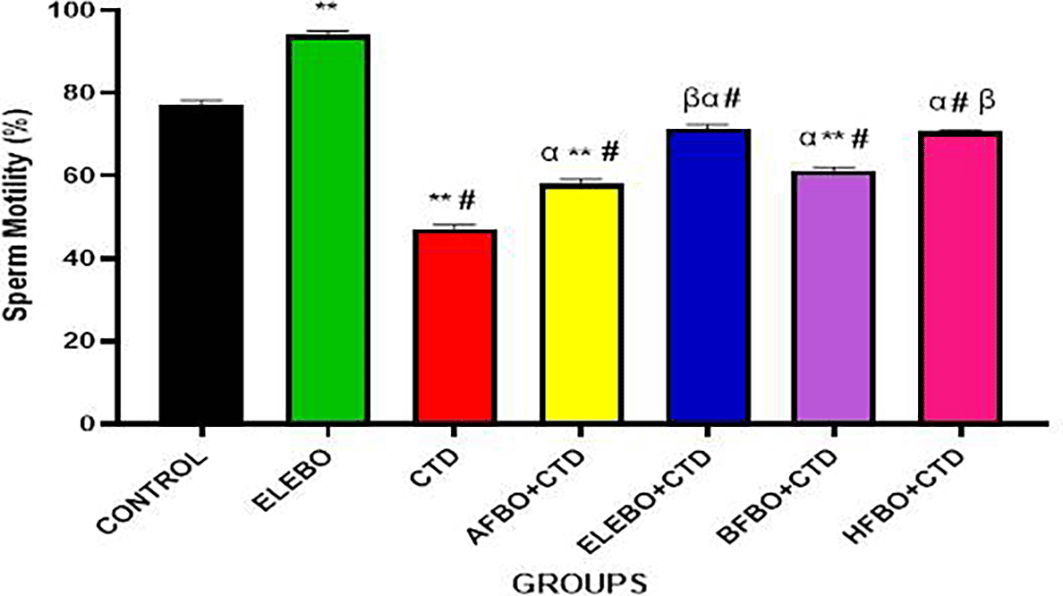
Data are presented as mean ± S.E.M (n = 5).
Statistical analysis was performed using Tukey's multiple comparisons test. There was a significant decrease in sperm motility in the cimetidine treated group when compared with the control; however this effect was reversed in the groups that received the solvent fractions groups. There was a significant increase in sperm motility in the ELEBO group when compared with other fractions at (p < 0.05) ** = p < 0.0001 compared with control, # = p < 0.0001 compared with ELEBO, α = p < 0.0001 compared with CTD, β = p < 0.0001 compared with AFBO+CTD A- control, ELEBO (Ethanol leaf Extract of Brassica oleracea), CTD (cimetidine), AFBO+CTD (Aqueous fraction of Brassica oleracea + Cimetidine) ELEBO +CTD (Ethanol leaf Extract of Brassica oleracea + Cimetidine), BFBO+CTD (n-Butanol fraction of Brassica oleracea + Cimetidine), HFBO+CTD (n-Hexane fraction of Brassica oleracea + Cimetidine).
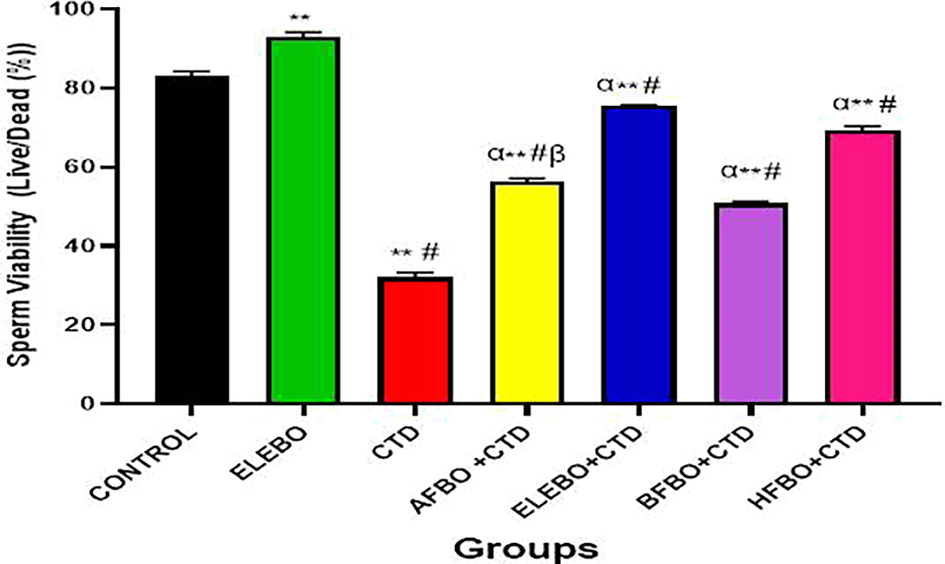
Data are shown as mean ± S.E.M (n = 5). Mean values were compared among one another using the Turkey multiple comparisons test. There was a significant decrease in sperm viability in the cimetidine treated group when compared with the control, however this effect was reversed in the groups that received the solvent fractions groups. There was a significant increase in sperm viability in the ELEBO group when compared with other fractions at (p < 0.05), ** = p <0.0001 compared with control, # = p <0.0001 compared with ELEBO, α = p<0.0001 compared with CTD, β = p <0.0001 compared with ELEBO+CTD, HFBO+CTD. A- Control, ELEBO (Ethanol leaf Extract of Brassica oleracea), CTD (cimetidine), AFBO+CTD (Aqueous fraction of Brassica oleracea + Cimetidine) ELEBO +CTD (Ethanol leaf Extract of Brassica oleracea + Cimetidine), BFBO+CTD (n-Butanol fraction of Brassica oleracea + Cimetidine), HFBO+CTD (n-Hexane fraction of Brassica oleracea + Cimetidine).
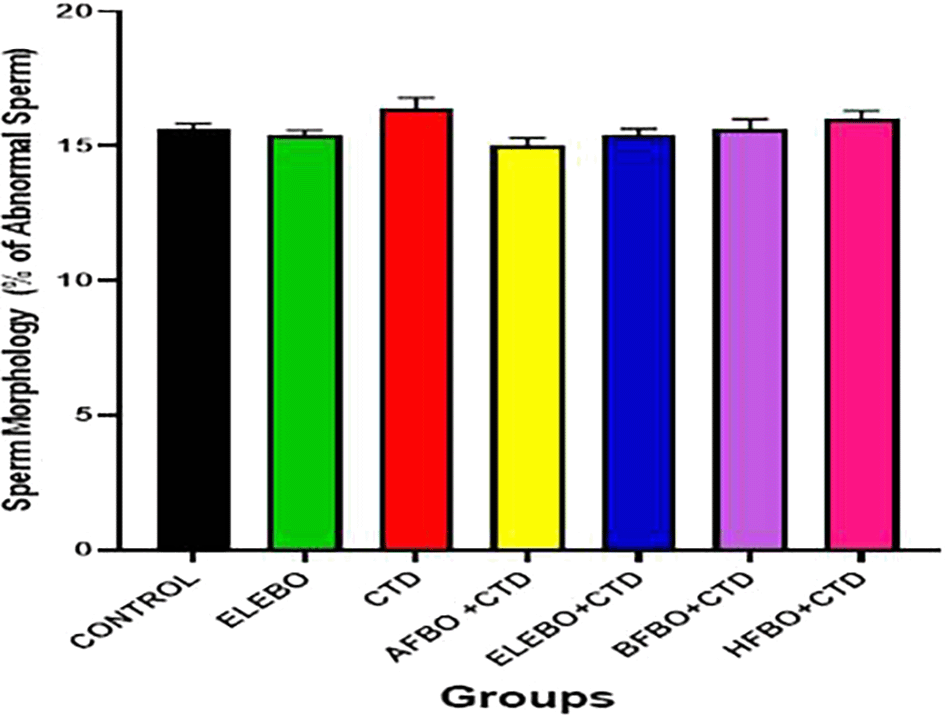
Administration of cimetidine in Wistar albino rats showed no significant (p > 0.0808) difference when compared with all groups.
Data are shown as mean ± S.E.M (n = 5).
Mean values are considered to be significant at p < 0.05. A- Control, ELEBO (Ethanol leaf Extract of Brassica oleracea), CTD (cimetidine), AFBO+CTD (Aqueous fraction of Brassica oleracea + Cimetidine) ELEBO +CTD (Ethanol leaf Extract of Brassica oleracea + Cimetidine), BFBO+CTD (n-Butanol fraction of Brassica oleracea + Cimetidine), HFBO+CTD (n-Hexane fraction of Brassica oleracea + Cimetidine).
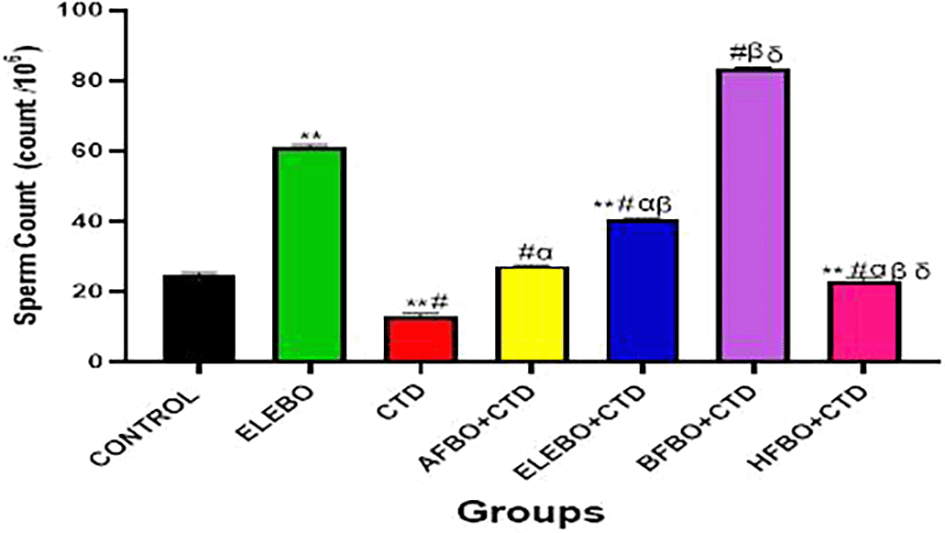
Data are shown as mean ± S.E.M (n = 5).
Mean values were compared among one another using the Turkey multiple comparisons test, revealing significant differences at p<0.05,
There was a significant decrease in sperm count in the cimetidine treated group when compared with the control; however this effect was reversed in the groups that received the solvent fractions groups. ELEBO and BFBO+CTD shows most significant increase when compared with other solvent fraction groups ** = p <0.0001 compared with control, # = p <0.0001 compared with ELEBO, α = p<0.0001 compared with CTD, β = p <0.0001 compared with AFEBO+CTD, δ = p <0.0001 compared with ELEBO+CTD.
A- Control, ELEBO (Ethanol leaf Extract of Brassica oleracea), CTD (cimetidine), AFBO+CTD (Aqueous fraction of Brassica oleracea + Cimetidine) ELEBO +CTD (Ethanol leaf Extract of Brassica oleracea + Cimetidine), BFBO+CTD (n-Butanol fraction of Brassica oleracea + Cimetidine), HFBO+CTD (n-Hexane fraction of Brassica oleracea + Cimetidine).
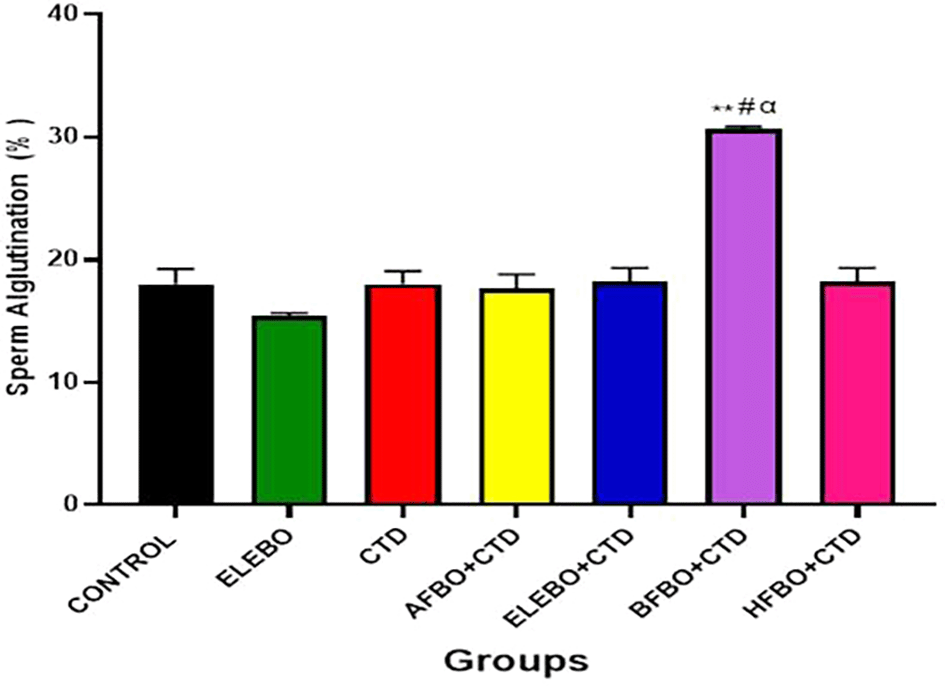
Data are shown as mean ± S.E.M (n = 5). Mean values were compared among each group using the Turkey multiple comparisons test, revealing significant differences at p < 0.05, there was no significant differences between the cimetidine group and the control, BFBO showed significant increase in sperm agglutination when compared with all groups at (p < 0.05) ** = p < 0.0001 compared with control, # = p < 0.0001 compared with ELEBO, α = p < 0.0001 compared with CTD. A- Control, ELEBO (Ethanol leaf Extract of Brassica oleracea), CTD (cimetidine), AFBO+CTD (Aqueous fraction of Brassica oleracea + Cimetidine) ELEBO +CTD (Ethanol leaf Extract of Brassica oleracea + Cimetidine), BFBO+CTD (n-Butanol fraction of Brassica oleracea + Cimetidine), HFBO+CTD (n-Hexane fraction of Brassica oleracea + Cimetidine).
The statistical analysis revealed significant differences (p < 0.05) in sperm parameters across treatment groups, with marked improvements observed in sperm motility ( Figure 10), sperm viability ( Figure 11), and sperm count ( Figure 13) following administration of B. oleracea var. viridis extracts and fractions. Figure 12 illustrates sperm morphology findings, which indicate that extract treatment mitigated structural sperm abnormalities induced by cimetidine. Lastly, Figure 14 highlights the effects on sperm agglutination, where treatment with extracts reduced pathological sperm aggregation, a known factor in reduced fertility.
These findings suggest that bioactive compounds in Brassica oleracea var. viridis may exert spermatoprotective effects, possibly through antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. Further investigations into the underlying molecular mechanisms are warranted.
The prevalence of male infertility due to drug-induced damage and oxidative pressure with hormonal disruption presents significant health problems.5,30,31 Histamine H2 receptor antagonist cimetidine causes spermatogenesis disruption that then lowers sperm count and reduces sperm motion and survival rates and produces more abnormalities alongside agglutination effects.32,33
This current research utilized Brassica oleracea var. viridis (B. oleracea var. viridis) ethanol extracts and fractions to study their spermatoprotective impact on cimetidine-induced reproductive toxicity in Wistar rats. The in-silico component of our study evaluates the physiochemical, drug-likeness and pharmacokinetic properties of phytocompounds identified abundantly in B. oleracea var. viridis. The in-silico molecular docking study was carried out to evaluate the ligand-receptor interactions of Gamma-sitosterol when docked against AKT1 and EGFR proteins. AKT1 is a key signaling molecule in the PI3K/AKT/mTOR pathway, which regulates cell proliferation and survival.34 Over activation of AKT1 leads to increased oxidative stress, apoptosis of spermatogenic cells, and disruption of Sertoli cell function.34 The inhibition of AKT1 reduces oxidative stress, restores Sertoli cell support, and promotes spermatogonia differentiation.35
The EGFR/ERK pathway plays a role in cell cycle regulation and testicular function. Hyperactivation of EGFR leads to inflammation, fibrosis, and impaired spermatogenic niche.36 EGFR inhibition helps reduce inflammation, restore blood-testis barrier integrity, and improve germ cell proliferation as shown in Figure 1.37
The in-vivo sperm analysis evaluates the reproductive potential by measuring sperm motility, viability, morphology, and total count and agglutination.
This research combined experimental methods and computational techniques to reveal the therapeutic potential, spermatoprotective effects, and fertility-enhancing abilities of B. oleracea var. viridis. This establishes a connection between traditional medicine and contemporary pharmacology as male reproductive health solutions.
Bioactive compounds need satisfactory physicochemical properties as well as drug-likeness and lipophilicity and solubility to achieve effective pharmacokinetic and pharmacodynamic characteristics.32,37 This study reported substantial findings regarding B. oleracea var. viridis selected compounds’ therapeutic capability alongside Blood-Brain Barrier (BBB) permeability.
The evaluated compounds in this study showed good drug-likeness properties since they adhere to the parameters established by Lipinski’s Rule of Five (RO5) which supports their potential for oral delivery. Lipinski’s Rule of Five helps predict oral bioavailability by assessing molecular weight, lipophilicity (LogP), and the number of hydrogen bond donors and acceptors.38 Compounds meeting these criteria are more likely to be absorbed efficiently, making the rule essential in early drug discovery.38,40
From B. oleracea var. viridis, phytocompounds Gamma-Sitosterol and Phytol achieved ideal molecular weights suitable for their use as pharmacological compounds. The data supports body acceptance and distribution of these compounds which confirms their potential as medical agents.38,39 However, the high molecular weight of Bacteriochlorophyll-c-stearyl and Hexatriacontane identified in this plant exceeded optimal thresholds so these compounds may face absorption barriers and reduced availability throughout the body.
The fundamental nature of lipophilicity influences drug distribution within the body while also affecting membrane permeability of drugs.41,42 Matrix interactions and permeability through the BBB expressed by Gamma-Sitosterol due to its moderate lipophilicity suggests both efficient cellular uptake and receptor binding. Higher WLogP values, especially among Hexatriacontane indicate excessive lipophilicity that leads to drug accumulation in tissues and results in problematic water solubility which hinders therapeutic use.43 Compounds including 1-(cyclopropylcarbonyl) piperidin-3-amine showed marked hydrophilicity through their -0.10 WLogP value, resulting in a better solution but administration might need modification of formulations due to a potential decrease in permeability.
Drug absorption depends greatly on solubility and the study discovers potential drug candidates with suitable solubility properties.44,45 The compound 6-Isobutyryl-2,2,4,4-tetramethylcyclohexane-1,3,5-trione exhibited an outstanding solubility showing potential for excellent absorption. The very low solubility level of Hexatriacontane may require lipid-based delivery systems to improve bioavailability when used as an active pharmaceutical ingredient. This aligns with previous reports by46,47 highlighting that compounds or therapeutic agents with low solubility require lipid-based delivery systems to enhance substantial bioavailability.
The metabolic stability along with the binding capacity to targets depends on the strength of hydrogen bonding.48 Hexatriacontane and gamma-Sitosterol displayed weak hydrogen bond potential that leads to reduced drug solubility and bioavailability regardless of their strong membrane binding properties. Phenol, 4-ethenyl-2,6-dimethoxy- shows both moderate hydrogen bonding potential and optimal WLogP value allowing potential BBB and CNS access.
Two drug development candidates with anti-inflammatory, antioxidant and cardioprotective properties identified in B. oleracea var. viridis include 9,12,15-Octadecatrienoic acid and gamma-Sitosterol. This suggests strong therapeutic potential. However, the therapeutic achievements and effectiveness of bioactive fatty acids and sterols could be enhanced by utilizing prodrug modifications together with nanoparticle-based delivery systems alongside lipid encapsulation for improving their bioavailability.49,50 Our research findings validate the existing knowledge about the essential equilibrium between drug solubility, lipophilicity and metabolic stability of compounds identified in B. oleracea var. viridis when considering drug development processes.
Concerning pharmacokinetic analyses, the studied compounds demonstrated suitable characteristics for medical applications when used in drug development and delivery systems. Most B. oleracea var. viridis compounds demonstrated excellent gastrointestinal absorption, indicating they can enter the bloodstream easily after swallowing. These predictions revealed that 6-Isobutyryl-2,2,4,4-tetramethylcyclohexane-1,3,5-trione, Phenol, 4-ethenyl-2,6-dimethoxy- along with 2,2,4,4-Tetramethyl-6-(2-methylbutanoyl) cyclohexane-1,3,5-trione exhibit strong absorption together with blood-brain barrier permeability. The proposed compounds have demonstrated suitable characteristics for neural and protective brain usage, highlighting promising delivery options for central nervous system disorders.
Cytochrome P1A2 (CYP1A2) inhibitors modulate the metabolism of drugs and endogenous compounds, offering therapeutic potential in reducing drug clearance, enhancing bioavailability, and minimizing toxic metabolite formation.51,52 The inhibitory effect of Pentadecanoic acid together with Dichloroacetic acid tridec-2-ynyl ester on CYP1A2 offers potential applications in drug metabolism control and drug interaction management. This suggests that these two compounds might potentially optimize drug therapies because they modify metabolic pathways particularly when administered with restricted therapeutic ranges. Hexatriacontane showed excellent skin permeability properties which suggests suitability for transdermal drug delivery systems.
Although these favorable properties exist some essential restrictions need to be taken into account. Phytol Hexatriacontane and gamma-Sitosterol showed poor GI absorption rates; hence, their system wide effectiveness may need advanced drug delivery systems comprising nanocarriers together with lipid-based formulations. Analysis showed that Phytol and Hexatriacontane act as P-glycoprotein (P-gp) substrates. Compounds with such profiles have been previously reported by9,53 to potentially leading to reduced bioavailability as well as deteriorated therapeutic effectiveness because of drug resistance through efflux mechanisms. This challenge can be achieved through liposomal encapsulation combined with structural modifications to enhance drug retention and absorption.54
This pharmacokinetic assessment demonstrates that these compounds present various therapeutic applications from CNS potency to permeable skin and metabolism regulators. The majority of compounds in B. oleracea var. viridis demonstrate encouraging pharmacokinetic properties, which support further research into pharmaceutical development, even if some structural improvements and formulation upgrades might be needed.
Using the molecular docking approach, this research generates important knowledge about B. oleracea var. viridis bioactive compounds which display upregulating properties against the signaling proteins; AKT serine/threonine kinase 1 (AKT1) and Epidermal Growth Factor Receptor (EGFR) that control male reproductive health. AKT1 plays a crucial role in sperm function by regulating cell survival, metabolism, and motility.55,56 It is involved in spermatogenesis, protecting germ cells from oxidative stress and ensuring proper sperm development.56 AKT1 also influences acrosome reaction and capacitation, key processes required for fertilization. Dysregulation of AKT1 signaling is linked to impaired sperm function and male infertility.57 Similarly, EGFR is a transmembrane protein that plays a crucial role in cell growth, survival, and differentiation. In sperm function, EGFR signaling is involved in sperm maturation, motility, and the acrosome reaction, which is essential for fertilization.58 Dysregulation of EGFR activity has been associated with impaired sperm function and male infertility.59
When docked against both AKT1 and EGFR targets, gamma-sitosterol showed the best binding properties with molecular docking energy values comparable to those of the co-crystallized ligands. This implies that gamma-sitosterol shows the potential to control these targets, which affects important pathways involved in spermatogenesis and male fertility. Phytol and 7,10,13-hexadecatrienoic acid showed intermediate binding capacities, suggesting it could influence the molecular binding process.
The two-dimensional (2D) interaction diagrams displayed essential hydrogen bond interactions together with hydrophobic forces that maintain the stability of ligand-receptor bonding systems. Concerning AKT1, Gamma-sitosterol formed hydrogen bonds with Asparagine (ASN), Glutamine (GLN), Serine (SER), and Tyrosine (TYR). These amino acids play key roles in stabilizing ligand binding and influencing AKT1’s biological activity. Similarly with EGFR, Gamma-sitosterol formed hydrogen bonds with Asparagine (ASN), Glutamine (GLN), Serine (SER), and Threonine (THR). These interactions contribute to the stability and potential modulatory effects of gamma-sitosterol on EGFR signaling pathways.
The research discoveries create important opportunities for pharmaceutical development aimed at creating therapeutics from plant sources for reproductive health conditions. Gamma-sitosterol demonstrated robust affinity toward AKT1 and EGFR receptors, which implies its ability to control essential cellular pathways linked to cell development and testicular function regulation. The signaling pathways of AKT1 and EGFR play vital roles in cell survival and programmed cell death regulation thus their disrupted functions lead to male infertility especially in situations that cause testicular dysfunction and oxidative stress.56,57,59 Gamma-sitosterol demonstrates upregulating properties toward these targets in keeping with research showing phytosterols enhance male reproductive features, including sperm motility and testosterone regulation.
Plant-derived compounds demonstrate superior potential as alternative activators against synthetic drugs because they present both biological compatibility and decreased susceptibility to adverse reactions. The molecular docking predictions of binding interactions need laboratory testing at the cellular and animal levels to prove biological properties and therapeutic value. Between its merits, the current research demonstrates extensive computational methods to support experimental work in the future. The research lacks experimental proof through enzymatic assays or reproductive toxicity studies to determine the full effectiveness and safety levels of these compounds.
Future research must concentrate on studying the pharmacokinetic properties as well as bioavailability of these compounds in biological environments. A detailed study of how these substances influence hormone control together with sperm characteristics and testicular tissue structure would establish their prospective use as male fertility treatment options. The study brings together experimental and computational approaches to support existing research in natural reproductive health while creating possibilities for creating next-generation nature-based fertility treatments.
In the in-vivo experiment evaluating key sperm parameters such as sperm motility, viability, morphology, count and agglutination; B. oleracea var. viridis ethanol extracts and fractions showed strong spermatoprotective and restorative properties against male reproductive toxicity in Wistar rats exposed to cimetidine. The histamine H2 receptor antagonist cimetidine causes substantial damage to spermatogenesis and leads to decreased sperm count together with reduced motility and viability.33
Testicular abnormalities stem from oxidative stress along with hormonal imbalances and drug-induced testicular toxicity and immune-mediated damage.60,61
Putting together, the fertilization potential of sperm cells increased substantially after treatment with B. oleracea var. viridis’ ethanol leaf extract (ELEBO) and its fractions (AFBO+CTD, BFBO+CTD, HFBO+CTD) showing the most significant improvement. The drug cimetidine disrupts mitochondria function which results in depleted ATP levels and impaired flagellar movement and thus causes sperm motility reduction.62 B. oleracea var. viridis enhanced motility in sperm cells implying a potential function in mitochondrial bioenergetics and oxidative stress management. This finding aligns with a previous report by63 showing that B. oleracea var. viridis can decrease Reactive Oxygen Species (ROS) accumulation while showing antioxidant properties.
The extract treatments resulted in significant improvements of sperm viability by enhancing the proportion of live functioning spermatozoa. The testicular damage from cimetidine treatment causes germ cell apoptosis and Sertoli cell malfunction that ultimately reduces total sperm survival.33 The enhanced viability results indicate that bioactive compounds from B. oleracea var. viridis have protective effects against cell death which may be achieved through their influence on PI3K/Akt survival signaling pathways. The major phytosterol gamma-sitosterol found in B. oleracea var. viridis suggests cell survival regulatory properties because it bounded strongly to Akt1 which serves as a fundamental kinase for sperm cell proliferation and differentiation.
The total sperm count improved significantly after treatment with B. oleracea var. viridis ethanol extracts and fractions. Sperm count is a marker that plays a vital role in spermatogenesis efficiency and male fertility.64,65 Cimetidine which exhibits antiandrogenic properties disrupts the hypothalamic-pituitary-gonadal (HPG) axis to reduce testosterone synthesis and causes impaired spermatogenesis and oligospermia.66,67 Our analysis suggestss that B. oleracea var. viridis exhibits endocrine regulatory properties since it restored sperm count in test groups. This might be through potential effects on steroidogenic enzymes and androgen receptors.
The sperm count enhancement was most significant when using the (BFBO+ CTD), ELEBO (ethanol extract) and (ELEBO+CTD) treatment which showed superior results when combined with other treatment groups (BFBO+ CTD). Shows most significant increase in sperm count may be possibly due to lipophilic compounds that better penetrate and protect testicular tissue. Future studies should focus on elucidating its precise mechanisms particularly those involving steroid biosynthesis, redox regulation, and androgen receptor signaling.
The research holds significant value because oligospermia stands as the primary reason behind male infertility yet pharmacological treatments are scarce. The sperm production restorative capability of B. oleracea var. viridis positions it as a promising natural remedy against male reproductive toxicity. Future investigations should analyze the complete therapeutic value of this plant in male infertility treatment by investigating its mechanisms that affect steroid biosynthesis and oxidative stress management and androgen receptor signaling pathways.
The essential fertility parameter, sperm morphology showed favorable changes in animals administered B. oleracea var. viridis. Structural abnormalities of sperm head, midpiece and tail arise from oxidative damage and improper chromatin packaging and disrupted cytoskeletal elements.68 The protective compounds found in B. oleracea var. viridis seem to safeguard DNA and stabilize chromatin which leads to normal spermatozoa morphology.
Sperm agglutination represents a vital yet underrecognized male fertility factor because sperm clumping affects their movement and makes fertilization less likely.69,70 Sperm agglutination typically occurs as a result of both immune-mediated infertility, the presence of antisperm antibodies and excessive ROS production.70,71 The substantial decrease in sperm agglutination findings from this research demonstrates that B. oleracea var. viridis may have anti-inflammatory and immunomodulatory properties that block the development of autoantibodies against sperm antigens.
The in-silico and in-vivo study results validate B. oleracea var. viridis as a plant containing phytocompounds with spermatoprotective properties. Gamma-sitosterol expressed sperm function-enhancing ability through reproductive signaling modulation and sperm analytical parameter improvement and oxidative stress reduction. The strong binding abilities between Gamma-sitosterol and AKT1 and EGFR receptors demonstrate its significance in spermatogenesis. The natural therapy of B. oleracea var. viridis shows promise as an effective solution for sperm health improvement and reproductive dysfunction treatment because of increasing infertility rates and limited existing treatment options.
This study provides compelling evidence that bioactive compounds in Brassica oleracea var. viridis may serve as potential inhibitors of AKT1 and EGFR, key regulators of male spermatogenesis. Through an integrated in silico and in vitro approach, we identified gamma-sitosterol as a lead compound with high binding affinity and drug-like properties, supporting its role as a natural modulator of reproductive signaling pathways.
The protective effects observed in cimetidine-induced reproductive toxicity models demonstrate the potential of B. oleracea extracts to enhance sperm quality and mitigate oxidative stress-induced damage. Notably, the ethanol (ELEBO), BFBO and AFBO (Aqueous fractions of brassica Oleracea) fractions exhibited the greatest improvements in sperm motility, viability, and count, reinforcing their therapeutic relevance.
These findings highlight the potential of dietary phytochemicals in managing male infertility and lay the foundation for further studies on the pharmacological mechanisms underlying their bioactivity. Future research should focus on validating these effects in clinical settings and exploring synergistic interactions with other therapeutic agents. This study advances the understanding of plant-based bioactive compounds in reproductive health and paves the way for the development of novel, cost-effective interventions for male infertility.
In light of the findings from this study, it is advisable that both preclinical and clinical trials be pursued to further establish the efficacy and safety of Brassica oleracea var. viridis extracts—particularly gamma-sitosterol—as promising therapeutic agents for male infertility. The significant improvements observed in sperm quality following treatment with the ethanol (ELEBO), BFBO and AFBO (Aqueous fractions of brassica Oleracea) fractions underscore their potential for development into natural, plant-based supplements. Furthermore, the creation of standardized nutraceutical formulations containing these bioactive compounds could provide a cost-effective and accessible strategy for addressing male reproductive dysfunction. Continued research should also investigate possible synergistic interactions with current fertility therapies and further elucidate the molecular mechanisms underlying their biological activity.
The authors confirm that all guidelines set by the University’s research ethics for plant collection, characterization, and documentation was duly followed. The plant specimen was identified by the Department of Botany, Faculty of Science, Mbarara University of Science and Technology, Uganda. The experimental protocols received approval from the Kampala International University Research Ethics Committee with the REC number (KIU-2024-389) and also Uganda National for Science and Technology with registration number (HSF5192ES) was also obtained. The plant collection process adhered to local guidelines and does not require further confirmation.
We confirm that all individuals named in the Acknowledgments and Methods sections have agreed to the inclusion of their names and institutional affiliations in this manuscript.
Open Science Framework: Unveiling the Fertility Potential of Brassica oleracea: In Silico and in vivo Insights into Protein Kinase B (PKB/AKT1) and Epidermal Growth Factor Receptor (EGFR) Inhibition. https://doi.org/10.17605/OSF.IO/3EXFW.73
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Conceptualization and Design: Emmanuel Orire Ikuomola. Data Curation: Emmanuel Orire Ikuomola. Methodology: Emmanuel Orire Ikuomola and Daniel Udofia Owu. Resources: Emmanuel Orire Ikuomola, Victor Otu Oka, Uthman Shehu and Ibe Micheal Usman, Writing - Original Draft: Emmanuel Orire Ikuomola and Ilemobayo Victor Fasogbon. Writing - Review & Editing: Emmanuel Orire Ikuomola, Ilemobayo Victor Fasogbon, Ekom Monday Etukudo and Patrick Maduabuchi Aja. Validation: Daniel Udofia Owu, Victor Otu Oka and Ismahil Adekunle Adeniyi. Supervision: Daniel Udofia Owu, Victor Otu Oka, Ibe Micheal Usman and Patrick Maduabuchi Aja. Final Approval of the Version to be Published: Emmanuel Orire Ikuomola, Daniel Udofia Owu, Victor Otu Oka, and Patrick Maduabuchi Aja.
Open Science Framework: Unveiling the Fertility Potential of Brassica oleracea: In Silico and in vivo Insights into Protein Kinase B (PKB/AKT1) and Epidermal Growth Factor Receptor (EGFR) Inhibition. https://doi.org/10.17605/OSF.IO/3EXFW.73
This project contains the following extended data
• AKT1-sorted.csv
• AKT1-sorted.xlsx
• EGFR-sorted.csv
• EGFR-sorted.xlsx
• ARRIVE Checklist - Full.docx
• IKUOMOLA EMMANUEL DATA FILE F1000.docx
• Table 2…….docx
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nutritional Genomics and Molecular Nutrition
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 09 Jul 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)