Keywords
Keywords: antimicrobial resistance, multidrug-resistant, Escherichia coli, Klebsiella pneumoniae, EDTA.
This article is included in the Antimicrobial Resistance collection.
The escalating global threat of antimicrobial resistance presents a critical challenge to public health. In Iraq, the increasing rise of resistance strains further confirms the need for innovative treatment strategies. Ethylenediaminetetraacetic acid (EDTA) has shown promise in enhancing antibiotic efficacy via destabilizing bacterial membranes and disrupting biofilms.
This study examines the effects of combining EDTA with commonly used antibiotics on multidrug-resistant Escherichia coli and Klebsiella pneumoniae isolates from a major hospital in Baghdad, Iraq. Additionally, it assesses the prevalence of antibiotic resistance in these bacterial strains. The main goal is to improve treatment outcomes and address the growing challenges of antimicrobial resistance in healthcare settings.
This experimental in vitro study evaluates the effects of combining EDTA with meropenem, amikacin, and piperacillin-tazobactam on multidrug-resistant Escherichia coli and Klebsiella pneumoniae isolates from clinical samples.
The results show that EDTA enhanced the antibiotics’ effectiveness, increasing inhibition zone diameters and improving bacterial susceptibility. The inhibition zone expanded by approximately 2 to 5 millimeters. Resistance patterns shifted, with some previously resistant or intermediate strains becoming susceptible when treated with the combined therapy. In conclusion, EDTA increases the efficacy of meropenem, amikacin, and piperacillin/tazobactam against multidrug-resistant Escherichia coli and Klebsiella pneumoniae strains.
This presents a promising approach to improve treatment options for infections caused by resistant pathogens. Further research is needed to understand EDTA’s mechanisms and clinical applications.
Keywords: antimicrobial resistance, multidrug-resistant, Escherichia coli, Klebsiella pneumoniae, EDTA.
The rising bacterial resistance to various antimicrobial agents is becoming a serious public health concern. This problem often leaves little or no effective treatment options to combat infections caused by pathogenic organisms. Antimicrobial resistance has escalated globally, affecting both Gram-positive and Gram-negative bacteria more widely.1 Establishing standardized definitions and classifications for multidrug-resistant (MDR) bacteria is essential for consistently collecting and comparing epidemiological data across diverse healthcare settings and countries.2 MDR organisms are pathogens that display in vitro resistance to at least two distinct classes of antimicrobial agents. Infections caused by these organisms frequently result in delayed or inadequate antimicrobial therapy, contributing to poorer patient outcomes, prolonged hospital stays, and increased healthcare costs.3 These resistant organisms present a significant challenge in hospital and community settings, leading to increased mortality rates, particularly among critically ill patients.4
Recent advancements highlight the importance of global initiatives, such as the World Health Organization’s (WHO) Global Antimicrobial Resistance and Use Surveillance System (GLASS), to monitor and combat this growing threat (WHO, 2022). Furthermore, research continues to explore innovative treatment approaches, such as combining therapies and utilizing supplementary agents, to effectively manage multidrug-resistant (MDR) infections.5 Addressing this issue requires implementing robust infection control measures, responsible antibiotic use, and developing new antimicrobial drugs to ensure public health protection. Studies also show that inadequate antimicrobial therapy in critical infections, such as bloodstream infections, significantly impacts patient outcomes, making timely and appropriate treatment essential.4
Escherichia coli (E. coli) is a gram-negative, facultative anaerobic, rod-shaped bacterium commonly found in the intestines of warm-blooded animals.6,7 Although the majority of E. coli strains are harmless, specific serotypes, such as Enteropathogenic E. coli (EPEC) and Enterotoxigenic E. coli (ETEC), can cause severe infections. EPEC is a leading cause of urinary tract infections, while ETEC is commonly responsible for traveler’s diarrhea and can lead to significant foodborne illnesses.8 Pathogenic strains occasionally cause food contamination events, which can lead to product recalls.9 The main treatment strategy focuses on addressing dehydration and restoring fluids and electrolytes. Although antibiotic treatment has been shown to reduce the duration of infection and the shedding of ETEC, especially in adults in endemic regions and travelers, the growing resistance to commonly used antibiotics has raised concerns. As a result, antibiotics are often discouraged unless necessary.10 The prevalence of multidrug-resistant E. coli strains in Iraq has grown, creating significant public health challenges. Many research studies have been carried out to meet the critical need to control infections caused by these resistant strains.11,12
Klebsiella pneumoniae (K. pneumoniae) is a gram-negative, non-motile, encapsulated, lactose-fermenting, facultative anaerobic, rod-shaped bacterium.13 When inhaled, it can cause significant damage to the lungs of humans and animals, particularly impacting the alveoli and producing sputum that may be bloody, brownish, yellow, or jelly-like.14,15 Recently, Klebsiella species have emerged as significant pathogens responsible for nosocomial infections, posing a considerable challenge to healthcare settings.16 These bacteria have developed multiple mechanisms of multidrug resistance (MDR), including the formation of plasmids and the production of extended-spectrum beta-lactamase (ESBL) enzymes, further complicating treatment options.8 The rise and transmission of multidrug-resistant (MDR) Klebsiella pneumoniae in Iraq is becoming an increasing concern, especially in healthcare environments, where it presents serious threats to patient outcomes. Klebsiella pneumoniae has developed resistance to several commonly used antibiotics, including extended-spectrum beta-lactams (ESBLs), which complicates treatment strategies and leads to prolonged hospital stays, increased healthcare costs, and higher mortality rates.12,17
Ethylenediaminetetraacetic acid (EDTA) is a chelating agent that binds divalent cations, such as magnesium (Mg2+) and calcium (Ca2+), essential for maintaining bacterial cell wall integrity and biofilm formation. EDTA destabilizes bacterial membranes by chelating vital ions, which compromises membrane integrity, increases permeability, and enhances both antimicrobial and antibiofilm properties.16,18,19 Studies have demonstrated that EDTA significantly improves the efficacy of various antibiotics, particularly in combating biofilm-associated infections caused by multidrug-resistant (MDR) bacteria.20,21 For example, EDTA, when combined with indocyanine green, enhanced photodynamic therapy (PDT) in diabetic foot infections, although the reduction in bacterial viability was modest, with bacterial counts decreasing by less than tenfold at 5 mM concentrations.22 The combination of EDTA with antibiotics, such as ceftriaxone, sulbactam, and gentamicin, has also proven to be effective against resistant Gram-negative infections isolated from clinical samples, including respiratory, blood, and urine cultures.23–25
This experimental study aims to investigate the effects of combining EDTA with Meropenem, Amikacin, and Piperacillin-tazobactam on multidrug-resistant Escherichia coli and Klebsiella pneumoniae isolates from hospital settings. The study will evaluate changes in bacterial susceptibility to these antibiotics after the addition of EDTA compared to their susceptibility before the combination. In addition, the research will examine the extent of antibiotic resistance at Ghazi Al-Hariri Surgical Specialties Hospital in Baghdad, Iraq, shedding light on the present status of antimicrobial resistance in the region.
This study was performed in accordance with Declaration of Helsinki, it is a prospective study Clinical isolates of Escherichia coli and Klebsiella pneumoniae were obtained from a pool of samples collected over six months (June to December 2024) as part of a year-long study. The samples, sourced from urine, stool, blood, respiratory specimens, and wound swabs, were collected at Ghazi Al-Hariri Surgical Specialties Hospital in Baghdad, Iraq.
Ethical Approval was obtained from the deanship of Baghdad college of medicine no. 23 in 4th of January 2025 and the Department of (Pharmacology), College of Medicine, University of Baghdad has approved the study protocol from the clinical and ethical points of view. Written informed consent was obtained from each patient involved in the study, also copyright was obtained in regard to assessment tools involved. The bacterial strains were identified using the Vitek 2 system (bioMérieux, Marcy-l’Étoile, France).
Following identification and selective medium isolation, individual colonies of each microorganism were inoculated into 10 mL of sterile Brain Heart Infusion Broth (BHI-B) and incubated aerobically at 37°C for 24 hours. For inoculum activation, 0.1 mL of pure isolates were transferred into 10 mL of sterile BHI-B and incubated aerobically at 37°C for 18 hours to a specific cell density, usually equivalent to 0.5 McFarland standard (approximately 1.5 × 10^8 CFU/mL) before conducting experiments. All culture media were sterilized by autoclaving at 121°C and 15 psi for 15 minutes, while clean glassware was sterilized in a hot air oven at 180°C for 1 hour.11,26,27 Laboratory surfaces, including benches and floors, were disinfected using a bleach-based antiseptic solution (Fas). For the study, ten purified strains from each microorganism were selected.
To prepare EDTA (ethylenediaminetetraacetic acid) for use in combination with antibiotics, a standard stock solution was created by dissolving 25 mg of EDTA powder in 5 ml distilled water. The pH of the solution was adjusted as required, and sterilization was achieved by filtration using a 0.22 μm filter. The resulting solution was stored at 4°C.28,29
Stock solutions of meropenem, amikacin, and piperacillin-tazobactam were freshly prepared before each experiment by dissolving their powders in distilled water. These antibiotic solutions were also sterilized by filtration through a 0.22 μm filter prior to use.
The minimum inhibitory concentration (MIC) is the lowest concentration of an antibiotic that can prevent the visible growth of a microorganism. It is a key factor in evaluating whether an antibiotic is effective against particular bacteria. In this research, the MIC values for meropenem, amikacin, and piperacillin/tazobactam were measured using the broth microdilution method.30,31 This method involves adding bacterial broth to a 96-well plate, where they are exposed to various concentrations of antibiotics. Bacterial growth is examined after incubating the plates at 37°C for 20 hours. The MIC for each bacterial strain was then determined as the lowest concentration that completely prevents visible growth. The median MIC (MIC50) is then calculated, representing the MIC value at the 50th percentile for the strains tested.31
The antibiotic resistance evaluation was conducted per the breakpoints established by the Clinical and Laboratory Standards Institute (CLSI) guidelines (Clinical and Laboratory Standards Institute, 2024). These breakpoints were applied to each antibiotic and bacterial species to determine minimum inhibitory concentration (MIC) values, categorizing bacteria as susceptible (S), intermediate (I), or resistant (R).
The antibiotic susceptibility of each microorganism was evaluated using the disc diffusion method. Three twofold dilutions near the MIC value were tested individually and in combination with EDTA. The results were assessed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines,32 which provided breakpoints to classify bacterial susceptibility based on observed growth inhibition zone for each antibiotic, both alone and in combination with EDTA.33,34
Over the six-month isolation period, E. coli was isolated from samples of 120 patients, while K. pneumoniae was isolated from 84 patients. Antibiotic susceptibility was assessed in accordance with CLSI guidelines. The summarized results are presented in ( Table 1) below, which reflects the percentage of resistance and susceptibility to each antibiotic.
The MIC values (μg/ml) for ten randomly selected E. coli strains were as follows: meropenem ranged from 0.06 to 16, amikacin from 1 to 64, and piperacillin/tazobactam from 0.06 to 128. The median MIC (MIC50) values were 0.5 μg/ml for meropenem, 4 μg/ml for amikacin, and 8 μg/ml for piperacillin/tazobactam.
For ten randomly selected K. pneumoniae strains, the MIC values (μg/ml) ranged from 0.06 to 128 for meropenem, 2 to 128 for amikacin, and 4 to 256 for piperacillin/tazobactam. The median MIC values were 0.5 μg/ml for meropenem, 8 μg/ml for amikacin, and 32 μg/ml for piperacillin/tazobactam.
Ten purified bacterial isolates were randomly selected for testing with individual antibiotics and in combination with EDTA at a concentration of 200 μg/mL. Antibiotics were tested at three concentrations close to their minimum inhibitory concentration (MIC) values. The results demonstrated an increase in the diameter of growth inhibition zones with higher antibiotic concentrations and when combined with EDTA, compared to the antibiotics alone.
For meropenem, the tested concentrations near the MIC were 0.5 μg/mL (C1), 1 μg/mL (C2), and 2 μg/mL (C3). Amikacin was tested at concentrations of 2 μg/mL (C1), 4 μg/mL (C2), and 8 μg/mL (C3). Piperacillin/tazobactam was tested at 4 μg/mL (C1), 8 μg/mL (C2), and 16 μg/mL (C3). In most cases, the combination with EDTA enhanced the inhibitory effect of the antibiotics, as reflected in larger inhibition zones. Combining the three antibiotics with EDTA increases the zone of inhibition by a significant margin, in a range of 2-5 mm. The results are summarized in ( Table 2) and ( Table 3) below. Figure 1 and Figure 2 demonstrate the change in the diameter of inhibition zones for the three antibiotics in combination with EDTA.
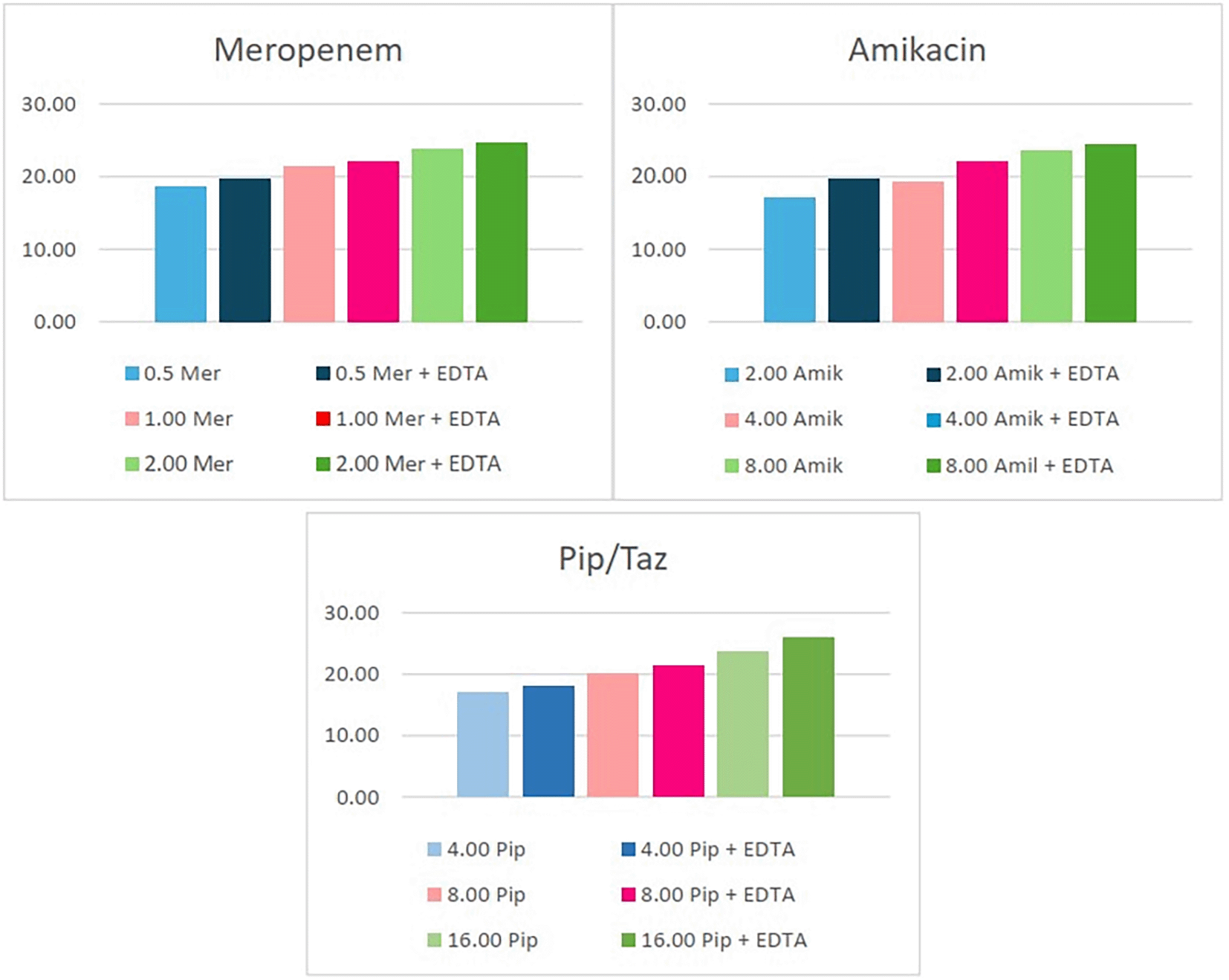
As demonstrated by the results in Tables 2 and 3, incorporating EDTA alongside antibiotics enhances the inhibition zones, indicating a marked improvement in antimicrobial efficacy. Another component of this study assesses the change in resistance resulting from adding an EDTA in combination. Furthermore, the study explores how this combination influences the overall resistance behavior. The diameters of growth inhibition zones for bacterial isolates at C1 concentrations were chosen to be evaluated following the Clinical and Laboratory Standards Institute (CLSI) 2023 guidelines to assess changes in resistance after the addition of EDTA. Among the ten E. coli isolates, shifts were observed: two resistant strains and one with intermediate sensitivity became susceptible to meropenem when combined with EDTA. Additionally, one resistant strain transitioned to intermediate sensitivity, and two intermediate strains became susceptible to amikacin under the same conditions. Similarly, one resistant strain and two intermediate strains shifted to susceptibility for piperacillin/tazobactam after its combination with EDTA.
For the ten K. pneumoniae isolates, a comparable effect was noted. One resistant strain and two intermediate strains became susceptible to meropenem following EDTA addition. Two resistant strains shifted to susceptibility for amikacin, and two resistant strains along with one intermediate strain became susceptible to piperacillin/tazobactam after EDTA combination. These findings are summarized in Table 4.
The results about the prevalence of resistance provide essential insights into the antimicrobial resistance patterns of E. coli and K. pneumoniae isolates in an essential Iraqi hospital setting like Ghazi Al-Hariri Surgical Specialties Hospital which occurs in the center of Baghdad and receives a large number of patients daily. The findings draw attention to the significance of growing antibiotic resistance and the challenges in treating infections caused by multidrug resistant pathogens. In Table 1 the case of E. coli, Meropenem remains practical mainly, with 86% of the isolates susceptible. The presence of 13% resistant and 1% intermediate strains points out the importance of continuous monitoring to control resistance spread. The results of K. pneumoniae show a susceptibility to meropenem of 56%, while 42% were resistant isolates. The high significance of resistance reflects a growing thread, lowering the available treatment options, and underscoring the need for more advanced strategies to overcome resistance spread. Resistance to meropenem can be developed primarily via the production of carbapenemases, enzymes that hydrolyze the β-lactam ring of carbapenems, making them ineffective. The most common types of carbapenemases in these organisms include KPC (Klebsiella pneumoniae carbapenemase) and NDM (New Delhi metallo-β-lactamase).35
When tested against both pathogens, amikacin shows susceptibility rates of 67% for E. coli and 64% for K. pneumoniae. There is a rising resistance rates (31% for E. coli and 35% for K. pneumoniae), which suggest that these organisms increasingly acquire resistance mechanisms. Resistance to amikacin is often due to drug enzymatic modification, which includes acetylation and adenylation mediated by aminoglycoside-modifying enzymes (AMEs).36 Bacterial ribosomal RNA mutations can also lead to decreased drug binding efficiency. Piperacillin/Tazobactam findings against E. coli shows 70% susceptible isolates, and a lower percentage against K. pneumoniae with 41%. The resistance then for K. pneumoniae was 56% of resistant isolates. Resistance mechanisms against piperacillin/tazobactam typically involve the production of β-lactamases, including ESBLs (extended-spectrum β-lactamases) and carbapenemases, that can inactivate piperacillin. Moreover, alterations in porin channels can prevent drug penetration into the bacterial cell.37
Looking after good antibiotic stewardship programs and optimizing the use of antibiotics is essential to improve clinical outcomes and reduce treatment failure among the growing antimicrobial resistance.38 The use of novel agents, in addition to combination therapies, is an important search area to combat resistant pathogens. Moreover, the high resistance rates observed in this study highlight the necessity to control and monitor the use of antibiotics and reflects the rising antimicrobial resistance in Iraqi healthcare settings. This deals with the global trend of antibiotic resistance and calls for coordinated efforts to address the issue effectively in Iraq.1
The antimicrobial activity results in Tables 1–3 highlights on the mean diameter of growth inhibition zones for three antimicrobial agents—Meropenem, Amikacin, and Piperacillin/Tazobactam—both alone and in combination with EDTA at varying concentrations on E. coli and K. pneumoniae isolates this was also clarified in Figures 1 and 2. The trend observed suggests that as the concentration of the antibiotics increases (C1 < C2 < C3), the effectiveness of the antibiotics improves, as shown by the larger inhibition zones. Notably, the addition of EDTA enhances the antimicrobial activity of each antibiotic, as the inhibition zones are consistently larger when EDTA is combined with the antibiotics compared to when each antibiotic used alone in a range of 2-5 mm. This suggests that EDTA can play a role in potentiating the effectiveness of these antibiotics.
EDTA is known to chelate divalent metal ions like calcium and magnesium, which are essential for bacterial cell wall integrity and function. By sequestering these ions, EDTA may increase bacterial permeability or make the bacteria more susceptible to the antibiotics, thus enhancing their overall antimicrobial action39 and this was summarized in Table 4. This finding is consistent with other studies, such as one published in 2011 in the Iraqi Journal of Medical Sciences which found that EDTA, when combined with antibiotics, significantly increased the antimicrobial activity against Pseudomonas aeruginosa isolates.40
These results also are in agreement with a 2022 study published in Antimicrobial Agents and Chemotherapy, which examined the pharmacodynamics of Piperacillin-Tazobactam/Amikacin combinations versus Meropenem on ESBL-producing K. pneumoniae strains and found that EDTA enhanced antimicrobial activity.16
The overall findings are especially significant in the context of the growing global thread of antimicrobial resistance. Studies continue to explore strategies to combat resistant bacterial strains, and the use of EDTA as an adjunct to antibiotics appears promising. Research by Al-Jumaili and Ahmed (2024) highlighted the growing issue of antimicrobial resistance in Iraq, emphasizing the need for effective control measures using a One Health approach.41 A study by Shamsee and Ibrahim (2024) also found that K. pneumoniae isolates exhibited significant resistance to various antibiotics, highlighting the need for novel solutions like combining EDTA with antibiotics.42 Additionally, Al-Jumaili and Almawla (2023)43 pointed out the importance of evidence-based antibiotic prescribing practices to combat multi-antibiotic resistance. Alkarkhi, Zeiny, and Ali (2016), Khan and Khan (2023), Khan et al. (2022) observed high antibiotic resistance in microorganisms isolated from surgical floors in hospitals, further reinforcing the need for new antimicrobial strategies.44–46
In conclusion, escalating antimicrobial resistance is a growing challenge. The data indicates that EDTA enhances the antimicrobial activity of Meropenem, Amikacin, and Piperacillin/Tazobactam against multidrug-resistant E. coli and K. pneumoniae isolates. This suggests that combining EDTA with these antibiotics could be an effective strategy for combating infections caused by multidrug-resistant pathogens. The larger inhibition zones observed reflect EDTA’s potential to boost antibiotic efficacy. Future studies should focus on understanding the mechanisms behind EDTA’s enhancement of antibiotic activity and its clinical applications.
Future research should focus on understanding how EDTA enhances antibiotic effectiveness, optimizing EDTA and antibiotic concentrations for resistant strains, and expanding studies to include other bacteria. Clinical trials are needed to evaluate safety and efficacy in humans. Additionally, exploring other potential enhancers, conducting comparative studies across different bacterial types, and assessing the cost-effectiveness and accessibility of EDTA in treatment are recommended.
Ethical Approval was obtained from the deanship of Baghdad college of medicine no. 23 in 4th of January 2025 and the Department of (Pharmacology), College of Medicine, University of Baghdad has approved the study protocol from the clinical and ethical points of view. Written informed consent was obtained from each patient involved in the study, and copyright was also obtained in regard to assessment tools involved.
Zenodo: The Antimicrobial Activity of EDTA in Combination with Meropenem, Amikacin, and Piperacillin-Tazobactam Against Hospital-Multidrug-Resistant Escherichia coli and Klebsiella pneumoniae Isolates, https://doi.org/10.5281/zenodo.1542810347
This project contains the following underlying data:
2024-12-25_final calculation - charts_E24O.xlsx
Data are available under the terms of Creative Common Attributes 4.0 International License (CC-BY 4.0) (https://creativecommons.org/licenses/by/4.0/)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: microbiology, antimicrobials, drug delivery, photodynamic therapy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 19 Sep 25 |
read | read |
|
Version 1 17 Jul 25 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)