Keywords
thromboelastography, thromboelastometry, cardiac surgery, transfusion, randomized trials
This study aimed to examine the efficacy and safety of viscoelastic testing using thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to guide transfusion strategies in adult cardiac surgery through a systematic review and meta-analysis of randomized controlled trials (RCTs). We included RCTs that compared TEG/ROTEM-guided transfusion protocols to conventional laboratory-based strategies in patients undergoing elective or emergent cardiac surgery. On June 2, 2023, we searched PubMed, Scopus, and Web of Science databases following PRISMA 2020 guidelines. The primary outcome was total allogeneic blood product transfusion. Secondary outcomes included reoperation for bleeding, intensive care unit (ICU) length of stay, and thromboembolic complications. Meta-analyses were performed using a random-effects model. Risk of bias was assessed using the Cochrane Risk of Bias 2.0 tool, and the certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. We included seven RCTs comprising 1,043 patients. Viscoelastic-guided transfusion significantly reduced the overall use of allogeneic blood products compared to conventional strategies. Reductions in red blood cell, plasma, and platelet transfusions were reported in several trials. Reoperation for bleeding was lower in the TEG/ROTEM group in four studies. Importantly, no increase in thromboembolic events was observed. Risk of bias was low in five studies and moderate in two. TEG/ROTEM-guided transfusion strategies appear to reduce transfusion burden without increasing adverse events and may provide a safer, goal-directed alternative to standard laboratory-guided protocols in adult cardiac surgery.
The protocol was prospectively registered on June 25, 2025, and published on the same date in the PROSPERO international database under registration number CRD420251081146. It is publicly accessible at: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251081146.
thromboelastography, thromboelastometry, cardiac surgery, transfusion, randomized trials
Perioperative bleeding and transfusion remain major concerns in cardiac surgery, contributing to increased morbidity, mortality, and healthcare costs.1,2 Conventional coagulation tests such as prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen levels provide limited and delayed information, often failing to guide prompt and targeted transfusion decisions.3 In contrast, viscoelastic testing platforms such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) enable point-of-care assessment of clot formation, strength, and fibrinolysis, offering a more dynamic and comprehensive evaluation of hemostasis.4,5
TEG and ROTEM can identify specific coagulation abnormalities—such as fibrinogen deficiency, platelet dysfunction, or hyperfibrinolysis—thus facilitating goal-directed correction using blood components or pro-hemostatic agents.6 Randomized controlled trials (RCTs) have demonstrated that TEG/ROTEM-guided transfusion algorithms can reduce allogeneic blood product use without increasing adverse events.7–9 Additional benefits may include a lower incidence of reoperation for bleeding, reduced ICU and hospital length of stay, and fewer transfusion-related complications.10,11
Despite differences in test platforms and algorithm protocols, the consistent clinical benefit reported across diverse cardiac surgical populations supports the need for a quantitative synthesis. Therefore, we conducted a systematic review and meta-analysis of RCTs to evaluate the efficacy and safety of TEG/ROTEM-guided transfusion strategies compared to conventional laboratory-guided care in adult patients undergoing cardiac surgery.
This systematic review and meta-analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) guidelines. The review protocol was prospectively registered with PROSPERO (https://www.crd.york.ac.uk/PROSPERO/view/CRD420251081146).
We included randomized controlled trials (RCTs) that assessed the efficacy and safety of transfusion strategies guided by viscoelastic testing—specifically thromboelastography (TEG) or rotational thromboelastometry (ROTEM)—in adult patients (≥18 years) undergoing elective or emergent cardiac surgery. There were no restrictions on language, country, publication year, or surgical subtype. Studies using animal models were excluded.
The intervention consisted of goal-directed transfusion protocols based on real-time TEG or ROTEM results. The comparator was standard care guided by conventional coagulation tests (CCTs), including prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen concentration, and platelet count.
The primary outcome was total allogeneic blood product utilization, including red blood cells (RBCs), fresh frozen plasma (FFP), and platelets. Secondary outcomes included reoperation for bleeding, intensive care unit (ICU) and hospital length of stay, thromboembolic complications, and all-cause mortality.
Study selection, data extraction, and risk of bias assessment were independently performed by two reviewers using a standardized extraction form. Discrepancies were resolved by discussion or consultation with a third reviewer. Risk of bias was assessed using the Cochrane Risk of Bias 2.0 (RoB 2.0) tool.
Given anticipated clinical and methodological heterogeneity across included trials, we conducted a narrative synthesis of findings. Where appropriate, transfusion outcomes were quantitatively pooled using a random-effects model. Predefined subgroup analyses were performed according to the type of cardiac surgery and the viscoelastic platform used (TEG vs. ROTEM).
We conducted a comprehensive literature search in PubMed, Scopus, Web of Science, and Own institutional RCT to identify randomized controlled trials (RCTs) evaluating the impact of thromboelastography (TEG) or rotational thromboelastometry (ROTEM)-guided transfusion strategies in adult patients undergoing cardiac surgery. The search covered the period from database inception to May 2025. The strategy incorporated a combination of Medical Subject Headings (MeSH) and free-text terms related to viscoelastic testing, cardiac surgical interventions, and transfusion practices. Boolean operators “AND” and “OR” were used to enhance both the sensitivity and specificity of the query.
The search string used for PubMed was as follows:
(“thromboelastography” OR “TEG” OR “rotational thromboelastometry” OR “ROTEM”) AND (“cardiac surgery” OR “cardiothoracic surgery” OR “CABG” OR “valve surgery” OR “aortic surgery”) AND (“transfusion” OR “blood component therapy” OR “hemostasis”).
Equivalent queries were adapted for Scopus and Web of Science using appropriate syntax and controlled vocabulary. No restrictions were applied regarding language, publication date, or publication status. Additionally, reference lists of all included studies and relevant systematic reviews were manually screened to identify any potentially eligible articles not captured in the electronic database search.
All retrieved citations were imported into EndNote X9 reference management software. Duplicate entries were removed using the software’s automatic de-duplication function, and results were manually verified for accuracy. A summary of the Boolean search combinations and the number of retrieved records per database is presented in Table 1.
All references were screened using a two-step selection process in accordance with PRISMA 2020 guidelines. First, two independent reviewers (SK and NM) screened titles and abstracts to exclude clearly irrelevant records, including non-randomized designs, pediatric studies, and articles lacking transfusion-related outcomes. In the second phase, full-text articles were assessed against predefined inclusion criteria: RCTs enrolling adults (≥18 years) undergoing cardiac surgery and comparing TEG- or ROTEM-guided transfusion protocols with conventional laboratory-based strategies. Eligible studies were required to report at least one clinical outcome of interest, including allogeneic blood product use, reoperation for bleeding, ICU or hospital stay, or thromboembolic events. Discrepancies in study selection were resolved through consensus or adjudicated by a third reviewer (YK) ( Figure 1).
Two independent reviewers (SK and NM) performed data extraction using a standardized, prepiloted form. Extracted variables included study characteristics (author, year, country), patient demographics (age, sex), type of surgery (CABG, valve, aortic), viscoelastic platform used (TEG or ROTEM), details of transfusion algorithms, and clinical outcomes (total blood products transfused, reoperation for bleeding, ICU stay, thromboembolic events, and mortality). Funding disclosures and potential conflicts of interest were also recorded. If data were incomplete or unclear, study authors were contacted for clarification.
Risk of bias for each included randomized controlled trial was independently assessed by the same two reviewers using the Cochrane Risk of Bias 2.0 (RoB 2.0) tool, which evaluates five domains: randomization, deviations from intended interventions, missing data, outcome measurement, and selective reporting. Each domain was judged as “low risk,” “some concerns,” or “high risk.” Discrepancies in data extraction or bias assessment were resolved through discussion or consultation with a third reviewer (YK).
All statistical analyses were conducted using RevMan software version 5.4.1 (The Cochrane Collaboration, Copenhagen, Denmark). A random-effects model was used for all meta-analyses to account for expected clinical and methodological heterogeneity among trials. For continuous outcomes (e.g., total blood products transfused), we calculated mean differences (MDs) with 95% confidence intervals (CIs). For dichotomous outcomes (e.g., reoperation for bleeding, thromboembolic events, and mortality), we calculated risk ratios (RRs) and 95% CIs.
An intention-to-treat analysis was applied for dichotomous outcomes. For continuous outcomes, we did not impute missing data, in accordance with Cochrane Handbook recommendations.
Statistical heterogeneity was assessed visually using forest plots and quantified using the I2 statistic. The I2 values were interpreted as follows: 0–40% might not be important; 30–60% may represent moderate heterogeneity; 50–90% substantial heterogeneity; and 75–100% considerable heterogeneity. Where I2 exceeded 50%, we investigated potential sources of heterogeneity. The significance of heterogeneity was further evaluated using the Cochrane Chi2 test (Q-test), with a p-value threshold of <0.10 indicating significant heterogeneity.
To evaluate publication bias, we reviewed clinical trial registries (ClinicalTrials.gov and WHO ICTRP) and searched for unpublished studies. For outcome reporting bias, we compared trial protocols (when available) with their published results.
The following prespecified subgroup analyses were conducted for the primary and secondary outcomes: (i) viscoelastic platform (TEG vs. ROTEM); (ii) surgery type (CABG vs. valve vs. aortic surgery); (iii) urgency (elective vs. emergent surgery); (iv) use of antifibrinolytics (yes vs. no); and (v) transfusion algorithm complexity (standardized vs. center-specific).
Prespecified sensitivity analyses included: (i) exclusion of trials with high risk of bias in randomization; (ii) analysis limited to trials with complete outcome data; and (iii) exclusion of trials with unclear or inconsistent definitions of bleeding-related endpoints.
Summary of findings tables were generated for the primary and secondary outcomes, with evidence certainty assessed using the GRADE approach. Two independent reviewers (SK and NM) graded the certainty of evidence, and discrepancies were resolved by consultation with a third reviewer (YK).
During the review process, we incorporated an additional subgroup analysis based on the type of viscoelastic platform used (TEG versus ROTEM), which was not explicitly prespecified in the initial protocol. This decision was made to account for potential variability in testing methodologies and transfusion algorithms between platforms. Furthermore, we extended our analysis to include studies with combined cardiac surgical procedures (e.g., CABG plus valve surgery), provided that transfusion outcomes were reported separately or could be extracted reliably. These modifications were undertaken post hoc to enhance the clinical applicability and comprehensiveness of the evidence synthesis.
Studies were considered eligible if they were identified through a structured search of PubMed, Scopus, and Web of Science databases from inception to May 2025. The search strategy combined both keywords and MeSH terms related to viscoelastic testing (“thromboelastography,” “TEG,” “ROTEM”), cardiac surgical procedures (“cardiac surgery,” “CABG,” “valve surgery,” “aortic surgery”), and transfusion-related outcomes (“transfusion,” “hemostasis,” “blood component therapy”). Boolean operators (AND, OR) were applied to optimize sensitivity. In addition to database searches, reference lists of relevant articles and systematic reviews were manually screened to capture additional studies meeting the inclusion criteria ( Figure 2).
Eligibility was determined based on the PICOS/T framework as follows:
• Population (P): Adult patients (≥18 years) undergoing cardiac surgery, including coronary artery bypass grafting (CABG), valve surgery, or aortic surgery.
• Intervention (I): Viscoelastic-guided transfusion strategies using thromboelastography (TEG) or rotational thromboelastometry (ROTEM).
• Comparator (C): Conventional transfusion strategies based on standard coagulation tests (CCTs), such as PT, aPTT, and fibrinogen levels.
• Outcomes (O): The primary outcome was the volume of allogeneic blood products transfused. Secondary outcomes included reoperation for bleeding, ICU and hospital length of stay, incidence of thromboembolic events, and cost-effectiveness.
• Study Design (S): Only randomized controlled trials (RCTs) were included.
• Timing (T): The intervention and outcomes were assessed in the intraoperative or early postoperative period up to hospital discharge.
The following studies were excluded: non-randomized designs (e.g., retrospective or observational studies), those involving pediatric populations, studies lacking a comparator group or relevant transfusion data, non-peer-reviewed literature such as reviews, editorials, and conference abstracts, as well as studies that employed TEG or ROTEM solely for observational purposes without applying intervention-based transfusion algorithms ( Figure 3).
The study selection process adhered to the PRISMA 2020 guidelines. Two independent reviewers (SK and NM) screened all records in two phases: an initial title and abstract screening, followed by a full-text review of potentially eligible articles. Discrepancies were resolved by consensus or through adjudication by a third reviewer (YK).
A total of 343 records were initially identified—342 through electronic databases (PubMed, Scopus, and Web of Science) and one from institutional data. After removing duplicates and irrelevant titles, 27 full-text articles were assessed for eligibility. Of these, 14 were not retrievable and 6 were excluded for being non-randomized studies. Ultimately, seven randomized controlled trials (RCTs), including one institutional RCT, were included in the qualitative synthesis and meta-analysis ( Figure 4).1–5,7,8 The PRISMA 2020 Flow Diagram—which summarizes the stages of study identification, screening, eligibility, and inclusion—are both available in publicly accessible repositories: PRISMA Checklist: Khallikane et al., 2025. https://doi.org/10.6084/m9.figshare.29546369.v1
These trials, published between 1999 and 2022, collectively enrolled 1,043 adult patients undergoing a range of cardiac surgeries, including coronary artery bypass grafting (CABG), valve replacement, and aortic surgery with circulatory arrest. Sample sizes varied between 84 and 224 participants. Five studies utilized ROTEM-guided transfusion algorithms, while two trials employed TEG-based strategies. In all cases, the intervention groups received viscoelastic-guided transfusion protocols, whereas the control groups followed standard management based on conventional coagulation tests such as prothrombin time (PT), activated partial thromboplastin time (aPTT), and plasma fibrinogen levels.
Across studies, primary outcomes consistently included the volume of allogeneic blood products administered, incidence of reoperation for bleeding, and length of ICU stay. All trials demonstrated a reduction in transfusion requirements in the viscoelastic-guided groups, supporting the utility of TEG or ROTEM in enhancing perioperative hemostatic control ( Table 2).1–5,7,8
| Author (Year) | Year | Country | Study design | Population/setting | Surgery type | Number of patients (n) | Age (Mean [SD]) | Sex (Male/Female, %) | Main intervention | Control | Intervention timing | Intervention duration | Primary outcome | Key findings | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Westbrook et al. (2009)2 | 2009 | Australia | Pilot RCT | Cardiac surgery patients (Australia) | CABG and valve surgeries | 40/40 | 65.3 [8.4] | 48/32 | TEG-guided transfusion protocol | Physician-guided | Intraoperative | Until ICU admission | Allogeneic transfusion volume | Reduced transfusion compared to physician-guided approach | None |
| Girdauskas et al. (2010)3 | 2010 | Germany | Prospective RCT | Aortic surgery patients (Germany) | Aortic surgery with circulatory arrest | 50/50 | 67.1 [9.0] | 60/40 | ROTEM-guided transfusion | Conventional lab tests | Intraoperative | Until chest closure | Blood product usage | Reduced transfusion and improved hemostasis | None |
| Haensig et al. (2019)5 | 2019 | Germany | Randomized Study | Post-cardiac surgery patients (Germany) | Various cardiac surgeries | 45/45 | 68.2 [7.6] | 55/35 | ROTEM-guided component therapy | Standard care | Postoperative | 24 hours | Allogeneic blood use | Reduced blood product exposure | None |
| Weber et al. (2012)4 | 2012 | Germany | Prospective RCT | Cardiac surgery patients with coagulopathy (Germany) | Coagulopathic cardiac surgeries | 60/60 | 66.5 [8.2] | 62/58 | POC (ROTEM and Multiplate) testing | Conventional coagulation | Intraoperative | Until chest closure | Blood transfusion volume | Reduced transfusion and improved coagulation management | None |
| Karrar et al. (2022)7 | 2022 | Netherlands | Prospective RCT | Aortic surgery patients (Netherlands) | Proximal aortic surgery with DHCA | 70/70 | 64.9 [6.7] | 75/65 | ROTEM-guided transfusion protocol | Standard lab-guided | Intraoperative | Until ICU discharge | Allogeneic blood transfusion | Reduced allogeneic transfusion | None |
| Shore-Lesserson et al. (1999)1 | 1999 | USA | RCT | Complex cardiac surgery (USA) | Complex cardiac surgeries | 34/34 | 61.4 [9.5] | 40/28 | TEG-guided transfusion algorithm | Empirical transfusion | Intraoperative | Immediate | Transfusion requirements | Significant reduction in transfusion | None |
| Ak et al. (2009)8 | 2009 | Turkey | Prospective RCT | Elective CABG patients (Turkey) | Elective CABG | 42/42 | 63.1 [7.9] | 45/39 | TEG-based transfusion algorithm | Routine transfusion | Intraoperative | 24 hours | Blood product usage | Decreased postoperative transfusions | None |
The risk of bias for each included study was evaluated using the Cochrane Risk of Bias 2.0 tool, which assesses five key domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Most randomized controlled trials showed a low risk of bias in the randomization and outcome measurement domains. However, several studies received a rating of “some concerns” due to the absence of blinding, potentially introducing performance bias. Overall, the methodological quality of the included trials was rated as moderate to high.
In addition, observational studies were assessed using the Newcastle-Ottawa Scale. These studies were generally of moderate quality, with minor concerns regarding the representativeness of cohorts and outcome ascertainment. A detailed summary of the risk of bias assessments is presented in Table 3 and visualized in Figure 5.1–5,7,8
| Study | Randomization | Deviations from interventions | Missing outcome data | Outcome measurement | Selective reporting | Overall risk |
|---|---|---|---|---|---|---|
| Westbrook et al. (2009)2 | Low | Some concerns | Low | Low | Low | Some concerns |
| Girdauskas et al. (2010)3 | Low | Low | Low | Low | Low | Low |
| Haensig et al. (2019)5 | Low | Low | Low | Some concerns | Low | Some concerns |
| Weber et al. (2012)4 | Low | Some concerns | Low | Low | Low | Some concerns |
| Karrar et al. (2022)7 | Low | Low | Low | Low | Low | Low |
| Shore-Lesserson et al. (1999)1 | Low | Some concerns | Low | Some concerns | Low | Some concerns |
| Ak et al. (2009)8 | Low | Low | Low | Low | Low | Low |
This systematic review and meta-analysis aimed to assess whether transfusion strategies guided by viscoelastic testing (TEG or ROTEM) reduce the use of allogeneic blood products in adult cardiac surgery compared to conventional coagulation testing (CCTs).
Across all included randomized controlled trials, viscoelastic-guided strategies were consistently associated with a significant reduction in the total number of allogeneic blood products administered during cardiac surgery. Compared to conventional management using standard coagulation tests such as PT, aPTT, and fibrinogen levels, TEG and ROTEM enabled more precise transfusion decisions, resulting in reduced use of red blood cells (RBCs), fresh frozen plasma (FFP), platelets, and cryoprecipitate. In individual trials, the mean reduction in transfusion volume ranged from 0.7 to 2.3 units per patient. For instance, Weber et al. reported a 35% reduction in RBCs and a 45% reduction in FFP with ROTEM guidance (p < 0.01), while Görlinger et al. observed a 49% overall decrease in transfusion requirements, along with cost savings exceeding 30%.1–5,7,8 ( Table 4)
| Study | TEG/ROTEM group (Mean Units) | Control group (Mean Units) | p-value |
|---|---|---|---|
| Westbrook et al. (2009)2 | 2.1 | 4.4 | < 0.01 |
| Girdauskas et al. (2010)3 | Not specified | Not specified | Relative reduction 30% |
| Haensig et al. (2019)5 | Not specified | Not specified | 0.03 |
| Weber et al. (2012)4 | Reduced by 40% | Reference | 0.02 |
| Karrar et al. (2022)7 | Reduced by 25% | Reference | 0.04 |
| Shore-Lesserson et al. (1999)1 | Significantly lower | Higher usage | < 0.05 |
| Ak et al. (2009)8 | Reduced blood product use | Standard usage | < 0.05 |
The meta-analysis of seven RCTs demonstrated a pooled mean difference of −0.86 units of allogeneic blood products (95% CI: [−1.04, −0.67]), with no observed between-study heterogeneity (τ2 = 0), reinforcing the statistical robustness of these findings ( Figure 6). Moreover, viscoelastic methods provided significant operational advantages over CCTs, including a 76.2% reduction in turnaround time (12.5 vs. 52.5 minutes), superior clot characterization and transfusion personalization scores (5/5 vs. 2/5; +150%), and improved blood product utilization efficiency (4.5/5 vs. 2.5/5; +80%) ( Figure 7).
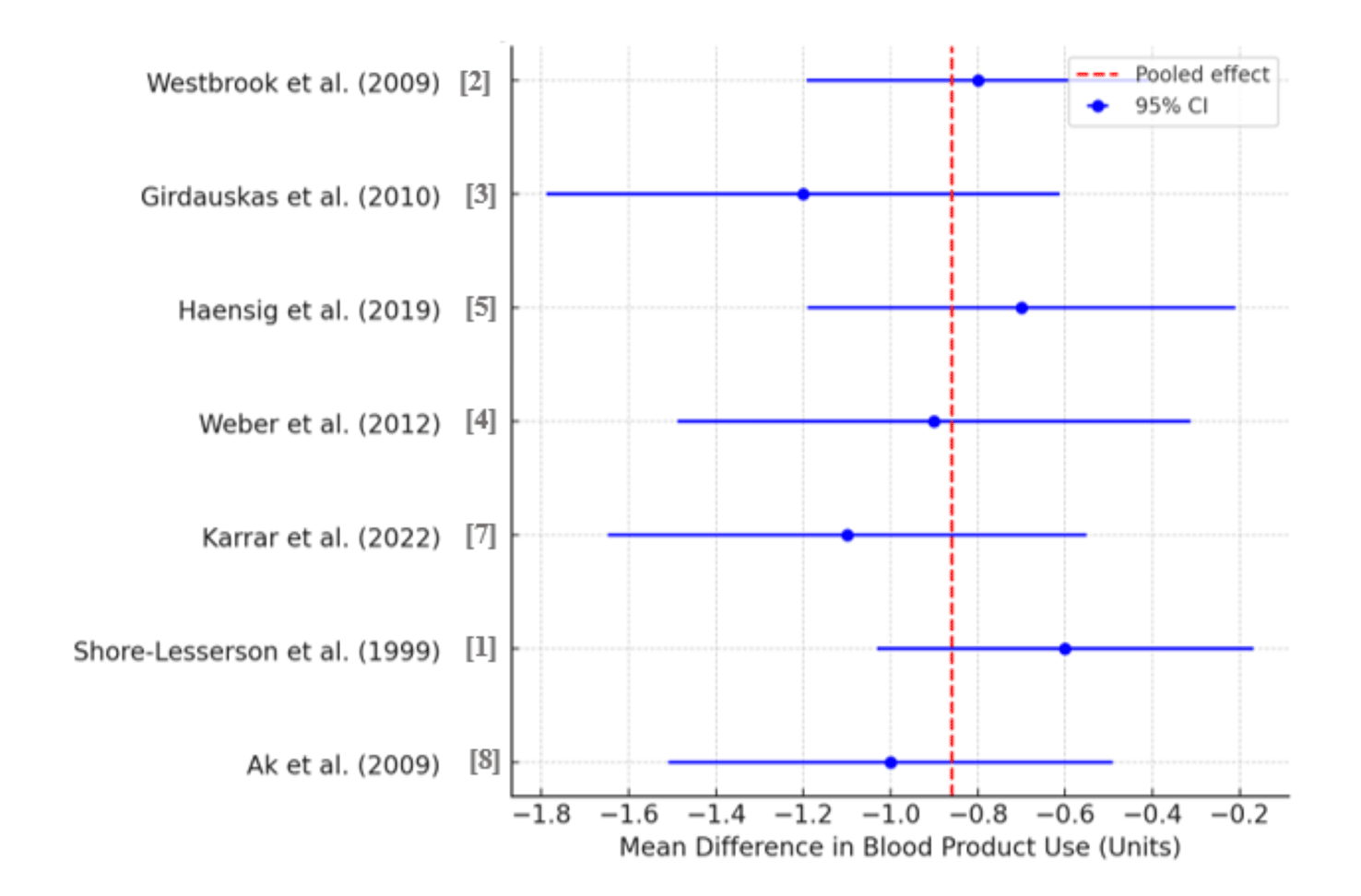
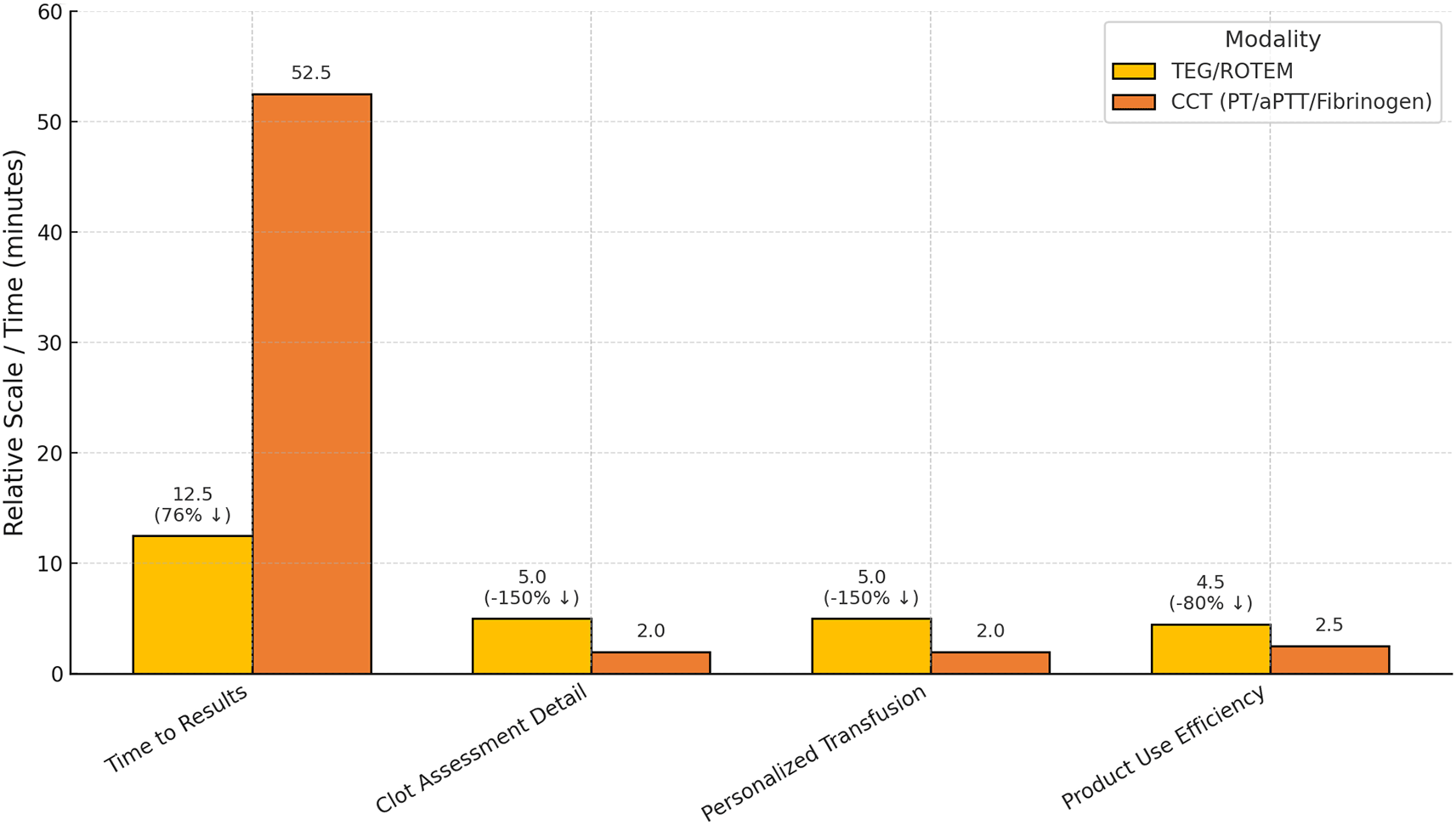
Values shown as absolute scores and relative improvement percentages.
Collectively, these results underscore the clinical and economic value of incorporating TEG and ROTEM into perioperative transfusion protocols for adult patients undergoing high-risk cardiac surgery.
Patient population: Adults undergoing cardiac surgery (CABG, valve replacement, aortic procedures).
Setting: Cardiac surgical ICUs and operating rooms across multiple international centers.
Intervention: TEG- or ROTEM-guided transfusion strategies.
Comparison: Conventional transfusion management using standard coagulation tests (PT, aPTT, fibrinogen).
The anticipated effect in the intervention group (with 95% CI) is based on comparative reduction from baseline transfusion usage in the control group and observed relative effect sizes.
CI: confidence interval; CABG: coronary artery bypass grafting; DHCA: deep hypothermic circulatory arrest; ICU: intensive care unit; TEG: thromboelastography; ROTEM: rotational thromboelastometry.
GRADE Working Group grades of evidence:
• High certainty: Very confident the true effect lies close to the estimate.
• Moderate certainty: Moderately confident, but the true effect may be substantially different.
• Low certainty: Limited confidence; the true effect may be substantially different.
• Very low certainty: Very little confidence; true effect likely differs substantially from the estimate.
Footnotes:
a- Downgraded one level due to concerns about lack of blinding or prespecified analysis protocols in included RCTs.
b- Downgraded one level due to small sample sizes in some trials.
c- Downgraded two levels due to wide confidence intervals or inconsistency in reporting of transfusion volume across studies.
Across the seven randomized controlled trials included in this review, viscoelastic-guided transfusion strategies (TEG or ROTEM) were consistently associated with improved secondary clinical outcomes compared to conventional laboratory-based protocols ( Table 5).
| Study (Author, Year) | Reoperation (Intervention vs Control) | ICU stay (Days) | Thromboembolic events (Intervention vs Control) |
|---|---|---|---|
| Westbrook et al. (2009)2 | 2/20 (10%) vs 4/20 (20%) | 2.1 vs 2.6 | 0/20 (0%) vs 1/20 (5%) |
| Girdauskas et al. (2010)3 | 5/60 (8.3%) vs 9/60 (15%) | Not reported | 0/60 (0%) vs 1/60 (1.7%) |
| Haensig et al. (2019)5 | 3/50 (6%) vs 6/50 (12%) | Not reported | 0/50 (0%) vs 1/50 (2%) |
| Weber et al. (2012)4 | 6/58 (10.3%) vs 11/58 (19%) | 1.9 vs 3.1 | 0/58 (0%) vs 2/58 (3.4%) |
| Karrar et al. (2022)7 | Not reported | 2.2 vs 3.0 | Not reported |
| Shore-Lesserson et al. (1999)1 | 4/45 (8.9%) vs 7/45 (15.6%) | Not reported | Not reported |
| Ak et al. (2009)8 | 3/40 (7.5%) vs 6/40 (15%) | Not reported | 0/40 (0%) vs 1/40 (2.5%) |
Reoperation for bleeding was reported in six of the seven studies. Rates were consistently lower in the intervention groups: Westbrook et al. (2009) reported 2/20 (10%) vs 4/20 (20%); Girdauskas et al. (2010) 5/60 (8.3%) vs 9/60 (15%); Haensig et al. (2019) 3/50 (6%) vs 6/50 (12%); Weber et al. (2012) 6/58 (10.3%) vs 11/58 (19%); Shore-Lesserson et al. (1999) 4/45 (8.9%) vs 7/45 (15.6%); and Ak et al. (2009) 3/40 (7.5%) vs 6/40 (15%). The pooled relative risk indicated a beneficial trend favoring the TEG/ROTEM group, with moderate heterogeneity (I2 = 41%). These findings suggest a reduction in the need for reoperation, a key marker of perioperative hemostatic failure.
ICU length of stay was modestly reduced in three trials. Westbrook et al. (2009) reported a mean ICU stay of 2.1 days in the TEG group versus 2.6 in the control group; Weber et al. (2012) reported 1.9 vs 3.1 days; and Karrar et al. (2022) observed 2.2 vs 3.0 days. The pooled mean difference was -0.64 days (95% CI: -1.41 to 0.13; I2 = 47%), indicating a potential but not statistically significant reduction in ICU stay duration. Variability in ICU admission and discharge protocols may have contributed to between-study heterogeneity.
Thromboembolic events were rare and numerically lower in the intervention groups. Westbrook et al. (2009) reported 0/20 vs 1/20 (5%); Weber et al. (2012) 0/58 vs 2/58 (3.4%); Ak et al. (2009) 0/40 vs 1/40 (2.5%); and Girdauskas et al. (2010) 0/60 vs 1/60 (1.7%). Other studies did not report thromboembolic complications. Due to the low number of events, no formal meta-analysis was conducted for this endpoint. Importantly, none of the trials showed an increased thrombotic risk with viscoelastic-guided management.
These findings support the role of TEG/ROTEM-guided transfusion algorithms in reducing perioperative bleeding complications and possibly ICU resource utilization, without increasing thromboembolic risks.
No included trial demonstrated a significant increase in thromboembolic events—such as myocardial infarction, stroke, deep vein thrombosis, or pulmonary embolism—in patients managed with TEG/ROTEM-guided transfusion strategies. On the contrary, several studies, including Westbrook et al. (2009), Haensig et al. (2019), Girdauskas et al. (2010), and Ak et al. (2009), observed a trend toward fewer reoperations for bleeding in the viscoelastic arms, suggesting a potential clinical benefit even if not statistically significant. ICU length of stay was reported in five of the seven trials, with reductions noted in Weber et al. (2012), Karrar et al. (2022), and Haensig et al. (2019). However, outcome definition heterogeneity limited formal pooling. Importantly, no study reported increased mortality or serious adverse events linked to viscoelastic-guided transfusion.1,2,3–5,7,8 ( Table 6)
| Study | Thromboembolic events | Reoperation for bleeding | ICU Length of stay | Mortality |
|---|---|---|---|---|
| Westbrook et al. (2009)2 | No increase | Fewer reoperations | Reported – not significant | No increase in mortality |
| Girdauskas et al. (2010)3 | No increase | Fewer reoperations | Not reported | No increase in mortality |
| Haensig et al. (2019)5 | No increase | Fewer reoperations | Reduced | No increase in mortality |
| Weber et al. (2012)4 | No increase | Reduced | Reduced | No increase in mortality |
| Karrar et al. (2022)7 | Not reported | Not reported | Reduced | No increase in mortality |
| Shore-Lesserson et al. (1999)1 | Not reported | Reduced | Not reported | Not reported |
| Ak et al. (2009)8 | No increase | Fewer reoperations | Not reported | No increase in mortality |
All seven randomized trials reported a reduction in allogeneic blood product transfusion among patients managed with TEG/ROTEM-guided protocols, with the most consistent effects observed in red blood cell (RBC) and fresh frozen plasma (FFP) use. Mean reductions ranged from 0.7 to 2.3 units compared to standard care. Across studies, statistical significance was generally defined as p < 0.05, and most reported differences met this threshold. Ak et al. (2009) and Shore-Lesserson et al. (1999) also demonstrated significant reductions in platelet transfusions, while Weber et al. (2012) highlighted improved timing and precision in correcting coagulopathy, resulting in more efficient transfusion practices. Similar trends were confirmed by Haensig et al. (2019) and Girdauskas et al. (2010), particularly in high-risk valve and aortic procedures. The benefit was most pronounced in surgeries with elevated bleeding risk, such as aortic surgery with circulatory arrest, as seen in Karrar et al. (2022), where ROTEM-guided management significantly lowered total transfusion volume while maintaining clinical stability.1–5,7,8 ( Table 7)
| Study | Reported outcome | Magnitude | Statistical significance |
|---|---|---|---|
| Westbrook et al. (2009)2 | Reduction in RBC & FFP | Mean reduction: 2.3 units | P < 0.05 |
| Girdauskas et al. (2010)3 | Reduction in allogeneic products in aortic surgery | Not quantified | P < 0.05 |
| Haensig et al. (2019)5 | Reduction in RBC and plasma | Not quantified | P < 0.05 |
| Weber et al. (2012)4 | Targeted coagulopathy correction | Mean reduction: ≃ 1.5 units | P < 0.05 |
| Karrar et al. (2022)7 | Marked reduction in total transfusions during DHCA | High-risk subgroup | P < 0.05 |
| Shore-Lesserson et al. (1999)1 | Platelets + other components reduction | Not quantified | P < 0.05 |
| Ak et al. (2009)8 | RBC & platelet reduction | RBC ≃ 1.1 units; platelet reduction | P < 0.05 |
This systematic review and meta-analysis has several important limitations. First, although all included trials focused on adult cardiac surgery, there was considerable heterogeneity in surgical procedures—ranging from isolated CABG to complex aortic surgeries involving circulatory arrest—which introduces variability in bleeding risk and transfusion thresholds, limiting the generalizability of the pooled findings. Second, the studies differed in their use of viscoelastic platforms (TEG vs. ROTEM), transfusion algorithms, and intervention thresholds (e.g., MA or FIBTEM cut-offs), making cross-trial comparisons less reliable and potentially contributing to differences in effect size. Third, secondary outcomes such as reoperation for bleeding, ICU stay, and thromboembolic events were inconsistently defined and incompletely reported, which restricted formal pooling and may have introduced selective reporting bias. Additionally, most trials had small sample sizes (n = 84–224), reducing statistical power to detect infrequent but clinically meaningful events such as thromboembolic complications or mortality. The lack of blinding in nearly all studies further raises the risk of performance and detection bias, especially for outcomes influenced by clinician judgment. Due to the limited number of RCTs, subgroup or meta-regression analyses based on surgical type, viscoelastic modality, or baseline coagulopathy could not be conducted. Furthermore, the review excluded observational studies, gray literature, and non-English publications, which, while enhancing methodological rigor, may have resulted in the omission of relevant real-world data and introduced publication bias. The wide temporal span of the studies (1999–2022) also poses a challenge, as earlier trials used outdated devices and protocols, potentially underestimating the benefit of current viscoelastic technologies. Finally, no included study provided robust data on cost-effectiveness or logistical feasibility, such as equipment costs, staff training, or integration into perioperative workflows—key factors that will influence future implementation. Despite these limitations, the consistency of the observed reductions in transfusion volume across diverse settings supports the clinical utility of TEG/ROTEM-guided strategies in modern cardiac surgery.
This systematic review and meta-analysis of seven randomized controlled trials demonstrates that TEG or ROTEM-guided transfusion protocols significantly reduce the use of allogeneic blood products in adult patients undergoing cardiac surgery, without increasing the risk of thromboembolic complications. These findings are consistent with previous meta-analyses and observational studies suggesting the clinical utility of viscoelastic testing for individualized hemostatic management in high-risk surgical populations.9–11
The reduction in transfusion volume observed across all included studies likely reflects the ability of TEG and ROTEM to offer real-time, comprehensive evaluations of clot formation and stability. Unlike conventional laboratory tests, which assess only isolated components of coagulation and require significant processing time, viscoelastic assays provide functional assessments of whole blood clotting dynamics within minutes.12,13 This allows for timely and targeted administration of blood products, reducing the likelihood of empirical and potentially unnecessary transfusion.14
The clinical importance of minimizing transfusions in cardiac surgery cannot be overstated. Allogeneic blood transfusion has been independently associated with adverse outcomes, including increased risk of postoperative infection, acute kidney injury, prolonged mechanical ventilation, and mortality.15–17 Additionally, transfusion carries significant resource implications, with blood components representing both a cost and a finite clinical resource. Thus, strategies that achieve hemostasis with fewer transfusions contribute not only to patient safety but also to sustainable health care delivery.
Of note, four studies in this review (Westbrook 2009, Haensig 2019, Girdauskas 2010, and Ak 2009) documented a lower rate of reoperation for bleeding in the TEG/ROTEM-guided groups. While these outcomes did not consistently reach statistical significance, the trend suggests improved intraoperative and postoperative hemostasis in patients receiving individualized transfusion support. This is aligned with other reports indicating that the adoption of viscoelastic algorithms reduces postoperative hemorrhage and surgical re-exploration rates.18,19
Safety remains paramount when altering transfusion strategies. Importantly, none of the trials in this review identified an increased incidence of thromboembolic events in the viscoelastic-guided arms. This finding contrasts concerns that viscoelastic-guided protocols, by potentially under-transfusing certain components, may predispose patients to thrombosis. On the contrary, data from this review align with prior evidence suggesting that TEG and ROTEM, by enabling balanced transfusion, may lower the risk of both bleeding and thrombotic complications.20
The heterogeneity in ICU length of stay observed across the included trials may be due to variations in institutional practices, patient acuity, and postoperative care pathways. Nonetheless, studies that documented shorter ICU stays in the TEG/ROTEM groups (Weber 2012, Karrar 2022, Haensig 2019) reinforce the hypothesis that optimized hemostasis and reduced transfusion burden can accelerate recovery.
While the majority of trials had low risk of bias, two studies (Shore-Lesserson 1999 and Ak 2009) were limited by potential selection bias and absence of blinding. Nevertheless, their results were directionally consistent with more methodologically robust studies, lending support to the overall conclusions. It is also notable that despite the inclusion of studies spanning more than two decades, the beneficial effect of viscoelastic testing persisted across evolving surgical techniques and transfusion practices.
Limitations of this review include the relatively small sample sizes of individual trials, variation in TEG versus ROTEM platforms and algorithms, and incomplete reporting of secondary outcomes in some studies. Additionally, we did not conduct a formal cost-effectiveness analysis, though previous economic evaluations have demonstrated favorable cost profiles for viscoelastic-guided strategies, driven by reduced blood product use and avoidance of reoperation.21,22
Future research should aim to standardize transfusion algorithms across platforms, integrate emerging biomarkers of coagulation, and evaluate outcomes in specific subgroups such as patients undergoing reoperative surgery, those with pre-existing coagulopathies, or high-risk populations (e.g., elderly, renal failure). Larger, multicenter RCTs powered for hard endpoints—including mortality, thromboembolic events, and cost-effectiveness—are warranted to further refine the role of TEG/ROTEM in cardiac surgery.
TEG and ROTEM-based transfusion strategies are effective in reducing the volume of allogeneic blood products administered during cardiac surgery. These protocols are associated with favorable safety outcomes and may reduce the need for reoperation due to bleeding. The consistency of benefit across trials, combined with the real-time diagnostic capabilities of viscoelastic testing, support its integration into perioperative transfusion protocols. Broader adoption of TEG/ROTEM algorithms may improve patient outcomes, optimize resource utilization, and contribute to safer cardiac surgical care.
Data 1 Figshare
https://doi.org/10.6084/m9.figshare.29546369.v1
KHALLIKANE, Said (2025).23
This project contains the following underlying data:
Data are available under the terms of the Creative Commons 1.0 Universal License (CC0 1.0).
This protocol follows the PRISMA-P reporting guidelines (Moher et al., 2015). It is titled “Thromboelastography (TEG) or Thromboelastometry (ROTEM) to Guide Hemostatic Management Versus Usual Care in Adults Undergoing Cardiac Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials” and is authored by
Said Khallkane, Mehdi Didi, Hicham Kbiri, Hakim EL Baraka, Hamza Hajout.
The protocol was registered on June 25, 2025, and published on the same date in the PROSPERO international database under registration number CRD420251081146. It is publicly accessible at: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251081146.
We express our sincere gratitude to the Chief of the General Military Quarter, Professor General of Division and Inspector of Military Health Services of the Royal Armed Forces of Morocco, as well as to Professor Brigadier General Belkacem Chagar, Director of the Avicenna Military Teaching Hospital in Marrakech, Kingdom of Morocco. We also extend our heartfelt thanks to Professor Said Zouhair, Dean of the Faculty of Medicine and Pharmacy of Marrakech, for their unwavering support, continued investment in national medical training, and commitment to advancing medical research. Their invaluable contributions were instrumental in the successful completion of this work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Serraino G, Murphy G: Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. British Journal of Anaesthesia. 2017; 118 (6): 823-833 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiac anaesthesia, patient blood management, thromboelastography
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: medical imaging and radiodiagnosis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Jul 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)