Keywords
Anterior cruciate ligament reconstruction, swelling, pain, maximum voluntary contraction, functional outcome
This article is included in the Manipal Academy of Higher Education gateway.
Postoperative knee swelling (PKOS) following anterior cruciate ligament reconstruction (ACLR) is known to contribute to deficits such as pain, swelling, reduced range of motion (ROM), and strength, impeding quadriceps activation, inducing arthrogenic muscle inhibition (AMI), and delaying rehabilitation. This study aimed to explore the association of PKOS with pain, ROM, maximum voluntary contraction (MVC), and functional outcome after ACLR.
Thirty-two subjects undergoing ACLR were recruited for this study. PKOS, pain, ROM, and MVC were measured on days 0, 1, 2, 3, 2nd week, and 6th week, while functional outcome was assessed using the Lower Extremity Functional Scale (LEFS) after 2 weeks and 6 weeks. Girth measurement was taken to assess swelling, pain was assessed using a visual analog scale (VAS), and MVC was analyzed using surface electromyography®.
Mean values for PKOS, VAS, ROM, MVC, and functional outcome showed overall improvement from day 0 to week 6, except for MVC values, which exhibited fluctuations from day 2 to day 3. However, no significant association (p > 0.05) was found between PKOS and pain, ROM, MVC, and functional outcome.
While PKOS improved along with other variables over the recovery period, it exhibited no significant correlation with pain, ROM, strength, or functional outcome post-ACLR, warranting further investigation.
Registration: Clinical Trial Registry (CTRI/2022/03/041101).
Anterior cruciate ligament reconstruction, swelling, pain, maximum voluntary contraction, functional outcome
Post-operative knee swelling (POKS) is a common impairment following anterior cruciate ligament reconstruction (ACLR) that can substantially affect recovery trajectories and long-term rehabilitation outcomes (Ong et al., 2023). In Indian epidemiological studies, ACL injuries have been reported as the most frequent sports-related knee trauma among young male athletes (John et al., 2016). ACL tears are among the most common ligament injuries of the knee and are often managed operatively, although nonoperative strategies remain viable in selected populations (Bogunovic & Matava, 2013). ACL reconstruction remains the standard surgical approach for restoring knee stability and function in active individuals (Musahl & Karlsson, 2019). Surgical stimuli can intensify the inflammatory cascade within the knee joint, leading to postoperative swelling. Additionally, the irrigation procedure conducted during ACLR, entailing the extraction of synovial fluid, could undermine joint lubrication, thus fostering an increase in knee swelling. Despite advancements in surgical techniques and rehabilitation protocols, PKOS remains elusive. Its association with pain, reduced range of motion (ROM), reduced quadriceps muscle strength, muscle inhibition, atrophy, and impaired functional outcomes remains poorly understood (Kochhal et al., 2019).
Swelling after ACLR is frequently linked with neuromuscular functional changes such as arthrogenic muscle inhibition (AMI), which is hypothesized to result from the inflammatory response triggered by tissue trauma associated with surgery (Palmieri-Smith et al., 2013; Sonnery-Cottet et al., 2019). Recent studies have also shown persistent impairments in quadriceps neuromuscular control and physical function extending well beyond the early post-operative phase (Hunnicutt et al., 2020). Non-contact ACL injuries, which are the most common type, are often attributed to faulty landing mechanics, muscle imbalances, and neuromuscular deficits (Shimokochi & Shultz, 2008). AMI is typically characterized by reduced activation and motor unit output in the vastus medialis and vastus lateralis muscles, leading to poor treatment outcomes, including persistent range of motion and strength deficits, postoperative stiffness (Palmieri-Smith et al., 2013; Sonnery-Cottet et al., 2019). Consequently, assessing maximum voluntary contraction (MVC) of these muscles using Electromyography (EMG) is essential to provide objective data on strength and activation of the quadriceps muscles. Documenting the changes in MVC values can indicate strength improvement or deficits, guiding the rehabilitation and monitoring patient progress. However, no previous study explored the association between PKOS and MVC over time in ACLR population.
Previous studies demonstrated that knee swelling leads to significant quadriceps inhibition and strength deficits (Palmieri-Smith et al., 2013). These findings are supported by a systematic review and meta-analysis confirming persistent deficits in quadriceps strength and volitional activation post-ACLR (Lisee et al., 2019). However, these studies largely focus on experimentally induced knee effusion and do not fully capture the impacts of acute and chronic effects of PKOS following ACLR, including deficits in pain, ROM, MVC and strength due to AMI, persistent impact on functional outcomes. Another study reported that quadriceps strength deficit observed following ACLR is primarily due to muscle atrophy rather than neuromuscular inhibition (Hobson, 2018). However, the study lacks objective measurement of maximum quadriceps strength and activation in the immediate postoperative period (<6 weeks).
Return to sport following ACLR has been shown to be influenced by knee impairments, pain, and functional limitations in the early post-operative period (Lentz et al., 2012). The need for this study arises from the recognized gap in the literature concerning the acute effects of post-operative swelling on key functional outcomes. While there is substantial evidence (Palmieri-Smith et al., 2013; Palmieri et al., 2004) indicating that knee swelling negatively affects muscle strength and functional outcomes in the later stages of recovery, there is limited data on its impact during the immediate post-operative period (Norte et al., 2018).
Therefore, the purpose of this study is to examine the time course of PKOS following ACLR and its association with pain, ROM, MVC, and functional performance in people undergoing ACLR for primary ACL injury. Examining the association of PKOS of ACLR with pain, ROM, MVC, and functional performance early in the rehabilitation process is critical for developing targeted interventions that can enhance recovery trajectories. Targeted neuromuscular strategies aimed at restoring knee extensor strength are essential components of evidence-based ACLR rehabilitation (Buckthorpe et al., 2019).
The Department of Physiotherapy, Kasturba Medical College, Mangalore, conducted this longitudinal study from February 2022 to January 2023. Ethical approval was obtained from the Institutional Ethics Committee, KMC Mangalore (IEC KMC MLR01/2022/12), and the study was registered with India’s Clinical Trial Registry (CTRI/2022/03/041101). Thirty-two participants, aged between 15 and 55, who underwent ACLR were recruited (see Table 1 for demographics) and provided written informed consent, compliant with the latest revision of the Declaration of Helsinki. Participants who underwent ACLR with meniscal repair, had associated fractures, injuries to the collateral ligaments, intraoperative complications like fractures, or postoperative infections were excluded. The overall participant screening and allocation process is depicted in Figure 1.
Following approval of the study, participants were recruited based on the eligibility criteria, and written informed consent was obtained from all participants. Demographic information for all participants was documented, and clinical assessments of PKOS, pain, ROM, and MVC were recorded on day 0, 1, 2, 3, during the 2nd week, and at the 6th week follow-up. The Lower Extremity Functional Scale (LEFS) was administered at the 2-week and 6-week intervals.
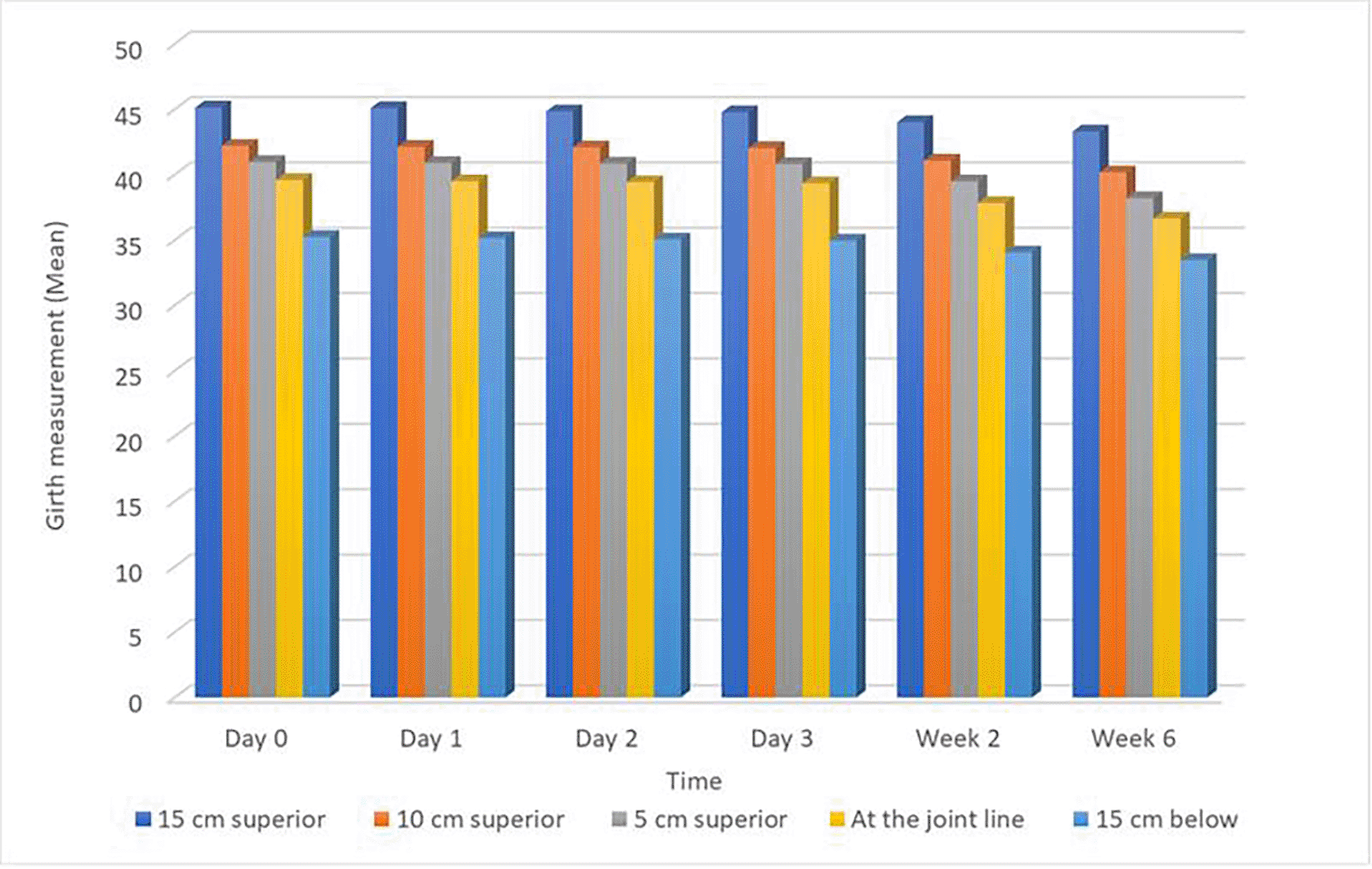
PKOS: Circumferential measurements of the limb were taken using an inch tape at specific levels: at the joint line ( Figure 2), 5 cm superior ( Figure 3), 10 cm superior ( Figure 4), 15 cm superior ( Figure 5), and 15 cm inferior to the joint line ( Figure 6). These measurements are proven to have a high level of reliability in patients recovering from ACLR surgery (Soderberg et al., 1996).
Sensor placement for surface EMG: The placement of the electrodes was according to the SENIAM® (Surface Electromyography for the Non-Invasive Assessment of Muscles) guidelines. The electrode for the rectus femoris is positioned on the imaginary line between the anterior superior iliac spine (ASIS) and the superior region of the patella at 50%. For the vastus medialis, the electrode is positioned at 80% on the line between the ASIS and the joint space in front of the anterior border of the medial ligament. The electrode for the vastus lateralis is positioned at the 2/3rd level on the line from the ASIS to the lateral side of the patella (SENIAM, 2020). The electrode placements used to assess MVC are illustrated in Figure 7.
MVC testing protocol: After the placement of sensors, all participants were asked to perform MVC of the identified muscles for 5 seconds. Participants were verbally encouraged to push as hard as possible for 5 seconds and were permitted to view the computer monitor during the MVC trials for visual feedback and motivation. During this process, MVC was recorded over three trials. EMG analysis was performed following the standard procedure outlined in the operating manual of the Data LITE® wireless system to determine the MVC of each muscle. The recorded data was rectified, and the MVC was documented using the Data LITE® wireless system: Biometrics Ltd application. Each trial was repeated thrice to ensure adequate motor unit data capture. Each trial followed a trapezoidal waveform: three-second ramp up, ten-second sustained contraction at the designated %MVC, and three-second ramp down. Each participant was instructed to follow the trapezoid as closely as possible, with real-time visual feedback displayed on a computer monitor.
Pain was assessed using the Visual Analog Scale (VAS), which has high test-retest reliability (ICC values ranging from 0.71 to 0.99) and moderate concurrent validity (0.71–0.78) (Kahl & Cleland, 2005).
ROM was measured using a standard long arm goniometer, which has high intra-tester reliability (0.99) and validity (0.98) ( Figures 8 & 9) (Brosseau et al., 2001).
The Lower Extremity Functional Scale (LEFS) was used to measure functional outcomes, with test-retest reliability coefficients ranging from 0.85 to 0.95 (Cupido et al., 2014).
The study employed various statistical methods to analyze continuous variables related to knee swelling and its associations with pain, ROM, MVC and LEFS. All continuous variables including pain, ROM, MVC and LEFS were presented as means with standard deviations (SDs) using descriptive statistics. Pearson correlation coefficients (r) was utilized to determine the correlation between PKOS and pain, ROM, MVC, and LEFS for all time points. A p-value of less than 0.05 was considered statistically significant. All analyses were performed with IBM SPSS version 25.0 (Statistical Package for Social Sciences).
The demographic characteristics of the study participants are summarized in Table 1 and Table 2. Thirty-two subjects, comprising 27 males and 7 females, were recruited for the study, with a mean age of 28.47 ± 8.613 years ( Table 2). The age-wise distribution of participants is illustrated in Figure 10. In Table 1, the distribution of participants by age reveals that the majority (62.5%) fell within the 21–30 age range, followed by 31–40 years (15.6%), and participants aged 20 and below accounted for 9.4%. Those above 40 years constituted 12.5% of the sample. Table 2 displays the gender distribution, with males comprising the majority (84.4%) and females representing 15.6% of the total sample. The gender-wise distribution is depicted in Figure 11. These findings suggest that the study sample predominantly consists of young to middle-aged adults, with a greater representation of males.
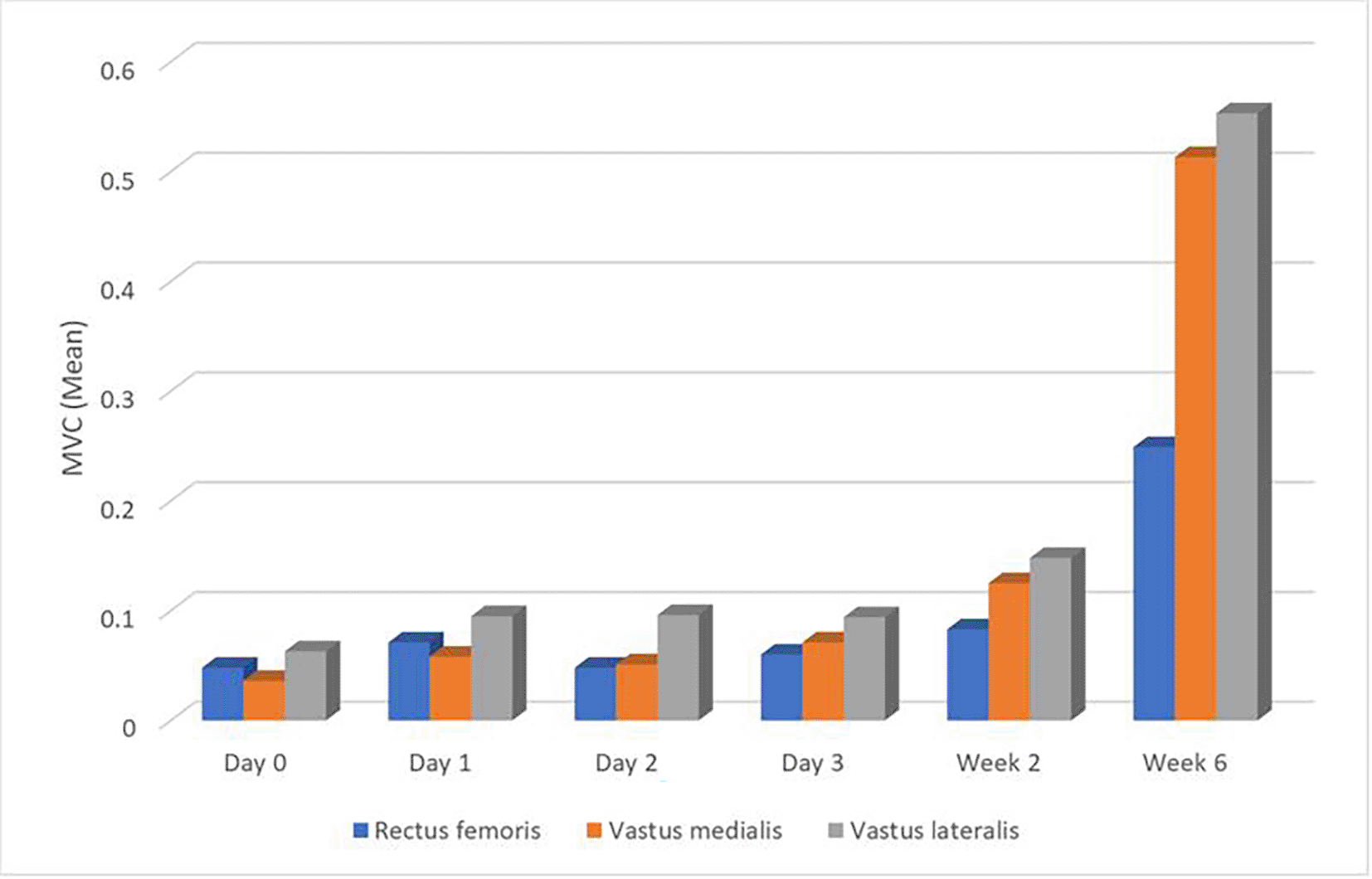
Table 3 presents mean values for various girth measurements, VAS scores, knee flexion ROM measurements, MVC measurements, and LEFS scores. A notable trend observed across multiple parameters is a decrease in measurements as one moves superiorly from 15 cm above the joint line to 15 cm below the joint line on Day 0. This trend persists throughout the entire data collection period, spanning Days 1-3 and Weeks 1 and 2. Specifically, girth measurements, VAS scores, and knee flexion ROM measurements exhibit this pattern consistently. The trend of pain reduction over time is illustrated in Figure 12. In contrast, MVC measurements for the rectus femoris, vastus medialis, and vastus lateralis muscles demonstrate a slight increase over the data collection period. Additionally, LEFS scores exhibit a general upward trend, indicating an overall improvement in lower extremity functional ability throughout the duration of the study. These trends in knee swelling reduction across anatomical sites and time points are visually depicted in Figure 13.
The correlation between PKOS as measured by girth at various locations around the knee, and clinical measures including pain (VAS), was examined from Day 0 to Week 6 post-surgery and is reported in Table 4. Across all time points, there was no significant correlation between knee girth measurements at different locations and VAS scores for activity or at rest on Day 0, suggesting that swelling may not strongly correlate with pain immediately post-surgery. However, as time progressed, a negative correlation between knee girth measurements at superior locations (15 cm, 10 cm, and 5 cm above the joint line) and VAS scores for activity was observed, albeit not consistently significant. This indicates that as knee swelling decreases, pain during activity tends to decrease as well, though not in a statistically significant manner for most time points.
| Day | VAS (activity) | VAS (rest) | |||||
|---|---|---|---|---|---|---|---|
| N | r value | p value | N | r value | p value | ||
| Day 0 | Girth- 15 cm superior | 32 | -0.001 | 0.994 | 32 | 0.176 | 0.334 |
| Girth- 10 cm superior | 32 | -0.144 | 0.432 | 32 | 0.119 | 0.518 | |
| Girth- 5 cm superior | 32 | -0.165 | 0.365 | 32 | 0.104 | 0.571 | |
| Girth- At the joint line | 32 | -0.147 | 0.421 | 32 | 0.070 | 0.705 | |
| Girth- 15 cm below | 32 | -0.100 | 0.584 | 32 | 0.312 | 0.082 | |
| Day 1 | Girth- 15 cm superior | 32 | -0.282 | 0.118 | 32 | -0.067 | 0.714 |
| Girth- 10 cm superior | 32 | -0.261 | 0.149 | 32 | -0.038 | 0.838 | |
| Girth- 5 cm superior | 32 | -0.234 | 0.198 | 32 | -0.015 | 0.935 | |
| Girth- At the joint line | 32 | -0.206 | 0.257 | 32 | -0.001 | 0.995 | |
| Girth- 15 cm below | 32 | -0.328 | 0.067 | 32 | 0.007 | 0.970 | |
| Day 2 | Girth- 15 cm superior | 32 | -0.256 | 0.158 | 32 | 0.257 | 0.155 |
| Girth- 10 cm superior | 32 | -0.285 | 0.114 | 32 | 0.254 | 0.161 | |
| Girth- 5 cm superior | 32 | -0.289 | 0.108 | 32 | 0.189 | 0.300 | |
| Girth- At the joint line | 32 | -0.221 | 0.225 | 32 | 0.178 | 0.331 | |
| Girth- 15 cm below | 32 | -0.399 | 0.024 * | 32 | -0.009 | 0.960 | |
| Day 3 | Girth- 15 cm superior | 32 | -0.351 | 0.049 * | 32 | 0.132 | 0.472 |
| Girth- 10 cm superior | 32 | -0.346 | 0.052 | 32 | 0.202 | 0.267 | |
| Girth- 5 cm superior | 32 | -0.374 | 0.035 * | 32 | 0.189 | 0.300 | |
| Girth- At the joint line | 32 | -0.326 | 0.069 | 32 | 0.276 | 0.127 | |
| Girth- 15 cm below | 32 | -0.272 | 0.132 | 32 | 0.065 | 0.725 | |
| Week 2 | Girth- 15 cm superior | 32 | -0.009 | 0.959 | 32 | 0.257 | 0.156 |
| Girth- 10 cm superior | 32 | -0.028 | 0.879 | 32 | 0.248 | 0.172 | |
| Girth- 5 cm superior | 32 | -0.088 | 0.632 | 32 | 0.177 | 0.333 | |
| Girth- At the joint line | 32 | -0.115 | 0.529 | 32 | -0.096 | 0.602 | |
| Girth- 15 cm below | 32 | -0.027 | 0.885 | 32 | 0.135 | 0.460 | |
| Week 6 | Girth- 15 cm superior | 32 | -0.046 | 0.803 | 32 | 0.187 | 0.307 |
| Girth- 10 cm superior | 32 | 0.005 | 0.980 | 32 | 0.233 | 0.200 | |
| Girth- 5 cm superior | 32 | -0.006 | 0.973 | 32 | 0.194 | 0.287 | |
| Girth- At the joint line | 32 | 0.162 | 0.374 | 32 | 0.230 | 0.206 | |
| Girth- 15 cm below | 32 | 0.075 | 0.684 | 32 | 0.143 | 0.435 | |
Table 5 presents the correlation between knee girth and knee flexion ROM, both active and passive, from Day 0 to Week 6 post-surgery. The study documented active and passive knee flexion ROM from Day 0 to Week 6 post-surgery, showing gradual increases in mean values from 23.44 to 96.63 for active ROM and from 29.13 to 105.09 for passive ROM ( Table 5). Initially, in the early days following ACLR (Day 0-1), no significant correlations were found between knee girth and ROM. However, weak positive correlations emerged over time, particularly at superior locations above the joint line. Significantly, on Day 2, negative correlations suggested a potential link between decreased knee swelling and slight improvements in ROM (15 cm below: r = 0.218, p = 0.231). By Week 6, negative correlations became more pronounced, indicating a potential association between reduced swelling and improved ROM (15 cm superior: r = -0.384, p = 0.030* for active ROM). The progression in active and passive knee flexion over time is visually represented in Figure 14.
| Day | ROM (Active) | ROM (Passive) | |||||
|---|---|---|---|---|---|---|---|
| N | r value | p value | N | r value | p value | ||
| Day 0 | Girth- 15 cm superior | 32 | 0.141 | 0.443 | 32 | 0.121 | 0.511 |
| Girth- 10 cm superior | 32 | 0.204 | 0.263 | 32 | 0.173 | 0.345 | |
| Girth- 5 cm superior | 32 | 0.211 | 0.247 | 32 | 0.195 | 0.284 | |
| Girth- At the joint line | 32 | 0.267 | 0.140 | 32 | 0.263 | 0.146 | |
| Girth- 15 cm below | 32 | 0.263 | 0.145 | 32 | 0.266 | 0.141 | |
| Day 1 | Girth- 15 cm superior | 32 | 0.103 | 0.576 | 32 | 0.067 | 0.715 |
| Girth- 10 cm superior | 32 | 0.111 | 0.545 | 32 | 0.083 | 0.652 | |
| Girth- 5 cm superior | 32 | 0.089 | 0.630 | 32 | 0.065 | 0.725 | |
| Girth- At the joint line | 32 | 0.171 | 0.349 | 32 | 0.154 | 0.401 | |
| Girth- 15 cm below | 32 | 0.331 | 0.064 | 32 | 0.299 | 0.096 | |
| Day 2 | Girth- 15 cm superior | 32 | -0.133 | 0.468 | 32 | -0.110 | 0.549 |
| Girth- 10 cm superior | 32 | -0.099 | 0.588 | 32 | -0.076 | 0.681 | |
| Girth- 5 cm superior | 32 | -0.098 | 0.595 | 32 | -0.067 | 0.715 | |
| Girth- At the joint line | 32 | -0.008 | 0.965 | 32 | 0.020 | 0.913 | |
| Girth- 15 cm below | 32 | 0.133 | 0.469 | 32 | 0.157 | 0.391 | |
| Day 3 | Girth- 15 cm superior | 32 | -0.121 | 0.508 | 32 | -0.095 | 0.605 |
| Girth- 10 cm superior | 32 | -0.081 | 0.659 | 32 | -0.060 | 0.744 | |
| Girth- 5 cm superior | 32 | -0.033 | 0.859 | 32 | -0.004 | 0.983 | |
| Girth- At the joint line | 32 | 0.042 | 0.821 | 32 | 0.072 | 0.696 | |
| Girth- 15 cm below | 32 | 0.121 | 0.511 | 32 | 0.109 | 0.553 | |
| Week 2 | Girth- 15 cm superior | 32 | -0.148 | 0.418 | 32 | -0.070 | 0.702 |
| Girth- 10 cm superior | 32 | -0.204 | 0.263 | 32 | -0.141 | 0.442 | |
| Girth- 5 cm superior | 32 | -0.143 | 0.435 | 32 | -0.086 | 0.641 | |
| Girth- At the joint line | 32 | -0.022 | 0.904 | 32 | 0.012 | 0.950 | |
| Girth- 15 cm below | 32 | 0.039 | 0.833 | 32 | 0.069 | 0.707 | |
| Week 6 | Girth- 15 cm superior | 32 | -0.287 | 0.111 | 32 | -0.347 | 0.052 |
| Girth- 10 cm superior | 32 | -0.294 | 0.102 | 32 | -0.374 | 0.035 * | |
| Girth- 5 cm superior | 32 | -0.277 | 0.124 | 32 | -0.359 | 0.044 * | |
| Girth- At the joint line | 32 | -0.113 | 0.538 | 32 | -0.233 | 0.199 | |
| Girth- 15 cm below | 32 | -0.263 | 0.147 | 32 | -0.338 | 0.058 | |
Table 6 illustrates the correlation between knee girth and MVC of the rectus femoris, vastus medialis, and vastus lateralis muscles from Day 0 to Week 6 post-surgery. Initially, on Day 0, no significant correlations were observed between knee girth and MVC measurements for all three muscles. However, as the recovery progressed, weak and inconsistent correlations emerged, with some time points showing positive or negative correlations, while others showed no significant associations. At Week 6, however, negative correlations between knee girth and MVC measurements were statistically significant for all three muscles: rectus femoris (r = -0.384, p = 0.030*), vastus medialis (r = -0.438, p = 0.012*), and vastus lateralis (r = -0.433, p = 0.013*). These changes in muscle activity over time are illustrated in Figure 15.
| Day | Rectus Femoris | Vastus medialis | Vastus Lateralis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | r value | p value | N | r value | p | N | r value | p value | ||
| Day 0 | Girth- 15 cm superior | 32 | 0.114 | 0.534 | 32 | 0.111 | 0.544 | 32 | -0.035 | 0.848 |
| Girth- 10 cm superior | 32 | 0.109 | 0.552 | 32 | 0.070 | 0.703 | 32 | -0.069 | 0.707 | |
| Girth- 5 cm superior | 32 | 0.077 | 0.677 | 32 | 0.070 | 0.703 | 32 | -0.115 | 0.531 | |
| Girth- At the joint line | 32 | 0.036 | 0.844 | 32 | -0.090 | 0.625 | 32 | -0.086 | 0.639 | |
| Girth- 15 cm below | 32 | -0.088 | 0.633 | 32 | -0.022 | 0.907 | 32 | -0.003 | 0.985 | |
| Day 1 | Girth- 15 cm superior | 32 | -0.038 | 0.835 | 32 | 0.004 | 0.983 | 32 | -0.190 | 0.298 |
| Girth- 10 cm superior | 32 | -0.105 | 0.566 | 32 | 0.011 | 0.954 | 32 | -0.157 | 0.391 | |
| Girth- 5 cm superior | 32 | -0.116 | 0.528 | 32 | -0.026 | 0.886 | 32 | -0.164 | 0.371 | |
| Girth- At the joint line | 32 | -0.082 | 0.654 | 32 | 0.035 | 0.848 | 32 | -0.146 | 0.424 | |
| Girth- 15 cm below | 32 | 0.008 | 0.963 | 32 | 0.121 | 0.509 | 32 | -0.111 | 0.547 | |
| Day 2 | Girth- 15 cm superior | 32 | 0.017 | 0.925 | 32 | -0.059 | 0.749 | 32 | -0.234 | 0.198 |
| Girth- 10 cm superior | 32 | 0.124 | 0.497 | 32 | 0.039 | 0.833 | 32 | -0.120 | 0.513 | |
| Girth- 5 cm superior | 32 | 0.058 | 0.751 | 32 | 0.078 | 0.671 | 32 | -0.080 | 0.662 | |
| Girth- At the joint line | 32 | 0.055 | 0.765 | 32 | 0.098 | 0.594 | 32 | -0.048 | 0.794 | |
| Girth- 15 cm below | 32 | 0.218 | 0.231 | 32 | 0.140 | 0.446 | 32 | -0.041 | 0.823 | |
| Day 3 | Girth- 15 cm superior | 32 | 0.025 | 0.891 | 32 | -0.259 | 0.152 | 32 | -0.209 | 0.252 |
| Girth- 10 cm superior | 32 | 0.130 | 0.479 | 32 | -0.189 | 0.300 | 32 | -0.084 | 0.649 | |
| Girth- 5 cm superior | 32 | 0.097 | 0.598 | 32 | -0.146 | 0.424 | 32 | -0.028 | 0.878 | |
| Girth- At the joint line | 32 | 0.111 | 0.545 | 32 | -0.088 | 0.633 | 32 | 0.047 | 0.799 | |
| Girth- 15 cm below | 32 | 0.227 | 0.211 | 32 | -0.047 | 0.800 | 32 | 0.074 | 0.689 | |
| Week 2 | Girth- 15 cm superior | 32 | -0.098 | 0.593 | 32 | -0.088 | 0.632 | 32 | -0.013 | 0.943 |
| Girth- 10 cm superior | 32 | -0.099 | 0.589 | 32 | -0.085 | 0.645 | 32 | 0.008 | 0.963 | |
| Girth- 5 cm superior | 32 | -0.108 | 0.555 | 32 | -0.144 | 0.431 | 32 | -0.001 | 0.997 | |
| Girth- At the joint line | 32 | -0.039 | 0.832 | 32 | 0.011 | 0.954 | 32 | 0.018 | 0.924 | |
| Girth- 15 cm below | 32 | 0.246 | 0.174 | 32 | 0.166 | 0.365 | 32 | 0.182 | 0.318 | |
| Week 6 | Girth- 15 cm superior | 32 | -0.384 | 0.030 * | 32 | -0.438 | 0.012 * | 32 | -0.433 | 0.013 * |
| Girth- 10 cm superior | 32 | -0.482 | 0.005 * | 32 | -0.456 | 0.009 * | 32 | -0.453 | 0.009 * | |
| Girth- 5 cm superior | 32 | -0.491 | 0.004 * | 32 | -0.427 | 0.015 * | 32 | -0.430 | 0.014 * | |
| Girth- At the joint line | 32 | -0.276 | 0.126 | 32 | -0.204 | 0.263 | 32 | -0.161 | 0.380 | |
| Girth- 15 cm below | 32 | -0.077 | 0.674 | 32 | -0.129 | 0.481 | 32 | -0.102 | 0.579 | |
Table 7 presents the correlation between knee girth and LEFS from Day 0 to Week 6 post-surgery. At Week 2, no significant correlations were found between knee girth and LEFS scores at any measurement location. However, a positive correlation was observed between knee girth 15 cm below the joint line and LEFS scores (r = 0.176, p = 0.335). By Week 6, negative correlations between knee girth and LEFS scores were observed at several measurement locations. Specifically, significant negative correlations were found for knee girth 5 cm superior (r = -0.375, p = 0.034*) and knee girth at the joint line (r = -0.340, p = 0.057)
| Day | LEFS | |||
|---|---|---|---|---|
| N | r value | p value | ||
| Week 2 | Girth- 15 cm superior | 32 | -0.134 | 0.465 |
| Girth- 10 cm superior | 32 | -0.123 | 0.501 | |
| Girth- 5 cm superior | 32 | -0.148 | 0.418 | |
| Girth- At the joint line | 32 | -0.043 | 0.815 | |
| Girth- 15 cm below | 32 | 0.176 | 0.335 | |
| Week 6 | Girth- 15 cm superior | 32 | -0.254 | 0.160 |
| Girth- 10 cm superior | 32 | -0.264 | 0.145 | |
| Girth- 5 cm superior | 32 | -0.375 | 0.034 * | |
| Girth- At the joint line | 32 | -0.340 | 0.057 | |
| Girth- 15 cm below | 32 | -0.200 | 0.273 | |
The objective of the study was to find an association between swelling at the knee joint with pain, ROM, muscle strength and functional outcome after ACLR.
In our study, the maximum number of participants who underwent ACLR were from the age group of 20-30 years; this could be attributed to their active lifestyle in comparison to those of the other age ranges. There were more male than female participants in our study despite the fact that the incidence rates of ACL injury are reported to be 2.4–9.5 times higher in females ( Sanders et al., 2016; Lew et al., 2021). This could be as a result of higher participation of males than females in contact or high-intensity sports, which is considered as a risk factor for ACL injury (Sanders et al., 2016).
The findings of our study suggested that girth of the knee and VAS on activity and at rest decreased from day 0 to week 6, however no significant association was seen between the two parameters, it was also noted that muscle inhibition persisted with presence of swelling, similarly studies have also suggested that in the absence of pain, quadriceps inhibition was noted in knee with artificially induced swelling (Hopkins & Ingersoll, 2000; Wood et al., 1988). Though the mechanism behind muscle inhibition has been poorly understood, it is believed that swelling on its own can cause considerable quadriceps AMI (Rice & McNair, 2010; Rice et al., 2014). Studies have revealed that intra articular swelling significantly reduces EMG activation and H reflex amplitude despite the absence of structural injury, pain or joint effusion (Wood et al., 1988; Rice et al., 2009) Additionally, joint infusion has been shown to significantly increase group II joint afferent discharge in animal studies by raising intra articular pressure and activating stretch and pressure-sensitive mechanoreceptors (Grigg & Hoffman, 1982; Wood & Ferrell, 1984). In the spinal cord, group I non reciprocal (Ib) inhibitory interneurons are known to be excited by group II joint afferents, inhibiting quadriceps alpha motor neurons and preventing complete activation of the muscle (Iles et al., 1990; Lundberg et al., 1978). Recent theoretical models further support these findings, emphasizing both spinal and supraspinal pathways contributing to persistent AMI and its impact on quadriceps activation (Norte et al., 2021). This is further corroborated by findings from experimental joint effusion models, which show that both pre- and post-synaptic spinal inhibition play key roles in arthrogenic quadriceps suppression (Palmieri et al., 2004).
AMI takes a central role in the injury cycle. Athletes who sustain joint injuries have mobility and ROM deficiencies. Reduced ROM can be caused by pain, swelling, muscle spasms, or the inability of the muscles around the joint to contract effectively. This causes muscle atrophy, weakness, and a slower rate of functional recovery (Hopkins & Ingersoll, 2000; Young, 1993). Recent studies have also shown that joint effusion can reduce corticomotor excitability of the quadriceps, contributing to persistent voluntary activation failure in the early phase of rehabilitation (Lepley et al., 2015).
In the present study, it was found that there was a statistically significant correlation between girth and passive ROM for knee flexion for week 6 when measured at 10 cm and 5 cm superior to the joint line. Though there was no statistically significant correlation noted between girth and ROM at other time points, there was an improvement in the knee flexion ROM from day 0 to week 6 which could be explained by the decreased levels of interstitial and intracapsular swelling following rehabilitation (Wilk et al., 2022). LEFS values were measured after 2 weeks and 6 weeks and it was seen that the mean values for LEFS increased from week 2 to week 6 for all the subjects. Similar longitudinal improvements in LEFS scores following ACLR have been reported previously, reflecting functional recovery over early rehabilitation periods (Alcock et al., 2012). However, the results showed that there was no association between girth measured at different points and LEFS after 2 weeks and 6 weeks.
The reduction in ROM following ACLR is due to multifactorial reasons, furthermore, pain catastrophizing and kinesiophobia could play a role in active avoidance of movement due to fear of recurring pain or injury and this could impair daily functioning and can have a detrimental effect on rehabilitation outcomes in the long-term (Österberg et al., 2013; Quartana et al., 2009). Patients may avoid actions that could potentially result in pain or re-injury which can in turn lead to unfavorable views towards one’s body and avoid participation in daily activities. After musculoskeletal injury and surgery, delayed recovery and discharge are frequently caused by pain catastrophizing and fear of pain (Leeuw et al., 2007; Tichonova et al., 2016). A higher level of pain catastrophizing was highly correlated with a greater level of pain in the knee while performing activities of daily living before and after rehabilitation. A higher degree of kinesiophobia was strongly correlated with more difficulties experienced while carrying out daily activities (Tichonova et al., 2016).
In the present study, it was seen that MVC values for rectus femoris, vastus medialis and vastus lateralis showed a gradual increase from day 0 to week 6 except from day 2 to day 3. A significant correlation was observed between girth measured at 15 cm, 10 cm and 5 cm superior to the joint line and MVC for the muscles at 6 weeks whereas other measurements for girth from day 0 to week 6 showed no association with MVC for rectus femoris, vastus medialis and vastus lateralis. This could be possibly due to swelling and alterations in skin impedance which can give rise to artifacts influencing EMG readings (Jun Kimura, 2013). Similarly, in a study done by Drechsler WI et al. (2006), changes in isometric muscle strength, voluntary activation and EMG parameters were monitored in relation to pain, swelling and stability in the knee joint in 31 patients after ACLR and it was found that there was no association between quadriceps activation levels and swelling, which was measured at 1 and 3 months after ACLR (Drechsler et al., 2006). The data from the current study supports this premise and expands on their work in that multiple factors could be responsible in influencing the MVC values after ACLR. Our study suggests that while swelling decreased over a period of time, parameters like pain, ROM, MVC and LEFS improved, but there was no statistically significant association found between these variables. These findings align with previous evidence showing that quadriceps function following ACLR may not always correlate directly with swelling or pain but can still influence patient-reported outcomes (Lepley et al., 2018; Loyd et al., 2019). Furthermore, a recent longitudinal study further supported these trends, showing weak correlations between swelling and strength or function in the early weeks following ACL reconstruction (Prabhakar, 2025). These observations highlight the clinical importance of restoring knee extensor strength through progressive neuromuscular rehabilitation after ACL reconstruction (Buckthorpe et al., 2019).
The variables that were examined, including swelling, pain, ROM, MVC, and functional outcome, all improved overall, however swelling by itself had no significant correlation with any of the variables. Though swelling has its own effect that might influence the rehabilitation outcomes, its management should not be given any lesser importance. Therapeutic modalities such as neuromuscular electrostimulation have been shown to reduce post-operative knee effusion, pain, and swelling following ACL reconstruction, thus supporting the need for proactive swelling management (Ediz et al., 2012).
The Department of Physiotherapy, Kasturba Medical College, Mangalore, conducted this longitudinal study from February 2022 to January 2023. Ethical approval was obtained from the Institutional Ethics Committee, KMC Mangalore (IEC KMC MLR01/2022/12), and the study was registered with India’s Clinical Trial Registry (CTRI/2022/03/041101).
Open Science Framework (OSF): Correlation Between Post-Operative Knee Swelling and Pain, Maximal Voluntary Muscle Contraction, and Function Following ACL Reconstruction: A Longitudinal Study, 10.17605/OSF.IO/BKUX4 (Prabhakar, A. J. 2025).
This project contains the following underlying data:
ACLR DATA ENTRY (2).xlsx
Data are available under the terms of the CC-By Attribution-NonCommercial-NoDerivatives 4.0 International (CC-BY 4.0).
We would like to thank Manipal Academy of Higher Education, Manipal, India and all the participants of the study. We would also like to acknowledge Dr. Sucharitha Suresh and Mr. Shyam Krishnan for their contribution towards data analysis and EMG analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)