Keywords
Chronic Myeloid Leukemia, Imatinib, Nilotinib, Hematologic Response
This article is included in the Oncology gateway.
Chronic Myeloid Leukemia (CML) is a myeloproliferative neoplasm driven by the BCR–ABL1 fusion gene. Tyrosine kinase inhibitors (TKIs), such as imatinib and nilotinib, have transformed CML management by significantly improving hematologic and molecular responses. However, real-world data from low- to middle-income countries, including Indonesia, remain limited.
This study aimed to evaluate early hematologic responses after three months of TKI therapy in CML patients and to assess the influence of demographic and clinical factors on treatment outcomes.
A retrospective cohort study was conducted at Wahidin Sudirohusodo Hospital, Makassar, reviewing medical records of 43 adult CML patients treated with imatinib or nilotinib from January to December 2024. Hematologic parameters were analyzed at baseline and monthly for three months. Associations between treatment response and patient characteristics were assessed using GLM-Repeated Measures and MANOVA.
Both TKI regiments significantly improved hematologic parameters (p < 0.001), with reductions in leukocyte counts and increases in hemoglobin and hematocrit. No significant interaction was found between time and treatment type (p > 0.05), indicating comparable efficacy. Patients receiving nilotinib showed a higher rate of complete response (60%) than those on imatinib (32.1%), though not statistically significant (p = 0.078). Body weight and BMI significantly influenced hematologic improvements (p < 0.05), while age did not.
Imatinib and nilotinib both effectively induce early hematologic responses in CML patients. While nilotinib showed a trend toward superior outcomes, statistical equivalence suggests both remain viable options. BMI and body weight are important predictors of response, highlighting the need for individualized treatment strategies. Further large-scale studies with extended follow-up are warranted to confirm long-term benefits and refine patient-specific therapy.
Chronic Myeloid Leukemia, Imatinib, Nilotinib, Hematologic Response
We have added the parameters in each figure and added the discussion regarding cost-effectiveness and possible drug reaction. We also added literatures specifically regarding haematological responses, albeit limited to non-comparison studies but we have tried to correlate to the current findings to further engage in a more feasible applicative outcome. in the discussion.
See the authors' detailed response to the review by George Nesr
See the authors' detailed response to the review by Deniz Ozmen
Chronic Myeloid Leukemia (CML) is a clonal myeloproliferative neoplasm caused by the BCR–ABL1 fusion gene, which results from a reciprocal translocation between chromosomes 9 and 22, known as the Philadelphia chromosome (t(9;22)(q34;q11)). This fusion gene encodes a constitutively active tyrosine kinase, leading to uncontrolled proliferation of myeloid cells and resistance to apoptosis.1–3 Globally, the incidence of CML is estimated to range from 0.6 to 2.8 per 100,000 population per year.4 Although the median age at diagnosis in Western countries is around 60 years, several studies indicate that the age at diagnosis in some Asian countries, including Indonesia, tends to be younger. In addition, many CML cases in Indonesia are detected in the late chronic phase while still asymptomatic.4
Over the past two decades, the management of CML has advanced dramatically with the advent of tyrosine kinase inhibitors (TKIs) such as imatinib, which have significantly improved patient outcomes.5–7 TKIs work by phosphorylating amino acids on enzyme substrates, thereby altering signal transduction and inducing cellular changes. Imatinib is a first-generation TKI that directly inhibits tyrosine kinase activity, affecting cell cycle and adhesion, ultimately leading to apoptosis. Second-generation TKIs such as nilotinib were developed to overcome resistance to imatinib by specifically and selectively inhibiting BCR-ABL autophosphorylation and suppressing cancer cell proliferation.8,9
Currently, second-generation TKIs like dasatinib, nilotinib, and bosutinib are available as first-line therapies, with the potential to induce faster and deeper molecular responses, especially in high-risk patients based on the Sokal or ELTS score.1,3 Although there is no conclusive evidence from large clinical trials showing significant survival benefits of second-generation TKIs over first-generation ones, real-world data suggest that second-generation TKIs can accelerate the achievement of early molecular response (EMR) and increase the likelihood of achieving treatment-free remission (TFR).3,10
TKIs have not only transformed the prognosis of CML into a controllable chronic disease but have also made TFR a realistic treatment goal for some patients. Nevertheless, around 20–30% of patients experience resistance or intolerance to TKIs, and 6–7% remain at risk of progressing to the blast phase, which continues to pose a challenge in long-term management. Moreover, most patients still require long-term therapy, as not all are eligible for TFR.11,12
Response to TKI therapy is assessed through hematologic, cytogenetic, and molecular parameters, with molecular response being the primary monitoring parameter due to its sensitivity and specificity for residual disease burden. Early molecular response, particularly achieving BCR–ABL1 ≤10% at 3 months, has been strongly correlated with better long-term outcomes such as improved progression-free survival (PFS) and overall survival (OS). Hematologic response is assessed via complete blood count during the first three months of therapy. Studies have shown that imatinib and nilotinib produce similar hematologic responses.2,3,10,13 Therapeutic response in CML patients is influenced by several factors. Higher body weight and BMI have been associated with poorer treatment response due to reduced drug levels in the blood. Other factors such as age and height were found not to affect treatment response.14
Although numerous large clinical trials have evaluated the efficacy of various TKIs, most of these studies were conducted in Western populations. Meanwhile, real-world data from Indonesia and other low- to middle-income countries remain minimally known. Considering regional differences in disease presentation, access to healthcare, drug availability, and cost, local evaluation of treatment outcomes becomes crucial. In Indonesia, the availability of TKIs is still uncommon and highly dependent on regional government support for procurement through local health budgets (APDB), particularly in primary care settings such as community health centers. Cost also plays a significant role, with imatinib being more accessible through the national health insurance due to its lower price, compared to nilotinib, which is more expensive and only available in major hospitals. Evaluating hematologic response at the third month as the most simple method is strongly associated with long-term prognosis which can provide valuable insights for optimizing treatment strategies for CML patients in Indonesia.2,10,15,16
Therefore, this study aims to evaluate the hematologic responses in CML patients after three months of TKI therapy and analyze the influence of demographic and confounding factors such as age, sex, disease phase at diagnosis, and baseline laboratory parameters on early treatment outcomes.
This analytical observational study employed a retrospective cohort design. Informed consent was not required for this study, as no direct contact was made with participants where data derived from patient medical records. The study was conducted at the Hematology Medical Oncology Outpatient Clinic of Wahidin Sudirohusodo Hospital, spanning from January to December 2024. Data management and statistical analysis were performed at the Department of Public Health, Faculty of Medicine, Hasanuddin University.
The target population was adult patients diagnosed with CML. The accessible population included all adult CML patients treated at Wahidin Sudirohusodo Hospital during the specified study period. Study participants were selected using non-random consecutive sampling. Inclusion criteria were: aged 18 years or older, diagnosed with CML based on history, physical and laboratories examination, and treated with TKIs either imatinib or nilotinib for at least three consecutive months conducted at the study site. Patients who had received hydroxyurea prior to TKI therapy were excluded.
The primary dependent variable was therapeutic response which is hematologic response after three months of treatment, classified as complete (WBC and PLT count back to normal, no immature granulocytes, no blasts in peripheral blood, no blasts and basophil in peripheral blood, and no sign or symptoms of disease), partial (improvement in blood counts, but not all complete response met), or failed (no improvement in blood counts) response based on hematologic parameters and NCCN guidelines. The independent variable was the type of TKI therapy—imatinib (400 mg once daily, orally) or nilotinib (300 mg twice daily, or 150 mg × 2 every 12 hours, orally). Confounding variables included age, sex, weight, height, and Body Mass Index (BMI), all derived from patients’ identity cards and standard measurements recorded at the first visit of the outpatient clinic. BMI was calculated by dividing body weight in kilograms by the square of height in meters (kg/m2).
Statistical analysis began with descriptive statistics, assessing qualitative data as frequency and percentage distributions, and quantitative data using means, standard deviations, medians, and ranges. Data normality was evaluated using the Kolmogorov–Smirnov or Shapiro–Wilk tests. For comparisons of hematologic parameters across four time points (diagnosed to three moths therapy), Repeated Measures ANOVA was applied to normally distributed data, while the Friedman test was used for non-normally distributed data. Post-hoc analysis (e.g., Bonferroni correction) was conducted when needed. To evaluate the effect of treatment type on changes over time, Mixed Between-Within Subjects ANOVA or Repeated Measures ANCOVA was employed, accounting for interactions between treatment and time. The General Linear Model (GLM) was used to assess covariate influences on laboratory outcomes. Chi-square tests were applied to examine associations between treatment regiment and therapeutic response category; McNemar’s test was considered when comparing paired binary outcomes. Statistical significance was defined as a p-value < 0.05 with a 5% alpha level and 80% statistical power. All analyses were conducted using SPSS version 22.
This study involved 43 subjects, all diagnosed with Chronic Myeloid Leukemia (CML) who received imatinib or nilotinib for a period of three months at Wahidin Sudirohusodo Hospital, Makassar, from January to December 2024. The sample was predominantly female (60.5%), with most patients receiving imatinib (65.1%) rather than nilotinib (34.9%). The mean age of the respondents was 42.28 years, with a mean body weight of 53 kg, mean height of 157 cm, and a mean body mass index (BMI) of 21.56 kg/m2 ( Table 1).
Table 1 shows the basic subject characteristic where dominantly (60.5%) are female gender with 65.1% with imatinib treatment. The average age of the subject was 42 years old, with 54 kg of bodyweight, 157 cm of height and 21.56 kg/m2 of body mass index.
Overall, the patients demonstrated a progressive improvement in hematological parameters over the three-month treatment course. A substantial reduction was observed in leukocyte counts, from a mean of 252,398 cells/μL at baseline to 17,219 cells/μL by the third month ( Figure 1). Similarly, hemoglobin levels increased from 9.42 g/dL to 11.48 g/dL ( Figure 2), and hematocrit improved from 28.45% to 35.16% ( Figure 3). The platelet count, initially high (640,953 cells/μL), decreased significantly to 318,186 cells/μL by the third month, indicating hematological stabilization in response to therapy ( Figure 4).
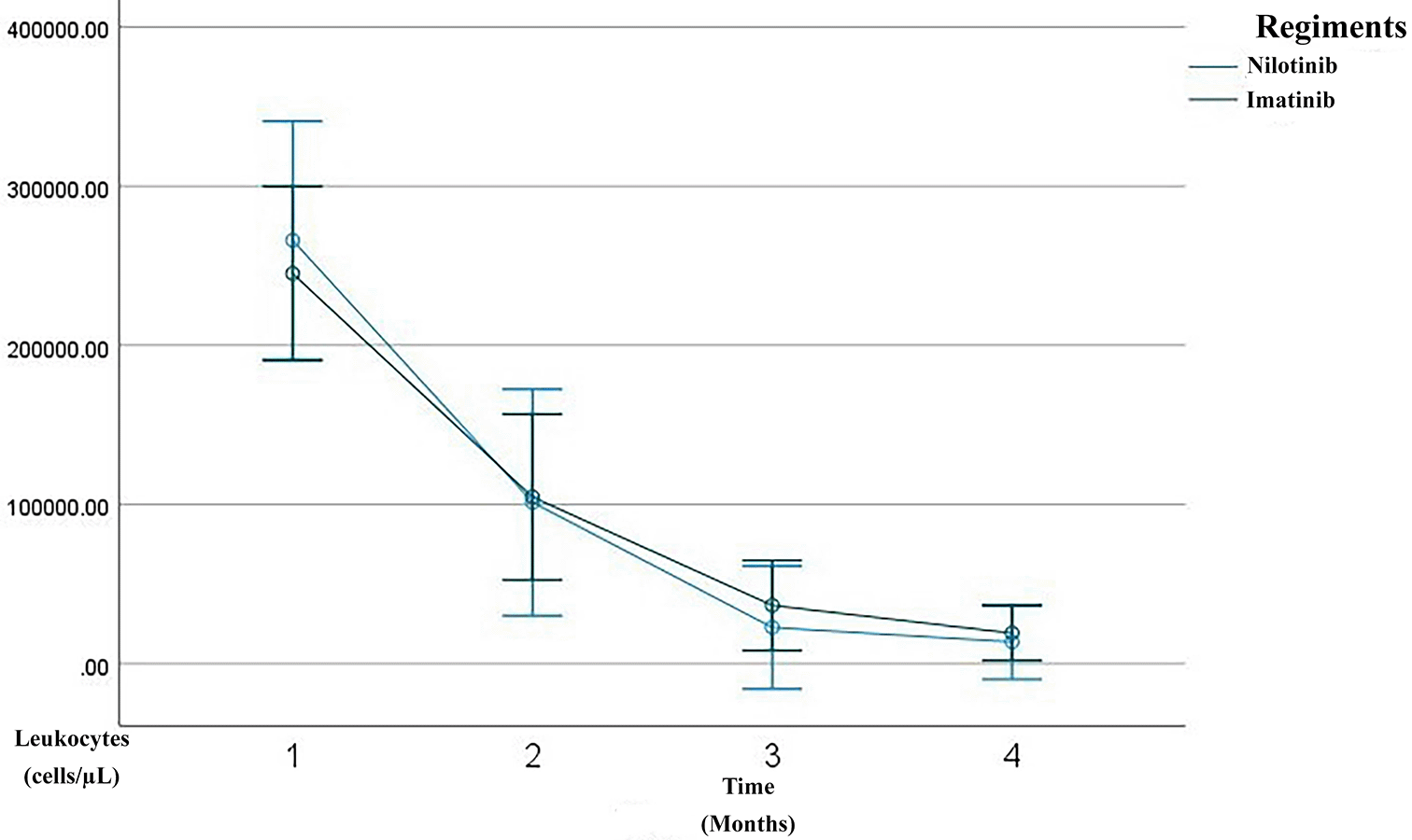
Line graph showing serial measurements of leukocyte count over the first 3 months of treatment. The imatinib group is depicted in green, the nilotinib in blue. A rapid decline in leukocyte count was observed within the three month of therapy, consistent with a complete hematologic response (CHR).
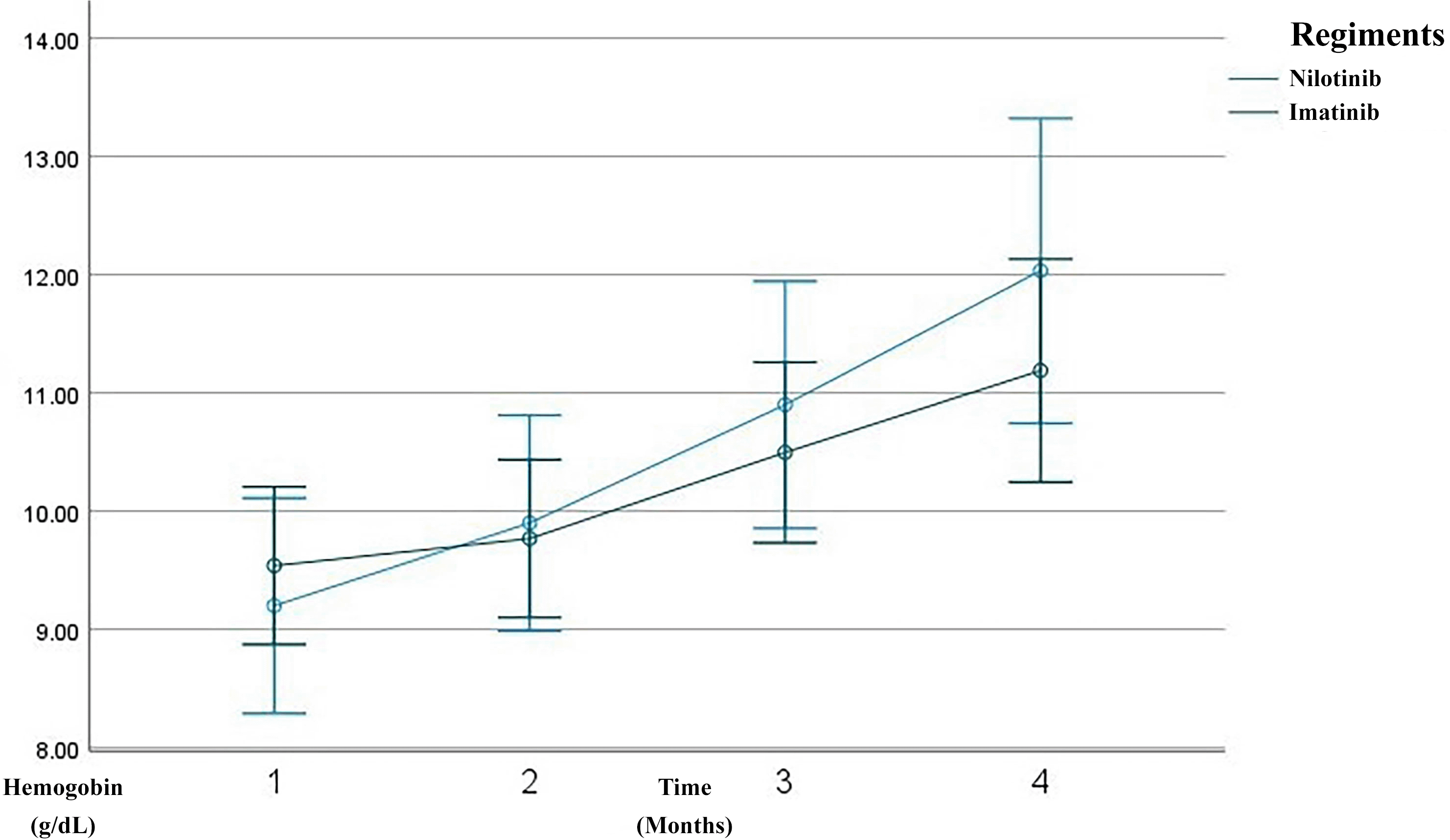
Line graph showing serial measurements of hemoglobin level over the first 3 months of treatment. The imatinib group is depicted in green, the nilotinib in blue. Hemoglobin gradually improved over time, reflecting recovery of normal hematopoiesis.
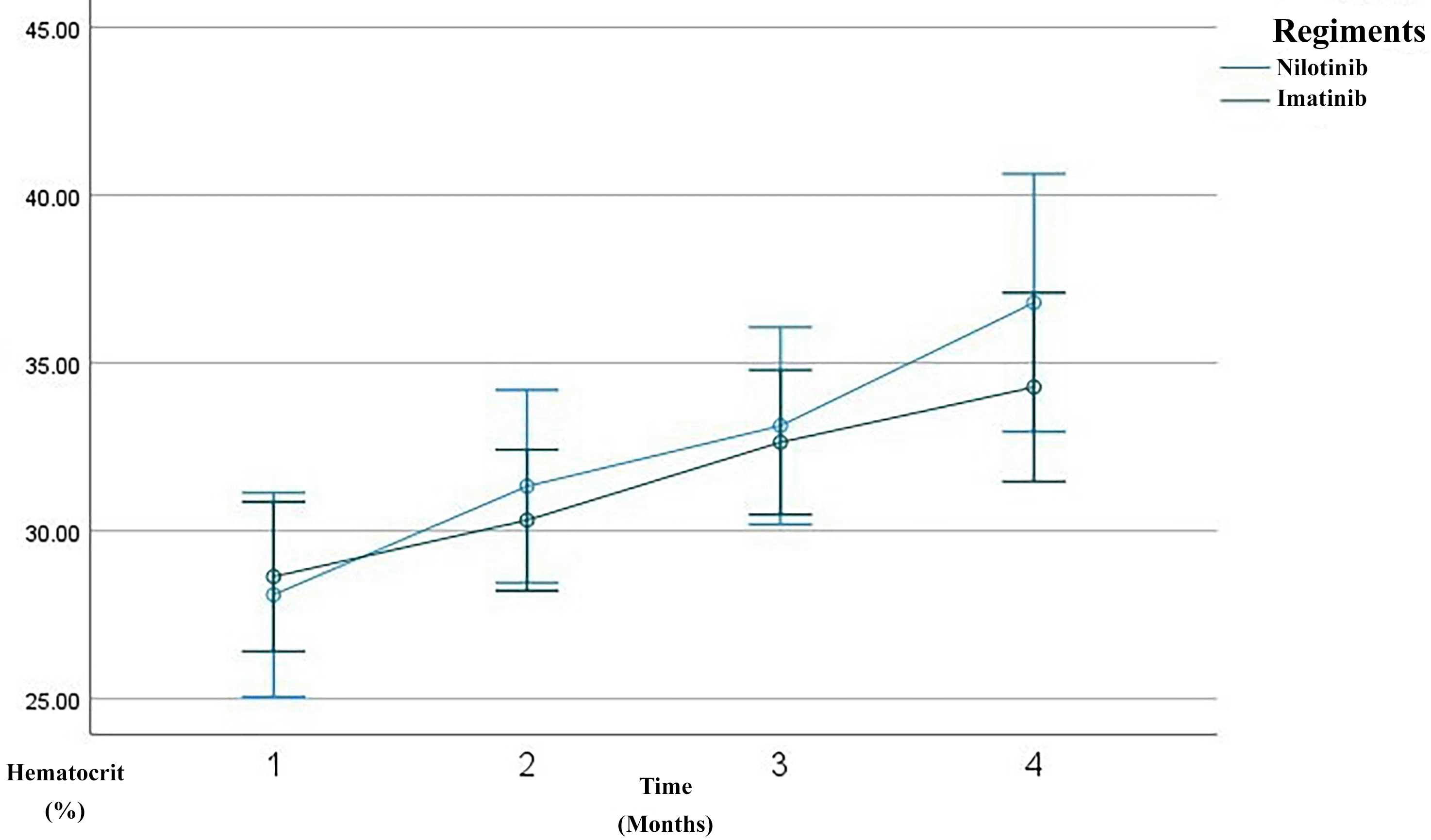
Line graph showing serial measurements of hematocrit level over the first 3 months of treatment. The imatinib group is depicted in green, the nilotinib in blue. Hemoglobin gradually improved over time, reflecting recovery of normal hematopoiesis.
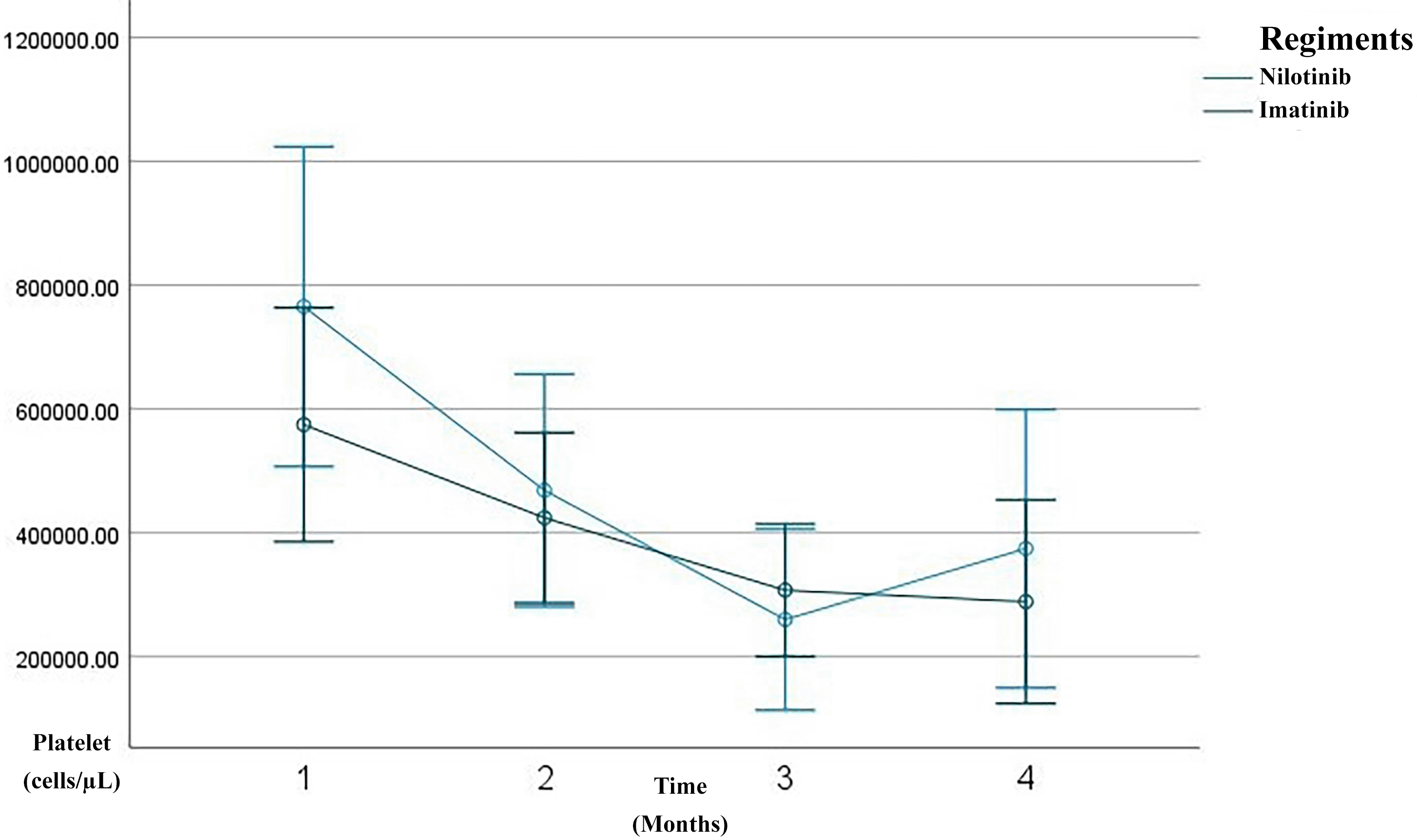
Line graph showing serial measurements of Platelet count over the first 3 months of treatment. The imatinib group is depicted in green, the nilotinib in blue. A rapid decline in platelet count was observed within the three month of therapy, consistent with a complete hematologic response (CHR).
Both imatinib and nilotinib regiments showed similar trends of improvement across major hematological indices, including decreased leukocyte and platelet count, increased hemoglobin, and hematocrit, over the course of three months. Using Generalized Linear Model Repeated Measures (GLM-RM) analysis, a significant main effect of time was observed for these parameters (p < 0.001), indicating that hematological values improved with continued therapy. However, the interaction between time and drug regiment did not yield statistically significant differences for most parameters (p > 0.05), suggesting that both imatinib and nilotinib had comparable efficacy in modifying hematological profiles within the longer observed period ( Table 2). Although slight variations in response dynamics were noted, such as for example, nilotinib showed a steeper decline in leukocyte count and a more pronounced increase in hemoglobin, these differences were not statistically significant. Likewise, changes in MCV, MCH, neutrophils, lymphocytes, monocytes, eosinophils, and basophils were observed over time, but again without meaningful distinction between regiments in their interaction with time.
Table 2 refers to the changes in the hematological parametes, where improvement in leukocyte and platelet counts over 3 months therapy, and increase in hemoglobin and hematocrit wither in imatinib or nilotinib group. The p-value for time is p < 0.05 while time and regiment p > 0.05.
Further analysis using Multivariate Analysis of Variance (MANOVA) examined the influence of confounding factors on hematological improvements. The study revealed that body weight and BMI significantly affected changes in leukocyte count, hemoglobin, hematocrit, and MCV during the therapy period (p < 0.05). Conversely, age did not show a statistically significant influence on any of the hematological parameters assessed. This indicates that patient anthropometric factors may play a role in hematological response to TKI therapy, whereas age alone does not appear to be a determinant of hematological recovery in the short-term therapeutic window ( Table 3).
Table 3 shows the relationship between hematological parameters with several confounding factors such as: age, body weight, body height, BMI and regiment type. Among all, body weight and BMI were significant with the hematological parameters.
After three months of treatment, patients receiving nilotinib exhibited a higher rate of complete response (60%) compared to those receiving imatinib (32.1%). Although this trend suggests a potential clinical advantage of nilotinib, statistical analysis using Chi-square and McNemar tests showed no significant difference across response categories (p > 0.05). When treatment outcomes were simplified into dichotomous categories (“response” vs “failure”), the results remained statistically non-significant (p = 0.894), indicating that both therapies were generally comparable in terms of achieving initial treatment success. Nevertheless, the observed trend toward better response in the nilotinib group warrants further investigation, particularly in larger cohorts or with extended follow-up periods ( Table 4).
Table 4 shows that more subjects reached complete response after 3 months in the nilotinib group compared to imatinib, however there were no relationship in the statistical analysis (p>0.05).
After three months of TKI therapy, both imatinib and nilotinib significantly improved hematological parameters in patients with CML. Overall, leukocyte counts markedly decreased from a baseline mean of 252,398/μL to 17,219/μL by month three, consistent with the known mechanism of TKIs in inhibiting BCR–ABL1 tyrosine kinase activity and promoting leukemic cell apoptosis.17,18 Hemoglobin levels increased from 9.42 g/dL to 11.48 g/dL, and hematocrit from 28.45% to 35.16%, reflecting recovery of erythropoiesis. This early hemoglobin improvement aligns with reports that imatinib therapy induces a significant rise in hemoglobin and hematocrit within the first months of treatment, even when red blood cell counts remain relatively stable.19 Platelet counts also dropped from 640,953/μL to 318,186/μL, aligning with the hematologic remission criteria established by the European LeukemiaNet (ELN) 2020.20 In the imatinib group, leukocytes declined from 245,123/μL to 19,155/μL, with hemoglobin rising from 9.54 g/dL to 11.19 g/dL and hematocrit from 28.64% to 34.29%. Prior studies report 60–80% of patients achieve complete hematologic response (CHR) within 3 months of imatinib therapy.18,21 These findings are consistent with observational cohorts showing rapid normalization of leukocyte counts and significant increases in hemoglobin and hematocrit after imatinib initiation, supporting its robust early hematologic efficacy.19 In the nilotinib group, leukocytes dropped more sharply, from 265,979/μL to 13,607/μL, with hemoglobin rising to 12.03 g/dL and hematocrit to 36.80%. Although nilotinib showed a trend of faster response, the difference was not statistically significant (p > 0.05), supporting evidence that it induces deeper early responses than imatinib.22 This hematologic safety profile is consistent with regional studies reporting that nilotinib-associated cytopenias, particularly thrombocytopenia and neutropenia, occur in a minority of patients and are generally manageable in the absence of severe bleeding or infection.23 Additional markers such as mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) were evaluated. MCV was significantly higher in the imatinib group (p = 0.024), suggesting mild macrocytosis, which may reflect active erythroid regeneration. Song et al. linked this macrocytosis to favorable molecular responses in TKI therapy.24 However, long-term data indicate that imatinib-related erythroid changes may evolve over time, with some patients developing persistent anemia despite adequate cytogenetic and molecular responses, likely due to c-KIT–mediated progenitor suppression.25 MCH showed a slight, non-significant decrease, possibly indicating early-stage erythrocyte production or compensatory hypochromia due to rapid erythropoiesis.26 This pattern is supported by studies demonstrating non-significant changes in MCV and MCH during early imatinib exposure, while hemoglobin and hematocrit increase significantly.19 Platelet counts progressively normalized in both groups, though nilotinib-treated patients exhibited rebound thrombocytosis at month three, possibly due to megakaryopoietic rebound or a drug-specific effect. This fluctuation is acceptable if not accompanied by thrombosis or bleeding, per Hughes et al.6,27 Consistent with prior reports, thrombocytopenia and thrombocytosis represent less frequent but recognized hematologic effects of imatinib therapy, typically remaining clinically manageable in early treatment phases. Importantly, platelet function studies indicate that nilotinib has minimal impact on platelet aggregation in response to ADP, collagen, and ristocetin, with isolated abnormalities to epinephrine considered clinically insignificant, supporting an overall low bleeding risk despite quantitative platelet fluctuations.23 These results suggest that TKIs improve not only leukocyte and hemoglobin levels but also secondary hematologic indicators like MCV, MCH, and platelets—reflecting hematopoietic recovery. Nevertheless, evidence from long-term cohorts highlights that imatinib-related myelosuppression, particularly anemia, may persist or become irreversible in a subset of patients, underscoring the importance of longitudinal hematologic monitoring.25 Taken together with platelet aggregation data, these findings support that early hematologic normalization under both imatinib and nilotinib generally occurs without clinically meaningful impairment of secondary hemostasis.23 MCV and MCH may offer early signals of therapeutic response, while differences in MCV trends between regiments suggest potential as pharmacodynamic biomarkers.
Body weight and body mass index (BMI) were significantly associated with hematologic response after 3 months of TKI therapy (p < 0.05). These factors influence drug pharmacokinetics and tissue distribution. Maroselli et al. found that patients with BMI <18.5 kg/m2 were at higher risk of imatinib overexposure, possibly due to altered metabolism.7,28 In this study, patients with above-average body weight (>53 kg) experienced greater leukocyte and platelet reduction, and more consistent hemoglobin improvement—especially in the nilotinib group. Similar findings by Abdulla et al. show better molecular responses in overweight patients.29 This observation aligns with head-to-head comparisons demonstrating nearly equivalent effectiveness between imatinib and nilotinib in achieving hematologic responses, suggesting that patient characteristics such as BMI may play a larger role than TKI selection alone. Although height showed no significant association with hematologic response, it influences body surface area (BSA), which historically guided chemotherapy dosing. However, studies including Kawaguchi et al. indicate that BSA does not correlate with imatinib trough levels, supporting the safety of fixed dosing strategies.30 Abdulla et al. also emphasized that BMI is more clinically relevant than height alone in determining TKI response.29 BMI was significantly associated with leukocyte (p = 0.042) and hematocrit (p = 0.035) improvements. Patients with normal or mildly elevated BMI responded better than those underweight, likely due to more stable hematopoiesis. Joseph et al. found plasma imatinib levels were 61.5% higher in underweight patients, increasing the risk of hematologic and systemic toxicities.27 Therapeutic drug monitoring (TDM) is thus advisable for patients with extreme BMI values. Yu et al. highlighted the link between poor nutrition and impaired hematopoiesis, reinforcing the role of BMI in drug efficacy and prognosis.11,31 Malnutrition impairs bone marrow regeneration and alters inflammatory profiles, contributing to suboptimal therapy response. Given the comparable short-term effectiveness of both TKIs, these findings have particular relevance in resource-limited settings, where drug cost is a major determinant of treatment choice and imatinib remains more accessible.32 In patients with higher BMI, nilotinib may be considered as a second-line option with caution, as its potential cardiovascular toxicity necessitates careful risk stratification despite favorable hematologic outcomes.33 Age ranged from 18 to 80 years (mean: 42.3), younger than typical Western cohorts. Younger patients have more regenerative hematopoietic capacity and fewer comorbidities.12 Although Jabbour and Kantarjian identified age <45 as a predictor of faster response, this study found no statistically significant association between age and hematologic response (p > 0.05).2
After 3 months of TKI therapy, both imatinib and nilotinib led to significant clinical responses. While 60% of nilotinib-treated patients achieved complete response (CR) versus 32.1% in the imatinib group, the difference was not statistically significant (p = 0.078). Imatinib, a first-generation TKI, effectively induces CHR and major molecular response (MMR), particularly in chronic-phase CML.19 Nilotinib, a second-generation TKI, binds BCR-ABL1 with 20–30 times greater affinity and achieves faster, deeper responses.34 Despite a shorter half-life, its higher plasma exposure and greater selectivity enhance its pharmacodynamic effects.35,36 Nilotinib also promotes early molecular response (EMR) at 3 months (BCR-ABL1 ≤10%), which is associated with improved long-term progression-free and overall survival, as well as greater potential for treatment-free remission (TFR).20,36
However, the lack of statistical significance in this study may be due to sample size limitations (n = 43), demographic variability, and comorbidities. Similar findings were reported by Belohlavkova et al., where short-term hematologic and molecular responses did not differ significantly between imatinib and nilotinib in newly diagnosed CML patients, despite a trend favoring nilotinib.22 Long-term use of nilotinib is associated with metabolic side effects, including hyperglycemia and cardiovascular risks.37 Thus, treatment selection should consider not only efficacy but also comorbidities. Imatinib may be preferable for patients with cardiovascular risk factors, given its safer long-term profile.
Overall, this study supports a personalized approach in CML management. Drug selection should account for cardiovascular risk, metabolic status, age, BMI, and therapeutic goals.38 Moreover, achieving CR at 3 months is only an early milestone—regular molecular monitoring through month 12 is essential, as recommended by ELN and NCCN.20,38
This study has several notable strengths. It provides real-world evidence on the early hematologic response to TKI therapy in Indonesian patients with CML, a population that is often underrepresented in large-scale clinical trials. The comparative analysis of first-generation (imatinib) and second-generation (nilotinib) TKIs offers clinically relevant insights, particularly in resource-limited settings where drug accessibility and cost are major concerns. Furthermore, the study tracked detailed hematological parameters over a three-month period, allowing for a comprehensive evaluation of treatment dynamics. Importantly, the investigation into anthropometric factors such as body weight and BMI as predictors of hematologic response adds a valuable perspective for individualized treatment approaches.
However, the study also has several limitations. The relatively small sample size (n = 43) may limit the statistical power to detect significant differences between treatment groups, particularly in categorical response rates. As a single-center, retrospective analysis, there is also a risk of selection bias and limited generalizability to other populations or healthcare systems. The absence of molecular response data, such as BCR–ABL1 transcript levels, prevents evaluation of deeper responses beyond hematologic remission. In addition, the follow-up period was limited to three months, which restricts conclusions about long-term outcomes, including progression-free survival, overall survival, and treatment-free remission. Lastly, potential confounding variables such as comorbid conditions, treatment adherence, nutritional status, and TKI plasma concentrations were not controlled for and may have influenced the observed responses.
In conclusion, both imatinib and nilotinib demonstrated significant improvements in hematological parameters within the first three months of therapy in patients with chronic myeloid leukemia. Although nilotinib showed a higher proportion of complete hematologic responses compared to imatinib, the difference was not statistically significant, indicating that both agents were comparably effective in the short term. The study highlights the potential influence of individual factors such as body weight and BMI on treatment response, reinforcing the importance of a personalized approach in CML management. These findings emphasize the need for larger, prospective studies with longer follow-up durations and molecular response assessments to better guide treatment decisions and optimize outcomes in diverse and resource-constrained settings.
Ethical approval was obtained from the Biomedical Ethics Committee on Human Research, Faculty of Medicine, Hasanuddin University, with clearance number 59/UN 4.6.4.5.31/PP36/2025. Patient confidentiality was maintained by omitting personal identifiers from all research documentation. Informed consent was not required for this study, as no direct contact was made with participants where data derived from patient medical records. The study was conducted at the Hematology Medical Oncology Outpatient Clinic of Wahidin Sudirohusodo Hospital, spanning from January to December 2024. Data management and statistical analysis were performed at the Department of Public Health, Faculty of Medicine, Hasanuddin University.
FigShare: A COMPARISON BETWEEN TREATMENT RESPONSE OF CHRONIC MYELOID LEUKEMIA PATIENTS RECEVING IMATINIB OR NILOTINIB. https://doi.org/10.6084/m9.figshare.29814944.v4 (Gosal D., 2025).39
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank to the staff of the Medical Hematology-Oncology outpatient clinic in Wahidin Sudirohusodo Hospital for the assistance in facilitating access to the patient records and supporting the data collection process.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hematology, all MPNs including CML, histiocytoses, CLL, benign hematology, MDS
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: CML
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hematology, all MPNs including CML, histiocytoses, CLL, benign hematology, MDS
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 31 Dec 25 |
read | |
|
Version 1 01 Sep 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)