Keywords
PSA Screening, Prostate Cancer, Systematic Review, Meta-Analysis, Screening frequency
This article is included in the Oncology gateway.
This article is included in the Health Services gateway.
International guidelines for prostate cancer screening vary, reflecting differences in healthcare systems, population risk profiles, and clinical practice preferences. A key difference among existing prostate cancer screening strategies is the frequency of screening; however, direct comparative evidence between different screening schemes remains limited. This study aimed to compare the effects of various prostate cancer screening intervals on clinical outcomes.
This systematic review and meta-analysis included major randomized controlled trials comparing prostate cancer screening methods with no screening. A meta-analysis was conducted to synthesize clinical evidence across various screening strategies. Sensitivity analyses were performed to assess the robustness of findings based on study quality. (PROSPERO ID: CRD42021265092),
Seven studies evaluating four main prostate cancer screening strategies were included in the meta-analysis. These strategies were categorized into four groups: 1) Two-year screening (e.g., Göteborg), 2) Four-year screening (e.g., ERSPC, Finnish), 3) Short-term screening (e.g., PLCO, Norrköping), and 4) One-time screening (e.g., CAP, Stockholm). In terms of prostate cancer detection, two-year interval screening showed the highest detection rate (RR = 1.88; 95% CI: 1.74–2.02), followed by four-year interval screening (RR = 1.42; 95% CI: 1.26–1.59). The overall analysis showed a significant increase in detection across all strategies (RR = 1.49; 95% CI 1.37–1.62). For prostate cancer-specific mortality, significant reductions were observed with two-year (RR = 0.54; 95% CI: 0.40–0.75) and four-year screening intervals (RR = 0.75; 95% CI: 0.63–0.90). Short-term and one-time screening strategies showed no statistically significant effect yielding an RR of 0.86 (95% CI: 0.73–1.02).
Based on the meta-analysis results, PSA screening conducted every two to four years appears to offer greater benefits, as both strategies significantly increase prostate cancer detection rates and reduce prostate cancer-related mortality. These findings may support decision making in countries where the optimal PSA screening strategy remains unclear. However, other factors such as targeted age range, cost effective and budget impact analysis should also be considered in the policy decision process.
PSA Screening, Prostate Cancer, Systematic Review, Meta-Analysis, Screening frequency
Prostate cancer is one of the major global health burdens. Its incidence consistently ranks among the highest of cancer in men and has shown a strong increasing trend over the past decade.1 According to cancer statistic report published in 2020,2 prostate cancer was diagnosed approximately 1.4 million people worldwide, ranking third among all cancers and ranking second among cancers in men, with estimated 375,000 deaths annually.
As PSA screening benefit has been studied in many randomized controlled trials and the result was inconsistency among each study. Results from latest systematic review and meta-analysis on PSA Screening is published in 2018,3 the results shown PSA screening have the benefit for increasing prostate cancer diagnosis rate, however, not show significant benefit on prostate specific mortality and all-cause mortality. Key variations between studies are commonly respected including difference in screening frequency and interval, PSA threshold and quality of the studies. Current guidelines vary between countries, and PSA-based screening remains a controversial topic due to concerns about efficacy in reducing mortality versus risks of overdiagnosis and overtreatment.
Key benefit of cancer screening is including the improvement in cancer diagnosis rate and many studies has shown survival benefit which related to reducing mortality, morbidity and reflect the overall cost of disease management.4 However, the risk of cancer screening includes anxiety of the result, have the chance for false positive test and false negative test, additional requirement of hospital resource including biopsy needed, hospitalization needed and overtreatment.5
The recommendation to adopt prostate cancer screening program varies among countries. Some countries such as United State, European, Australia recommended prostate screening program with informed decision program, while some others such as Japan and Asian countries including Thailand still not recommended it for used as broad population screening and suggest for specific population only (Ex. Private Hospital, premium insurance scheme checkup). Psychological burden, chance of overtreatment and economic burden were reported major obstacles for applying prostate cancer screening programs.6–10
In this study, we conducted systematic review followed by meta-analysis to summarize the available evidence and make comparison between different type of screening scheme compare including short-term and frequence screening with no screening program.
To evaluate the clinical performance of four prostate cancer screening strategies, this study applied the Population, Intervention, Comparison, and Outcomes (PICO) framework to guide article selection. The population of interest comprised individuals eligible for PSA-based screening programs. The intervention was prostate-specific antigen (PSA) screening, compared with no screening as the control. Only randomized controlled trials (RCTs) were included. The primary outcomes were prostate cancer detection rates and prostate cancer–specific mortality.
Key electronic databases used in the search included PubMed, Elsevier-Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL). In addition, we conducted a manual search of dissertation databases, reviewed reference lists of included studies, and screened recent abstracts from major annual meetings in urology and oncology. The list of potentially eligible articles was reviewed and discussed with domain experts to identify any additional relevant studies.
Keywords used in the search included “Prostate-Specific Antigen,” “Screening Program,” “Prostate Cancer,” and “Randomized Controlled Trial.” The detail of search strategies was displayed in Appendix 1. The search was limited to studies published between 1990 – 2025. Literature research was conducted between June 2020 and June 2025. In the case of updated literature within the same cohort population, we will decide to use the latest publication which longer time to follow-up.
This study was approved by the Ethical Committee of Chulalongkorn University (IRB No. 228/61). The protocol was registered in the PROSPERO database (ID: CRD 42021265092; 30 June 2021), and the report was written in accordance with the PRISMA 2020 guidelines.10
The key inclusion criteria for our review were as follows:
1. Studies with randomized controlled trials (RCTs) design comparing a prostate cancer screening method with no screening or with another screening strategy.
2. Studies that reported specifically prostate cancer detection rates and prostate cancer-specific survival.
3. Studies with an adequate follow-up period, defined as at least 10 years.
Studies were excluded if they met any of the following criteria:
1. Studies published in a language other than English and could not be interpreted.
2. Studies that full texts were not accessible.
3. Studies judged by the investigators to be of insufficient methodological quality.
Two independent reviewers (CR and ON) conducted the identification, selection, and data extraction processes for each included study. A standardized data extraction form was used to collect key study components, including study characteristics (e.g., study design, year, country, population size, setting), intervention and comparator details, screening frequency and duration, key outcomes (e.g., prostate cancer detection rate, prostate cancer–specific mortality), follow-up period, and risk of bias assessments. Discrepancies between the two reviewers were resolved through discussion and, if necessary, consultation with additional reviewers (BC, PA) to reach consensus.
Quality assessment of each study was conducted using the JADAD score, a quantitative tool that can be applied for classifying study quality. In addition, criteria were adapted from GRADE and CONSORT guidelines to complement the evaluation process. The assessment was independently performed by two reviewers (CR, ON). Any discrepancies between the reviewers were resolved through discussion, and if consensus could not be reached, additional reviewer (BC, PA) was consulted to make the final decision.
In the pooled data synthesis step, we performed a meta-analysis to summarize and compare effects of each prostate cancer screening scheme. Outcomes were reported in a risk-ratio format with 95% confidence interval (CI).12 Data collection was carried out using Microsoft Excel, and the meta-analysis was conducted using STATA statistical software.13
Heterogeneity was assessed and reported using chi square test and I2 statistic. The I2 threshold was defined as follows: I2 < 25% indicating low heterogeneity, I2 ranges between 25-50% indicated moderate heterogeneity and I2 > 50% indicated high heterogeneity. To address potential clinical heterogeneity that might occur from differences in screening strategies, the study conducted subgroup analysis based on screening intervals. A random-effect model was used to minimize statistical heterogeneity.
We searched the databases using pre-specified keywords and initially identified 68,968 articles. We initially perform primary screening from reviewing abstract and 68,943 record were excluded according to duplication between each database and not match with study criteria. After assessed the full text, 10 records were passed for full-text eligibility assessment. Three records are excluded from data extraction process according to inadequate data for extraction. The flowchart of searching strategy was presented in Figure 1.
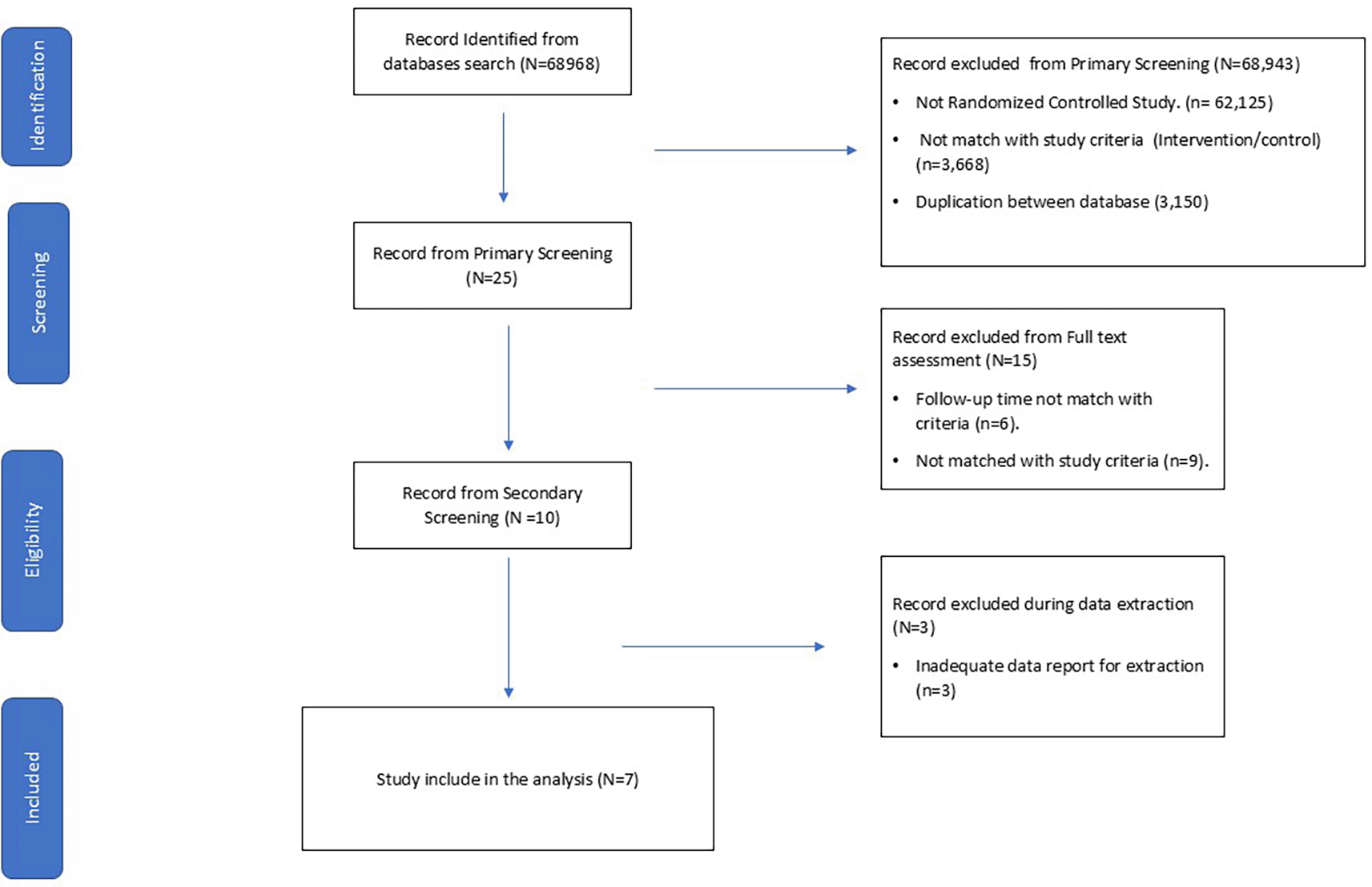
Following the eligibility check, seven large randomized controlled trials were included for data extraction. The included studies were classified into four groups based on the screening strategy: short term screening group (n = 2), two-year interval screening group (n = 1), four- year interval screening group (n = 2), and one-time screening group (n = 2). A summary of the study characteristics and classification groups were presented in Table 1. Quality assessment results shown included studies have moderate quality, all study lacking blinding process according to the nature of intervention. Large randomized controlled trials including PLCO and ESRPC showed low risk of bias, while others showed moderate risk of bias. Quality assessment using JADAD score and Cochrane Risk of Bias Tool were presented in Table 2 and Table 3.
| Study title | First author/publication year | # studied population | Follow-up (Yrs) | Age group | Screening interval | Identified group |
|---|---|---|---|---|---|---|
| Mortality Results from a Randomized Prostate-Cancer Screening Trial (PLCO)14 | Gerald L. Andriole, Pinsky (2017) | 76,683 | 15 | 55-74 | PSA annually for 5 yrs, DRE annually for 3 yrs | Short-Term Screening |
| Screening and Prostate-Cancer Mortality in a Randomized European Study (ESRPC)15 | Fritz H. Schroder (2019) | 162,389 | 16 | 50-74 | Screening every 4 yrs | Four Year Interval |
| Mortality results from the Göteborg randomized population-based prostate-cancer screening trial (Göteborg)16 | Jonas Hugosson (2017) | 20,000 | 18 | 50-64 | Screening every 2 yrs | Two Year Interval |
| Randomised prostate cancer screening trial: 20 year follow-up (Norrköping)17 | Gabriel Sandblom (2011) | 9,026 | 20 | 50-69 | 4 screening sessions #1-#2 DRE only #3-#4 PSA+DRE | Short-Term Interval |
| 15-Year Follow up of a Population Based Prostate Cancer Screening Study (Stockholm)18 | Anders Kjellman/Lundgren (2009/2018) | 27,146 | 15 | 55-70 | One time PSA+DRE screening | One Time Screening |
| Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality The CAP Randomized Clinical Trial (CAP Study)19 | Richard Martin (2018, 2024) | 419,582 | 15 | 50-69 | One time PSA Screening invitation | One Time Screening |
| Prostate Cancer Mortality in the Finnish Randomized Screening Trial (Finnish)20 | Tuomas P. Kilpeläinen (2013) | 80,144 | 11 | 55-70 | PSA Screening every 4 years until 70 years old | Four Year Interval |
We conducted a subgroup meta-analysis based on the screening strategy. For the prostate cancer diagnosis rate shown in Figure 2, the result showed significant improvement across all subgroups as well as overall analysis (RR 1.37; 95% CI: 1.19-1.57: I2 = 98.2%, p value < 0.001). Prostate cancer-related mortality was not significant in overall analysis shown in Figure 3 (RR 0.86; 95% CI: 0.74-1.00: I2 = 78.3%, p value < 0.001). However, from subgroup analysis, prostate cancer related mortality significantly reduced in groups with more frequent screening; namely the four-year and two-year interval screening group (RR 0.75; 95% CI: 0.63-0.90: I2 = 51.5%, p value = 0.151 in four-year interval group and RR 0.54; 95% CI: 0.40-0.75: I2 not reported in two-year interval group). Nevertheless, heterogeneity remained high (I2 = 78.6%, p-value < 0.001).
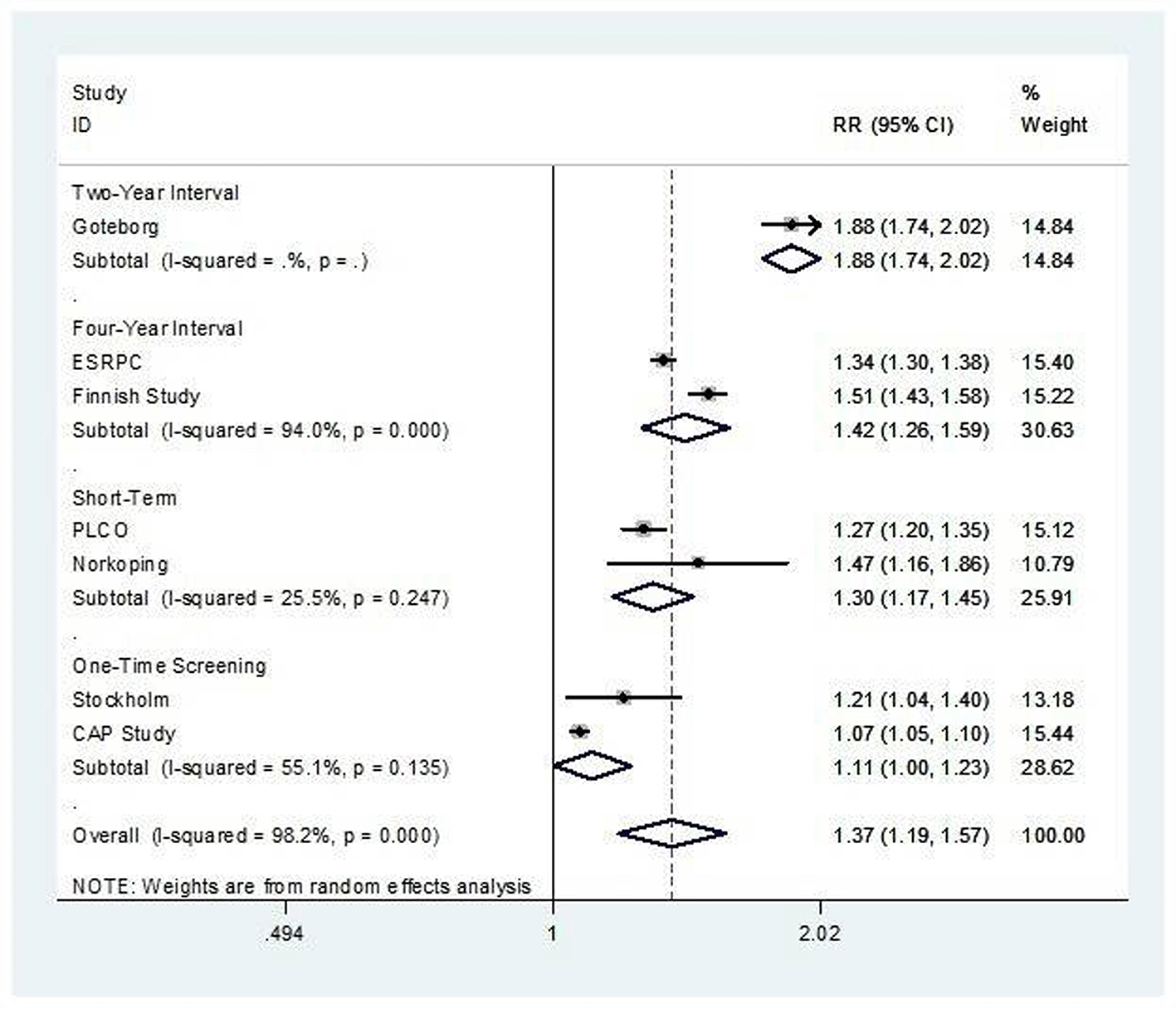
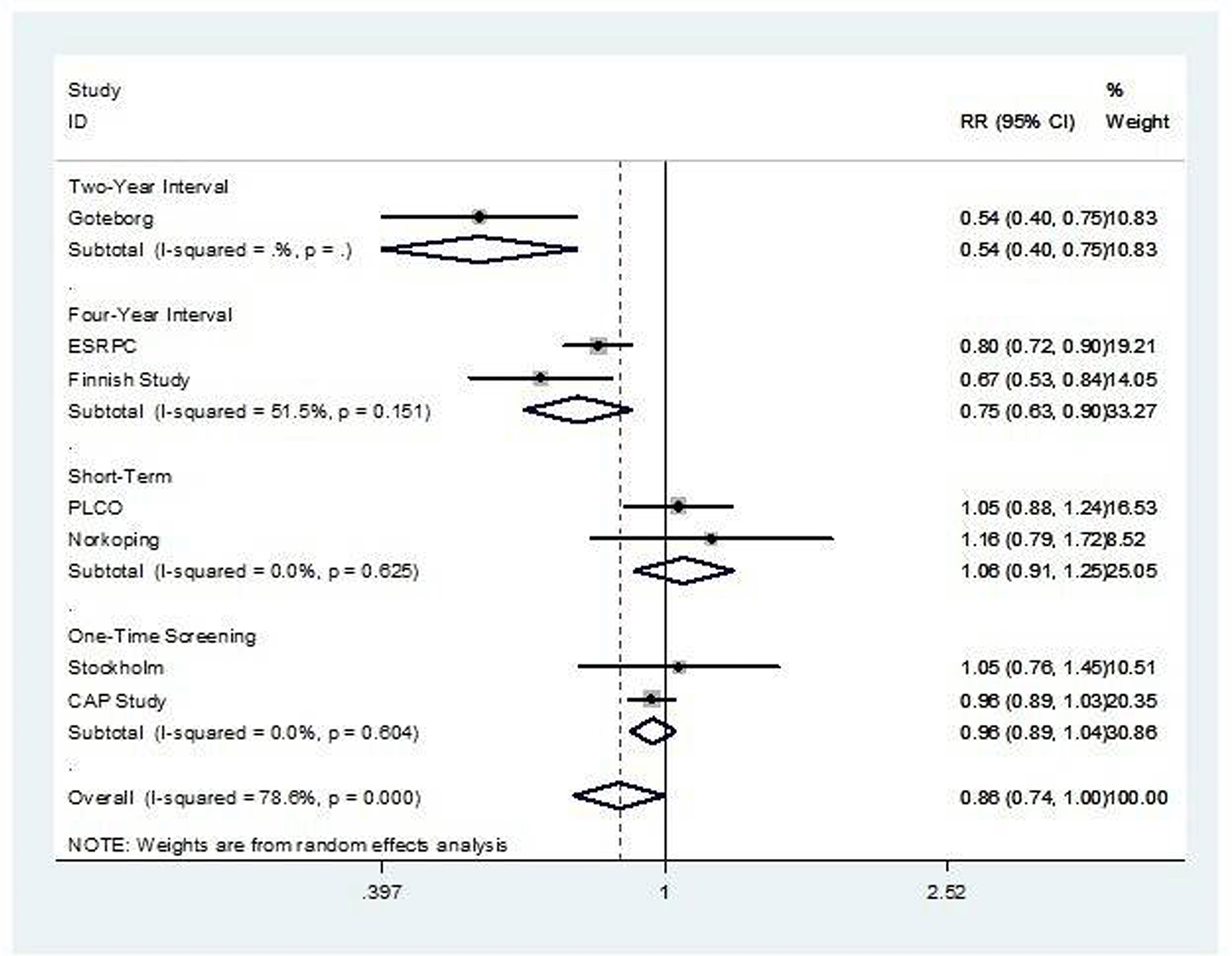
The source of heterogeneity in the pooled analysis presumably came from two studies: the CAP Study and the Göteborg study. The CAP study involved one-time screening and was affected by a high level of contamination, while the Göteborg study implemented a two-year screening interval, in contrast to other frequent- screening schemes.
To our knowledge, this is the first meta-analysis to evaluate the effects of prostate cancer screening stratified by screening frequency, which added value by providing additional insight beyond previous study.5 This meta-analysis suggested that the two-year screening scheme yielded the most favorable outcomes, followed by four-year screening scheme. Both schemes significantly reduced prostate cancer-related mortality compared to other screening schemes and no screening. The two-year screening strategy yielded the best probability to achieve outcome in both of prostate cancer related mortality and improved probability of prostate cancer detection.
From the result of our study, factors shown most important for varying treatment outcome were the frequency of the screening method. As two-year screening yields the best result while four years screening group also shown the good screening outcome. In contrast, study scheme which only do screening for a limited duration shows inferior outcome compared with frequent screening scheme. The result suggested that screening could have benefit in improving prostate cancer diagnosis and survival but need to be done as optimal frequency (ex. 2 year or 4-year interval).
As prostate cancer is a slow-progressing disease, short term and infrequent screening schemes may not be effective in detecting cancer at a very early stage.16 In contrast, frequent screening schemes increase the possibility of earlier detection. This approach may shift the stage distribution toward earlier diagnoses, which are related with improved long-term outcomes, including reduced prostate cancer-related mortality.17 However, the benefit of screening programs must be weighed against potential risks such as overdiagnosis, unnecessary screening costs, psychological impact, and limited generalizability across different age groups or ethnicities.
While this study identifies the optimal screening strategy based on clinical outcomes, policy decisions require consideration of broader factors, including cost-effectiveness, budget impact, and system capacity.
In conclusion, a frequent screening scheme conducted every two years demonstrated the most favorable outcomes as 88% increase in prostate cancer detection and 46% reduction in prostate cancer-related mortality. These findings support the adoption of a regular prostate cancer screening program to improve outcomes in prostate cancer management.
This research was supported by Graduate 100th year study scholarship program and Graduate 90th research scholarship program, Chulalongkorn university. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
All relevant data are within the paper and its Supporting information files. All data used in this paper is shared under an open license CC-By Attribution 4.0 International including Final manuscript, Supplementary appendix, Prisma Checklist, Figures, Data extraction form, Data Analysis file. All underlying data used in the analysis—including values behind means, figures, and extracted image data—are included and openly available.
All data can be accessed at URLs OSF | Comparative Effectiveness Analysis of Different Prostate Cancer Screening Strategies: A Systematic Review and Meta-Analysis with DOI Identifier as DOI 10.17605/OSF.IO/H2AE8.20
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Minor points:
Minor points: