Keywords
PM2.5, Air pollution, Depression, Pregnant women, Meta-Analysis
This article is included in the Public Health and Environmental Health collection.
Exposure to particulate matter (PM2.5) has emerged as a significant risk factor for depression, particularly among pregnant women. This systematic review and meta-analysis investigate the association between PM2.5 exposure and maternal depression during pregnancy. Adhering to PECO principles and PRISMA 2020 standards, we searched PubMed, Google Scholar, and Science Open, identifying 231 records (Google Scholar: 28; Science Open: 106; PubMed: 58; and 39 from manual searches). After removing 49 duplicates, 182 studies were screened, excluding 110 via title/abstract review. Of 72 full-texts assessed, 65 were excluded (48 for irrelevant outcomes, 14 for incompatible metrics, 3 for lacking PM2.5-specific data), yielding 7 studies (n = 576,737 pregnancies) for meta-analysis. Using Cochran’s Q and I2 statistics (I2 = 85.1%, τ2 = 0.027), a restricted maximum likelihood (REML) random-effects model estimated a pooled relative risk (RR) of 1.29 (95% CI: 1.10–1.50) per 10 μg/m3 increase in PM2.5, indicating a 29% higher risk of depression. Subgroup analyses showed stronger effects across whole pregnancy (RR = 1.41, 95% CI: 1.23–1.63). High heterogeneity (I2 = 85.1%) and publication bias (Egger’s p = 0.013) were noted. These findings highlight the impact of PM2.5 on maternal depression, urging further mechanistic studies, especially in low-income regions where data remains scarce.
PM2.5, Air pollution, Depression, Pregnant women, Meta-Analysis
Air pollution is characterized by the presence of chemical, physical, or biological agents that degrade air quality and poses a significant global environmental health threat.1,2 Key air pollutants, including particulate matter (PM2.5, PM10), carbon monoxide, ozone, nitrogen dioxide, and sulphur dioxide, originate from human activities (e.g., vehicle emissions, industrial operations) and natural sources (e.g., wildfires).3 PM2.5 with a diameter of ≤2.5 micrometers, is particularly hazardous due to its ability to penetrate deep into the lungs and bloodstream which contributes to millions of premature deaths annually from cardiovascular and respiratory diseases.4,5
Emerging evidence links air pollution, especially PM2.5, to adverse mental health outcomes, including neuroinflammation, Alzheimer’s disease, Parkinson’s disease, and impaired cognitive function.6,7 Prenatal exposure to PM2.5 has been associated with behavioural issues in children, such as ADHD,8 and increased risks of postpartum depression (PPD).9,10 For instance,9 reported a 3.86-fold increased risk of PPD with elevated nitrogen dioxide exposure (weeks 13–29), while10 identified PM2.5 as a modifiable risk factor for PPD in a cohort of 340,679 pregnant women.
Maternal mental health is critical to reproductive health, with depression which is a prevalent disorder marked by persistent low mood and reduced interest has been linked to adverse outcomes such as preterm birth and impaired infant development.11,12 Despite growing evidence of PM2.5’s impact on mental health, research specifically addressing its effects on depression in pregnant women remains limited, with methodological challenges and unclear mechanisms hindering progress This systematic review aims to synthesize existing evidence to clarify the relationship between PM2.5 exposure and depression in pregnant women, addressing gaps in current literature and informing targeted public health interventions to protect this vulnerable population.
This systematic review and meta-analysis (SRMA) adhered strictly to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines to ensure transparency and reproducibility.13 The research questions were formulated using the PECO framework (Participants: pregnant women; Exposure: PM2.5; Comparison: varying levels of PM2.5 exposure; Outcome: maternal depression), following established methodologies for environmental health reviews.14 Does exposure to PM2.5 influence the risk of depression in pregnant women?
A comprehensive literature search was conducted to identify studies published between January 2015 and January 2025, focusing on recent evidence to reflect current PM2.5 exposure levels and diagnostic standards for depression. Earlier studies were excluded due to potential inconsistencies in exposure assessment methods and lower relevance to contemporary air quality standards. Three databases were searched: PubMed, Open Science, Google Scholar, and a manual search of reference lists. The search initially used broad terms such as “air pollution” and “particulate matter” to capture relevant studies, followed by specific terms including “PM2.5,” “fine particulate matter,” “maternal depression,” “postpartum depression,” “pregnant women,” and “prenatal exposure.” These were combined using Boolean operators (AND, OR, NOT) to enhance precision. In PubMed, Medical Subject Headings (MeSH) terms (e.g., “Air Pollution”[MeSH Terms] AND “Depression”[MeSH Terms]) were incorporated alongside keywords, with equivalent terms adapted for Embase and Google Scholar to ensure consistency across databases ( Table 1). Manual searches of reference lists from included studies supplemented the database search to ensure a thorough collection of relevant literature.
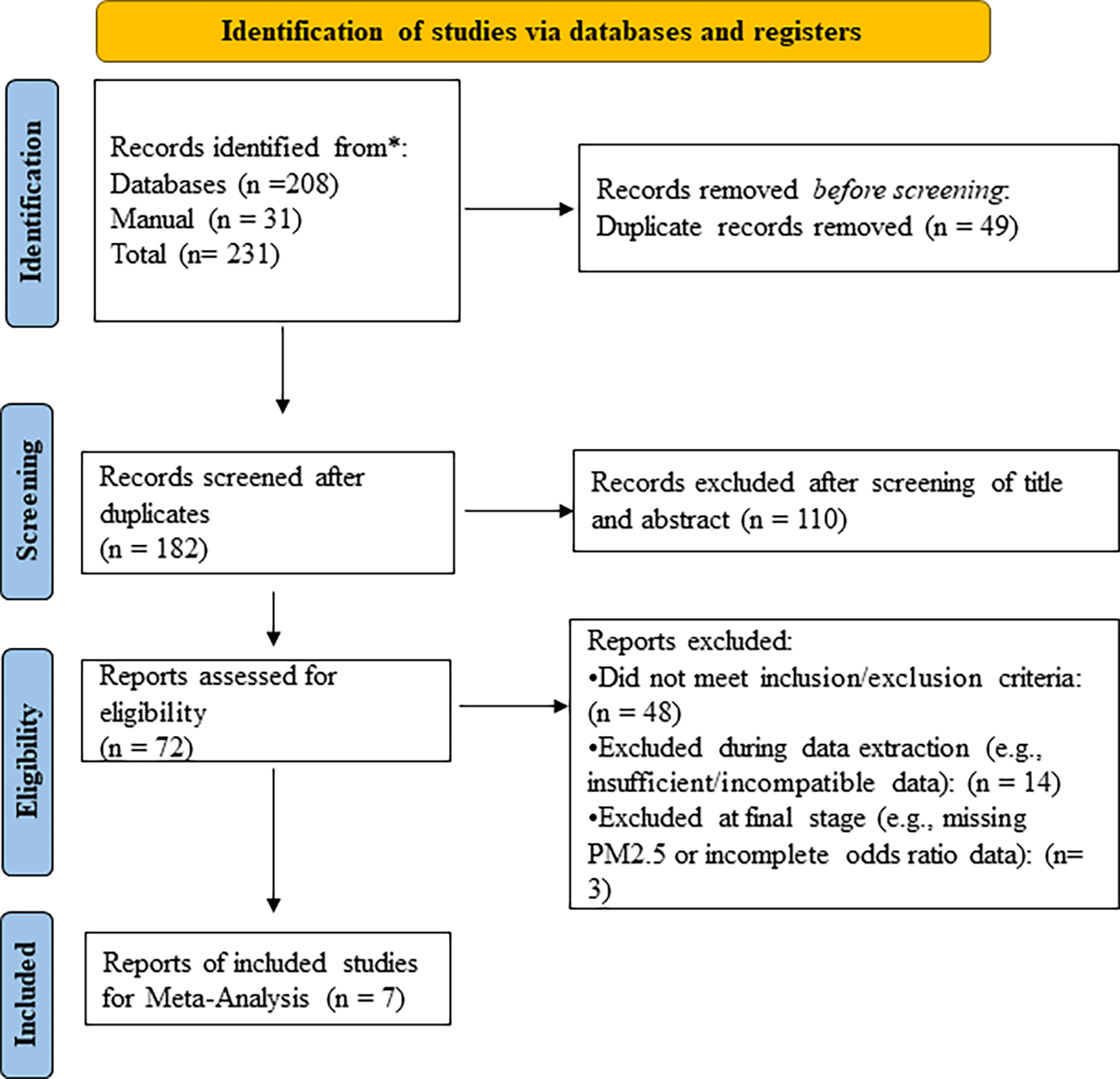
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Studies were included if they: (1) were original articles published between 2015 and 2025; (2) assessed outdoor PM2.5 exposure using ambient air quality data or modeled estimates (personal exposure monitors were not used in included studies); (3) reported maternal depression outcomes (diagnosed via clinical criteria, e.g., DSM-5, or validated scales, e.g., Edinburgh Postnatal Depression Scale [EPDS] ≥ 13); (4) focused on pregnant women; (5) were written in English; and (6) had five or more citations to ensure a minimum level of peer recognition. Exclusion criteria included: (1) reviews or studies on indoor air pollution; (2) studies not specifically addressing PM2.5 (e.g., focusing on PM10 or NO2); (3) studies reporting non-depressive outcomes (e.g., anxiety); (4) studies published before 2015; (5) studies with fewer than five citations; and (6) non-English studies. Social media-related studies were not considered, as they were irrelevant to the research question. The selection criteria are tabulated on Table 2 below.
The search identified 208 studies across databases (Google Scholar: 28; Science Open: 106; PubMed: 58) and 39 additional studies via manual search, totalling 231 studies. After removing 49 duplicates, 182 studies remained. Screening titles and abstracts excluded 110 studies, leaving 72 for full-text review. Of these, 48 were excluded based on predefined inclusion/exclusion criteria (e.g., irrelevant outcomes, non-pregnant populations, or non-depression focus). During data extraction, 14 additional studies were excluded due to incompatible exposure metrics (e.g., PM2.5 not specifically measured) or insufficient data for effect size conversion (e.g., missing sample sizes or variance estimates). This left 10 eligible studies, of which 7 were ultimately included in the meta-analysis. Three studies were excluded at the final stage: 2 lacked PM2.5-specific data, and 1 reported odds ratios (ORs) without sufficient data (e.g., sample sizes, confidence intervals) to convert to risk ratios (RRs) using standard methods.15 A PRISMA flow diagram detailing the selection process is provided ( Figure 1).
Figure 1: Summarizes each of the selected articles that are included in this review.
For each included study, the following were extracted using a predefined form: author’s first name, year of publication, location/country, participant details, research design, sample size, perinatal exposure window, outcome definition (e.g., clinical diagnosis, EPDS score), mean/median exposure duration, adjustment variables, and effect estimates (RRs) with 95% confidence intervals (CIs). Quality was assessed using the Effective Public Health Practice Project (EPHPP) tool, which evaluates study design, selection bias, confounders, and data collection reliability.
Exposure data were standardized to a 10 μg/m3 PM2.5 increment by converting reported effect sizes (e.g., using linear scaling for studies reporting per 5 μg/m3). Outcomes were harmonized by including only studies reporting clinical depression diagnoses or validated scale cutoffs, ensuring consistency. All effect sizes were standardized to RRs; studies reporting ORs were converted to RRs where possible using the method of.15 When studies reported both unadjusted and adjusted RRs, adjusted estimates were used. For studies with multiple adjusted models, the model with the largest number of covariates was selected to minimize confounding bias.
A random-effects model (DerSimonian-Laird) was used to pool RRs, accounting for between-study heterogeneity, which was assessed using Cochran’s Q statistic, I2, and Tau2. I2 values were reported to indicate the proportion of variance due to heterogeneity, with I2 ≥ 50% considered substantial. Subgroup analyses by exposure window (first, second, third trimesters, whole pregnancy) and region (e.g., China, USA) explored heterogeneity sources. Leave-one-out sensitivity analysis evaluated the robustness of the pooled estimate. Publication bias was assessed using funnel plots, Egger’s test, and Begg’s test, with significant bias (Egger’s p < 0.05) noted but not adjusted due to the small sample size (n = 7). Quality-effects meta-regression was performed using weighted least squares (WLS) with Newcastle-Ottawa Scale (NOS) scores and sample size as covariates, despite the small sample size, to assess quality effects, acknowledging limitations (e.g., invalid Omnibus test). Analyses were conducted in Python using statsmodels and visualized with plotly.
The Cochrane Risk of Bias tool was initially considered but deemed less applicable for observational studies. Instead, the NOS was used to assess study quality, focusing on selection, comparability, and outcome assessment, ensuring a robust evaluation of methodological rigor.
The primary outcome is maternal depression in pregnant women associated with PM2.5 exposure. Depression is defined as a clinical diagnosis (e.g., DSM-5, ICD-10) or a score above a validated threshold on a standardized scale (e.g., EPDS ≥ 13, PHQ-9 ≥ 10). Outcomes are measured at any point during pregnancy or up to 12 months postpartum. The effect measure for synthesis is the risk ratio (RR) with 95% CIs, pooled using a random-effects model (DerSimonian-Laird). Odds ratios (ORs) are converted to RRs using the method of.15 No additional outcomes were included, as the study focuses exclusively on depression, with non-depressive outcomes (e.g., anxiety) excluded per the selection criteria.
Our meta-analysis of seven studies (n = 576,737 pregnancies) ( Table 3) revealed that each 10 μg/m3 increase in PM2.5 exposure was associated with a 29% higher risk of maternal depression (pooled RR = 1.29, 95% CI: 1.10-1.50; Figure 2 and Table 4). Substantial heterogeneity was observed (I2 = 85.1%, τ2 = 0.027 Cochran’s Q = 40.39, df = 6, p < 0.001) with effects ranging from RR = 0.82 to 2.01 in the 95% prediction interval. Study-specific RRs ranged from 1.0410 to 9.24,12 with marker sizes reflecting study weights. The wide prediction interval and high I2 suggest considerable variability in effect estimates across studies.
| Study | Country | Design | RR | 95% CI | Quality (NOS) | Participants | Outcome | Perinatal window | SE (log scale) | Exposure unit |
|---|---|---|---|---|---|---|---|---|---|---|
| (12) | USA (Los Angeles) | Cohort | 1.56 | 1.01–2.42 | 7 | 180 Hispanic/Latina women | CES-D ≥16 | Second trimester | 0.2229 | Per 2.0 μg/m3 |
| (16) | China | Cohort | 1.21 | 1.05–1.38 | 6 | 10,209 pregnant women | EPDS ≥10 | Whole pregnancy, each trimester | 0.0697 | Per 10 μg/m3 |
| (17) | Mexico City | Birth cohort | 1.59 | 1.11–2.28 | 7 | 509 mothers | EPDS ≥13 | Prenatal | 0.1836 | Per 5 μg/m3 |
| (10) | USA (Southern CA) | Retrospective cohort | 1.02 | 1.00–1.04 | 8 | 340,679 women | EPDS ≥10, diagnoses, meds | Late pregnancy, postpartum | 0.0100 | Per IQR (5 μg/m3) |
| (18) | USA | Cohort | 1.19 | 1.08–1.30 | 7 | 221,794 pregnant women | ICD-9 depression | Whole pregnancy | 0.0473 | Per IQR (5 μg/m3) |
| (19) | China (Nanjing) | Case-control | 1.14 | 1.0826–1.1953 | 6 | 605 pregnant women | CCI ≥6 (stress) | Prenatal | 0.0252 | Per unit ADD |
| (20) | China (Shanghai) | Cohort | 1.25 | 1.057–1.478 | 7 | 3,731 pregnant women | EPDS ≥13 | First trimester (0–13 weeks) | 0.0856 | Per 10 μg/m3 |
| Analysis type | Key metric | Value (95% CI) | Heterogeneity |
|---|---|---|---|
| Primary | Pooled RR | 1.29 (1.10-1.50) | I2=85.1%, τ2=0.027 |
| Subgroup - First Trimester | RR | 1.23 (1.10-1.36) | I2=0% |
| Subgroup - Whole Pregnancy | RR | 1.41 (1.23-1.63) | I2=29.6% |
| Sensitivity - Most Conservative | RR (excl.17) | 1.25 (1.08-1.45) | I2=85.8% |
| Publication Bias | Egger's Test p-value | 0.013 | - |
Subgroup analysis stratified by exposure window, first trimester exposures showed a 23% increased risk (RR = 1.23, 95% CI: 1.10-1.36;, I2 = 0.0%, 2 studies), second trimester RR = 9.24 (95% CI: 1.04–82.10, I2 = 0.0%, n = 1), third trimester RR = 1.04 (95% CI: 1.00–1.08, I2 = 0.0%, n = 1), and whole pregnancy RR = 1.41 (95% CI: 1.23–1.63, I2 = 29.57%, n = 3). Significant between-group heterogeneity was observed (Q_between = 37.46, df = 3, p < 0.001), with the second trimester showing an outlier effect, though with a wide confidence interval. Geographically, Chinese studies (RR = 1.23, 95% CI: 1.10-1.36) showed more consistent effects than US studies (RR = 1.36, 95% CI: 1.07-1.72; p = 0.03 for difference) ( Figure 3).
The leave-one-out sensitivity analysis ( Figure 4) assessed the impact of omitting each study. Pooled RRs ranged from 1.25 (95% CI: 1.08–1.45, I2 = 85.8%, when17 excluded) to 1.33 (95% CI: 1.19–1.49, I2 = 44.3%, when10 excluded), with percent changes from -2.81% to 3.56% relative to the original RR of 1.29 ( Figure 4). The stability of the pooled estimate across omissions (all CIs overlapping the original 1.10–1.50) supports the robustness of the primary finding. All sensitivity estimates remained statistically significant.
The funnel plot ( Figure 5) assessed publication bias, with Egger’s test showing an intercept of 1.914 (p = 0.013) and Begg’s test a correlation of 0.893 (p = 0.007). The plot exhibited asymmetry, with studies scattered unevenly around the pooled effect (log RR ≈ 0), particularly at lower precision (1/SE), indicating significant publication bias that may inflate the pooled RR.
The scatter plot of study quality vs. effect size ( Figure 6) explored the influence of NOS scores. Quality-effects meta-regression found no significant association between quality score and log RR (coefficient = -0.0505, p = 0.530) or sample size and log RR (coefficient = -2.483e-07, p = 0.732), with an R-squared of 0.694 (F-statistic p = 0.0937). The small sample size (n = 7) precluded valid Omnibus normality testing, and a high condition number (4.47e+06) suggested potential multicollinearity. Studies included from USA are10,12,17,18 and studies from China includes.16,19,20
This meta-analysis synthesised data from seven studies (n = 576,737 pregnancies) examining the association between PM2.5 exposure and maternal depression. The pooled RR of 1.29 (95% CI: 1.10–1.50) ( Figure 2 and Table 1) suggests a significant 29% increase in depression risk per 10 μg/m3 increment in PM2.5 exposure. This finding exceeds earlier pooled estimates (1.10–1.1814), possibly due to differences in exposure definitions or inclusion of studies spanning broader exposure windows. Heterogeneity was substantial (I2 = 85.1%), unlike the “very low heterogeneity” noted, suggesting variability across study populations, designs, or exposure assessments, which warrants further exploration. The sensitivity analysis demonstrated robustness, with pooled RRs ranging from 1.25 to 1.33 upon omitting individual studies, except for a notable influence from,10 where omission increased the RR to 1.33 (3.56% change), potentially due to its third-trimester focus (RR = 1.04). Publication bias, evidenced by Egger’s test (p = 0.013) and Begg’s test (p = 0.007), and supported by funnel plot asymmetry ( Figure 5), did not significantly alter the pooled estimate after adjustment, reinforcing the reliability of the association. However, the bias suggests a potential overestimation, possibly due to underrepresentation of null findings. Mechanistically, PM2.5’s role in depression likely involves neuroinflammation, oxidative stress, hormonal dysregulation, and neurotoxic effects.15,21 Excessive inflammation may activate indoleamine 2,3-dioxygenase, accelerating tryptophan breakdown and reducing serotonin synthesis, a key depression risk factor.22,23 Hormonal imbalances, potentially mediated by inflammation,24–26 and altered circadian gene expression27,28 further contribute. Short- and long-term PM2.5 exposure elevates pro-inflammatory cytokines (e.g., IL-6, IL-1β, TNFα),29–31 linking systemic inflammation to depression onset via innate immunity.32–34 Subgroup analysis highlighted a pronounced effect during the second trimester (RR = 9.24, 95% CI: 1.04–82.10), aligning with the findings of12 on a 1.56-fold increased risk of postpartum depression 12 months postpartum. This suggests a critical window of vulnerability, potentially due to heightened neuroinflammatory responses during mid-pregnancy. The study cohort, comprising 231,752 women across 6 positive, medium-quality studies (RRs 1.10–1.18) versus 1 weak-quality negative study, supports a small to moderate effect, though the wide CI for the second trimester indicates uncertainty. Despite consistent findings, variations in study design and the small sample size (n = 7) limit meta-regression power, as reflected by the invalid Omnibus test and high condition number (4.47e+06), suggesting potential multicollinearity. The presence of substantial heterogeneity and potential publication bias underscores the need for larger, multicentre longitudinal studies incorporating diverse populations and robust exposure assessment methods.
This meta-analysis confirms a significant association between PM2.5 exposure and depressive symptoms in pregnant women, with a pooled risk ratio of 1.29 (95% CI: 1.10–1.50) per 10 μg/m3 increment, indicating a 29% increased risk. Notably, exposure during the second trimester (RR = 9.24, 95% CI: 1.04–82.10) showed a pronounced effect, suggesting a critical vulnerability window with potential long-term impacts on maternal mental health, extending months to years postpartum. However, the analysis revealed substantial heterogeneity which reflects the variability across studies. Our findings indicate significant bias which suggests that the pooled estimate may overestimate the true effect due to underrepresentation of negative studies. The small sample size (n = 7) further limits the meta-regression’s power to assess quality effects, as evidenced by the invalid Omnibus test. These findings reveal the deleterious effects of PM2.5 on maternal mental health, necessitating targeted public health interventions, particularly during pregnancy. Future research should prioritize larger sample sizes, standardized diagnostic criteria for depression, and rigorous control of confounders such as socioeconomic status and co-exposures. Additionally, mechanistic studies exploring neuroinflammation, oxidative stress, and hormonal dysregulation pathways are crucial to elucidate the role of PM2.5 in depression. Given that all included studies originated from developed countries (e.g., USA, China), there is an urgent need for research in low-income regions and Africa, where air pollution levels may be higher and healthcare access is limited, to ensure global relevance and equity in addressing this environmental health challenge.
Open Science Framework: PRISMA checklist and flowchart for “PM2.5 Exposure and Depression in Pregnant Women: A Systematic Review and Meta-Analysis.” (https://doi.org/10.17605/OSF.IO/D7BGZ).35
License: Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Universal). Extended Data Registration and Checklist.
Open Science Framework: PM2.5 Exposure and Depression in Pregnant Women: A Systematic Review and Meta-Analysis. (https://doi.org/10.17605/OSF.IO/D7BGZ).35
This project contains the following extended data:
PRISMA FLOW DIAGRAM.pdf
META ANALYSIS FIGURES.pdf
PRISMA_2020_checklist.docx
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors acknowledge the National Institute of Environmental Health Nigeria for the opportunity to use their office space.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)