Keywords
Essential oils; biofilm; caries; gingivitis; mouthrinse.
This article is included in the Pathogens gateway.
This study aimed to investigate the antibiofilm effect of coconut and frankincense oils.
Different types of coconut (organic refined, cocos nucifera, organic virgin, and organic extra virgin) and frankincense (frankincense pure essential oil in fractionated coconut oil and uplifting frankincense pure essential) oils were investigated. Serial dilutions (1:3, 1:6, 1:12, 1:24, 1:48, 1:96, and 1:192) were created from each oil and incubated with an overnight culture of S. mutans. The total growth and biofilms absorbance were measured at 595 and 490 nm, respectively. One-way ANOVA and Tukey tests were used for data analysis.
The greatest biofilm inhibition was observed in the uplifting frankincense pure essential oil at 1:3 dilution (0.67±0.12), which was significantly lower (p<0.01) than the control (1.51 ± 0.07). In addition, organic refined (0.96±0.13), organic virgin (1.21±0.28), and organic extra virgin (1.07±0.17) were associated with less biofilms compared to the control, but without a statistical significance. Frankincense pure essential oil in fractionated coconut oil and cocos nucifera coconut oil did not show biofilm inhibition.
Organic refined, organic virgin, and extra virgin coconut oils, and uplifting frankincense essential oil, effectively reduced S. mutans levels in vitro, with the highest amount of biofilm reduction associated with uplifting frankincense essential oil.
Essential oils; biofilm; caries; gingivitis; mouthrinse.
Dental caries is a disease influenced by multiple factors. Elements such as diet, the shape and structure of teeth, the oral environment, and the presence of certain microorganisms all contribute to the development of dental caries.1,2 Among such microorganisms, Streptococcus mutans (S. mutans) is a facultative anaerobic, gram-positive bacterium known for its strong association with dental caries due to its high virulence.3 Its ability to adhere to tooth enamel, produce acidic byproducts, store glycogen, and generate extracellular polysaccharides contributes significantly to its role in tooth decay.4 Several methods have been investigated to prevent dental caries by targeting the formation of cariogenic biofilms through the application of antimicrobial agents.5
Mechanical tooth cleaning is the most widely accepted and dependable approach for maintaining oral hygiene. In addition to mechanical tooth cleaning, research has concentrated on chemotherapeutic agents aimed at reducing or preventing dental caries.6,7 With the growing concern regarding the emergence of pathogens with antimicrobial resistance in dentistry and medicine, several investigations have explored the capabilities of natural products in preventing biofilm-triggered diseases.8,9 Oil pulling is a traditional oral hygiene practice, with coconut oil (cocos nucifera) being one of the most frequently used oils for this method.10 Oil pulling, traditionally referred to as ‘Kavalagraha’ or ‘Kavala Gandoosha’ in the ancient Ayurvedic text Charaka Samhita, is a practice that involves swishing natural oils in the mouth. This technique is believed to help eliminate harmful bacteria, fungi, and other microorganisms from the teeth, gums, throat, and oral cavity.11
Coconut oil is rich in medium-chain saturated fatty acids, which compared to long-chain fatty acids, are more readily absorbed and metabolized by the body. This is due to their smaller size, greater solubility, and enhanced resistance to oxidation.10,12 Coconut oil is composed of about 92% saturated fats, with lauric acid making up nearly half of that content. Lauric acid is known for its antibacterial and antifungal properties.10,13 Research has demonstrated that coconut oil exhibits strong antimicrobial effects against various microorganisms.14 Studies evidenced that coconut oil is also effective against S. mutans and C. albicans in an in vitro oral biofilm model.14,15
Frankincense oil, derived from Boswellia species, is another pulling oil that has shown a long-standing historical and medicinal significance, notably in traditional medicine, due to its aromatic properties and therapeutic potential.16 Its active component, boswellic acids like acetyl-11-keto-β-boswellic acid (AKBA), plays a significant role in supporting oral and dental health by exhibiting antimicrobial and anti-inflammatory properties.17 Research shows that frankincense, especially from Boswellia serrata, effectively inhibits key oral pathogens such as Porphyromonas gingivalis and Enterococcus faecalis, which are involved in periodontitis.17
Coconut oil and frankincense oil pulling has been reported to help reduce plaque-related gingivitis and bad breath.18 However, there is limited scientific evidence regarding their impact on S. mutans, the bacteria primarily involved in the onset and development of dental caries. Besides, with the presence of different forms of these two oils, investigate the most anticariogenic form worths to be investigated. Therefore, the current study aimed to evaluate the effect of different types of coconut and frankincense oils against S. mutans biofilm growth. Besides, this study investigates the synergetic effect of the two oils against S. mutans biofilms. We hypothesized that coconut and frankincense oils, and their combination would significantly reduce the S. mutans biofilm growth.
In this study, four different types of organic coconut oil were used: (A) organic refined coconut oil, (B) fractionated liquid coconut oil (cocos nucifera), (C) organic virgin coconut oil, (D) organic extra virgin coconut oil. Also, two types of frankincense oil were used: (E) frankincense pure essential oil in fractionated coconut oil, (F) uplifting frankincense pure essential oil. The details of the selected oils and their manufacturers’ information are found in Table 1. Prior studies19–21 indicated that the standard deviation for absorbance measurements associated with biofilm formation is roughly 0.15. As a result, this study was structured to attain an 80% power level for detecting a significant difference at a 5% significance threshold. To achieve this, three separate experiments were conducted, each involving 3 to 4 samples, culminating in a total of 9 to 12 samples per group.
Each type of oil was mixed with brain-heart infusion (BHI) broth supplemented with 2% sucrose at the following dilutions: 1:3, 1:6, 1:12, 1:24, 1:48, 1:96, 1:192. The mixtures were used immediately after mixing. The experimental design included six main experimental groups according to the type of oil then another seven subgroups according to the dilution. A group consisting of BHI supplemented with 2% sucrose only was used as a control.
An overnight culture of S. mutans was prepared in BHI broth. The optical density (OD) of the culture was adjusted to 0.9 to standardize the bacterial concentration. All experimental and control samples were incubated with an overnight culture of S. mutans for 24 hours at a ratio of 20:1 (broth to bacterial culture). Subsequently, 10 μL of the adjusted S. mutans culture was added to 190 μL of either different type of oil dilution (oil mixed with BHI broth supplemented with 2% sucrose) at 1:3, 1:6, 1:12, 1:24, 1:48, 1:96, and 1:192 dilutions or the control group (BHI with 2% sucrose only) inside the wells of a 96-well plate. A sterility control of only BHI with 2% sucrose was also applied to detect any possible contamination. The 96-well plates were then incubated aerobically at 5% CO2 for 24 hours. After incubation, the total absorbance (planktonic and biofilm) was measured at 595 nm using a spectrophotometer (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA).19–21
Following this, the planktonic cells were removed, and the remaining adherent biofilm was treated with 200 μL of 10% formaldehyde for 30 minutes. The wells were then rinsed three times with deionized water to eliminate residual formaldehyde. To visualize the biofilm, 200 μL of 0.5% crystal violet solution was added and left to stain for 30 minutes. Afterward, excess dye was removed through three additional washes, leaving only the stained biofilm. And the spectrophotometer measurement at 490 nm was used to estimate the biofilm absorbance.19–21
Data were expressed as means ± standard deviations, based on at least three biological replicates to provide descriptive statistics. The Shapiro-Wilk test was employed to assess the normality of the data distribution. To compare the effects of coconut and frankincense oils on the biofilm formation and total growth of S. mutans, one-way ANOVA followed by Tukey’s post hoc tests were utilized (using Sigma Plot 12.0; SYSTAT). A p-value of less than 0.05 was deemed statistically significant.
Figure 1 illustrates the effect of coconut and frankincense oils on total growth. Organic refined coconut oil ( Figure 1A) showed the absorbance values decrease with increasing concentrations, showing significant reduction (p < 0.05) at the 1:12, 1:6, and 1:3 dilutions compared to the control. Similar downward trends were observed for cocos nucifera oil ( Figure 1B), with significant reductions at all the dilutions except for the 1:192 dilution. For organic virgin coconut oil ( Figure 1C), absorbance levels decline with increasing concentration, with significant reduction (p < 0.05) starting from the 1:96 to 1:12 dilutions when compared to the control, but no significant change in the absorbance at 1:6, and 1:3 dilutions. In addition, organic extra virgin coconut oil ( Figure 1D), particularly at the 1:48, 1:24, 1:12, 1:6, and 1:3 dilutions, showed significant reduction (p < 0.05) in the total growth compared to the control.
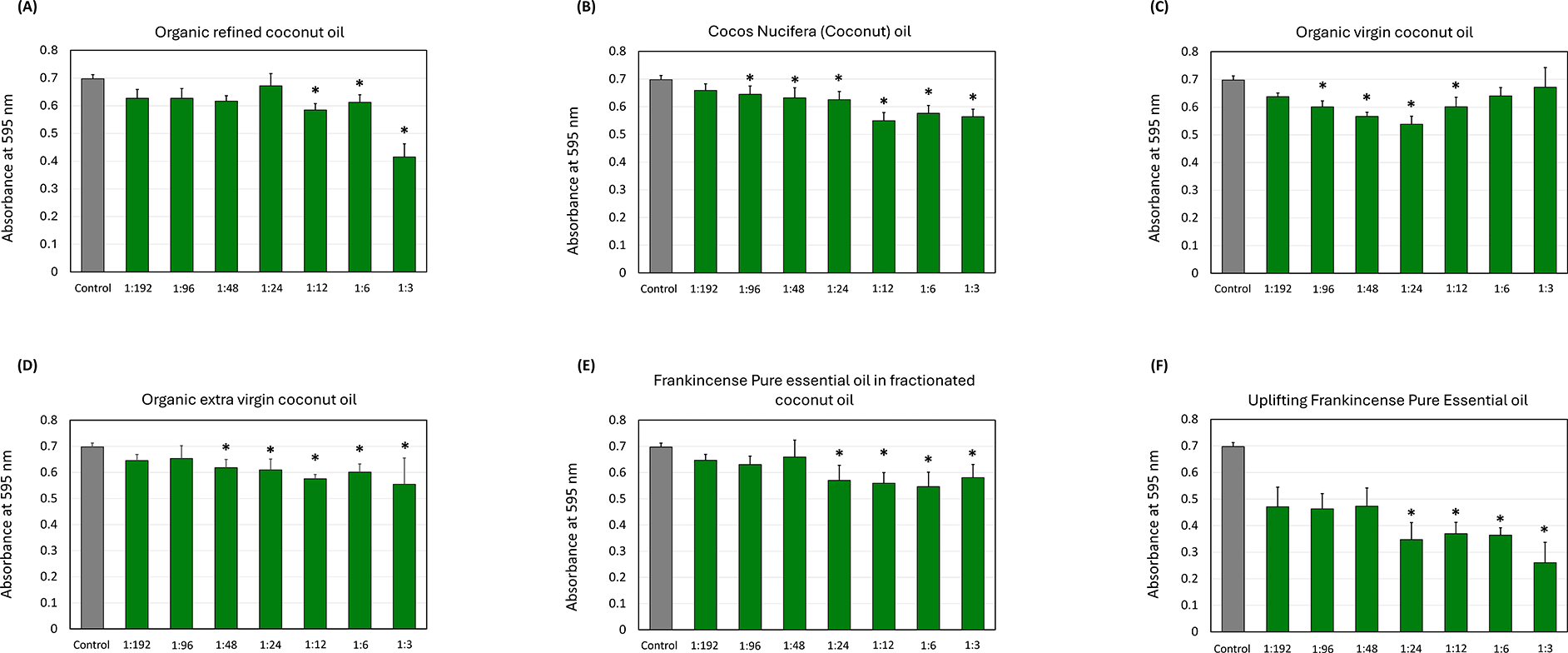
The impact of (A) organic refined coconut oil (B) cocos nucifera, (C) organic virgin coconut oil, (D) organic extra virgin coconut oil, (E) frankincense pure essential oil in fractionated coconut oil, and (F) uplifting frankincense pure essential oil on S. mutans total growth. Each group consisted of 3 wells, and the experiment was repeated three times (n = 9). Asterisks indicate a significant difference compared to the control samples with no treatment.
Frankincense pure essential oil in fractionated coconut oil ( Figure 1E) showed a marked decrease in the OD across all concentrations, with the significant reduction at 1:24, 1:12, 1:6, and 1:3. Similarly, the uplifting frankincense pure essential oil ( Figure 1F) demonstrated the most amount of total growth reduction, which was observed with increased concentration, especially at the 1:24, 1:12, 1:6, and 1:3 dilutions (p < 0.05).
Figure 2 illustrates the impact of oils on the biofilm growth of S. mutans. The organic refined coconut oil ( Figure 2A) led to a decrease in biofilm absorbance as the concentration increased, with the only significant reductions observed at the 1:3 dilution (p < 0.05). Cocos nucifera and organic virgin coconut oils ( Figure 2B & 2C) exhibited a progressive decline in OD with higher concentrations, but the amount of reduction was not significant at any dilution. The organic extra virgin coconut oil ( Figure 2D) showed only significant reduction (p < 0.05) at the 1:3 dilution.
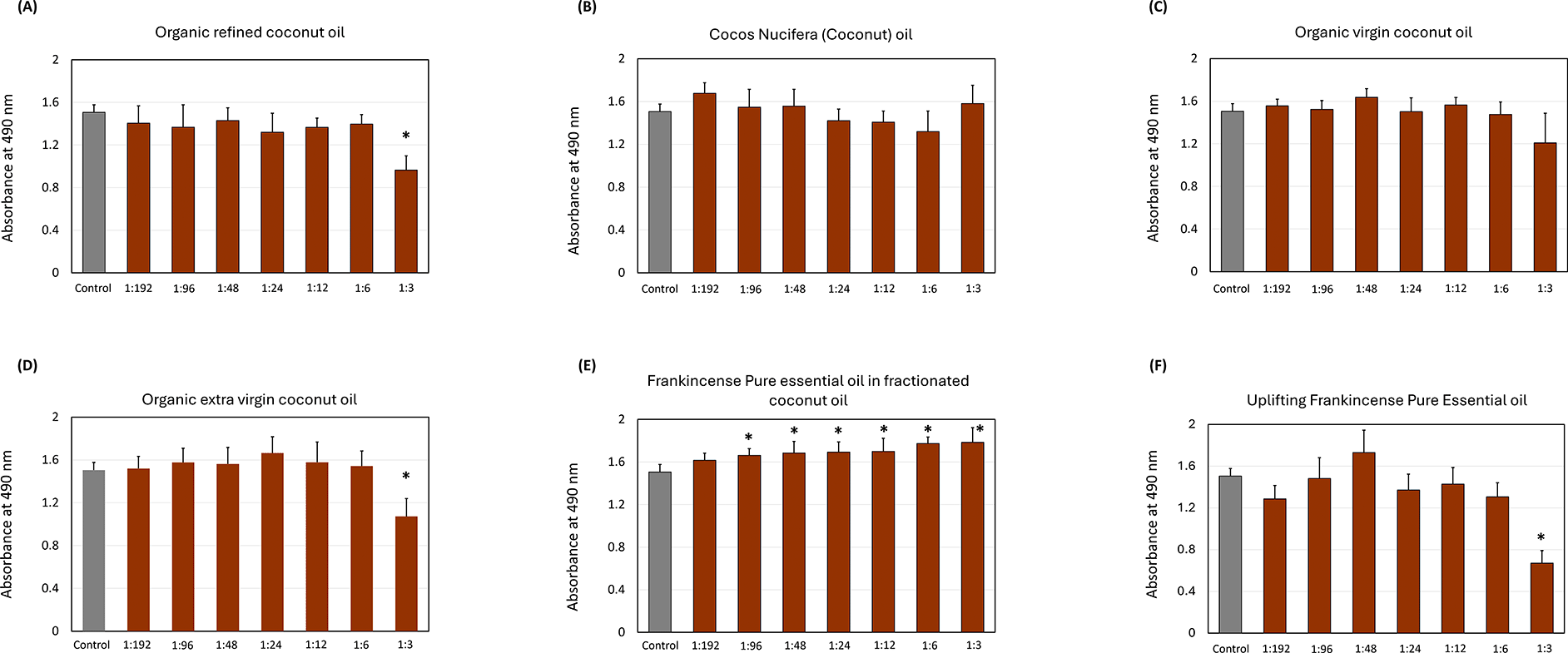
The impact of (A) organic refined coconut oil (B) cocos nucifera, (C) organic virgin coconut oil, (D) organic extra virgin coconut oil, (E) frankincense pure essential oil in fractionated coconut oil, and (F) uplifting frankincense pure essential oil on S. mutans biofilm formation. Each group consisted of 3 wells, and the experiment was repeated three times (n = 9). Asterisks indicate a significant difference compared to the control samples with no treatment.
Among the frankincense oils, pure frankincense essential oil in fractionated coconut oil ( Figure 2E) significantly increased the biofilm growth across most tested concentrations, with the strongest effects at 1:6, 1:12, and 1:3. Opposingly, the uplifting frankincense essential oil ( Figure 2F) reduced the biofilm growth at all the dilutions except at the 1:48 dilution, with the only significant biofilm, inhibition at the 1:3 dilution (p < 0.05).
Figure 3 illustrates the effects of various oils on S. mutans levels at the 1:3 dilution. The highest reduction observed in the group was treated with uplifting frankincense pure essential oil, which had an absorbance of 0.67 nm ± 0.12 nm, the lowest among all groups, and it was significantly lower (p < 0.01) compared to the control (1.51 nm ± 0.07 nm). In addition, organic refined (0.96 nm ± 0.13 nm), organic virgin (1.21 nm ± 0.28 nm), and organic extra virgin (1.07 nm ± 0.17 nm) were associated with less biofilms compared to the control, but without a statistical significance. The group treated with frankincense pure essential oil in fractionated coconut oil and cocos nucifera coconut oil were associated with anincrease in the biofilm growth compared to the control.
This study evaluated the antimicrobial effects of different coconut and frankincense oils preparations on S. mutans in both planktonic and biofilm forms. Notably, organic virgin coconut, extra virgin coconut, organic refined coconut, and uplifting frankincense essential oils showed the strongest inhibitory effects, particularly at higher concentrations. While the other oils were associated with no reduction against the S. mutans biofilms. Therefore, the hypothesis was partially accepted. The findings of this study highlight the potential of natural oils as alternative or adjunctive agents in oral health care, especially in reducing cariogenic bacteria.
Given the rising interest in natural products and growing concerns about antimicrobial resistance, the use of compounds such as coconut oil and frankincense oil presents a promising alternative to conventional oral antiseptics. Alcohol-containing mouthwashes, while effective, are associated with drawbacks such as mucosal irritation, altered taste, and patient discomfort, particularly with long-term use.22,23 Furthermore, widespread reliance on synthetic agents like chlorhexidine has raised concerns about the potential for promoting antimicrobial resistance.24 In contrast, natural oils have not been linked to resistance development and may offer a safer profile.25 They may also preserve the balance of the oral microbiome more effectively than alcohol-based products.26 Their multifunctional properties, including anti-cariogenic effects, low toxicity, and potential for long-term use, are valuable tools in preventive dentistry, particularly for individuals seeking holistic and gentler alternatives.27–29
Dental biofilms are the pathogenic form for oral microbes when causing dental caries or other biofilm-induced diseases.30 Opposite to their planktonic form, bacteria embedded within the biofilms are 1,000 resistant to therapeutic agents and bioactive compounds, which can complicate controlling the biofilm and reducing its pathogenicity.31,32 Therefore, we intended in this study to investigate the effect of oils on S. mutans planktonic and biofilms forms to explore the potential of these oils against more different forms of oral microbes. Our findings support and expand upon existing evidence regarding the antimicrobial properties of coconut oil against S. mutans. Previous studies have attributed these effects to the high concentration of medium-chain fatty acids—particularly lauric acid—which disrupt bacterial cell membranes, leading to cell lysis and reduced viability.10 Moreover, our observation that organic extra virgin coconut oil showed stronger biofilm reduction than other coconut oil types adds to findings by Alyami et al.,33 who emphasized medium-chain fatty acids ability to disrupt S. mutans within biofilms. This suggests that oil purity and extraction methods may influence antimicrobial efficacy, an area that has received limited attention in prior studies. By comparing different forms of coconut oil, our study refines current understanding and highlights the importance of oil quality in optimizing natural oral health interventions.
In contrast to coconut oil, frankincense oil is less extensively studied for its antimicrobial effects against S. mutans, though initial evidence suggests potential activity. Previous studies have highlighted frankincense’s anti-inflammatory and biofilm-disrupting properties, but direct evidence targeting S. mutans remains limited.34,35 Our findings help fill this gap by demonstrating that uplifting frankincense essential oil significantly reduced S. mutans biofilm levels, indicating promising antibacterial potential. However, the unexpected increase in OD observed with the pure frankincense essential oil in fractionated coconut oil suggests that formulation and carrier oils may influence efficacy. This highlights the complexity of essential oil interactions and the need for standardized preparations in oral applications. Compared to coconut oil, which has a well-established inhibitory effect on S. mutans in both planktonic and biofilm forms,10 frankincense oil shows emerging but less consistent results. Thus, while our data supports its potential, further investigation is needed to clarify its mechanisms, optimal concentrations, and formulation for oral health use.
The results of this study have potential applications in the creation of oral care products that include coconut and frankincense oils. Given their demonstrated antimicrobial activity against S. mutans, these natural oils could be formulated into mouthwashes, toothpastes, or oil-based rinses aimed at caries prevention. Essential oils are already used in commercial dental products due to their broad-spectrum antimicrobial effects and lower side effect profiles compared to synthetic agents like chlorhexidine.36 Some mouthwashes contain a combination of essential oils, including eucalyptus, tea tree, and clove oils, which have been shown to be effective against plaque and gingivitis.37 Moreover, their inclusion may appeal to patients seeking alcohol-free and holistic oral hygiene solutions, especially those with sensitivities to conventional formulations. To ensure clinical efficacy, future product development should focus on optimizing delivery systems and conducting in vivo studies.
This study has some limitations that should be considered when interpreting the results. First, the experiment was conducted in vitro, which may not fully replicate the complex conditions of the oral cavity, including salivary flow, oral microbiome diversity, and host immune responses. Second, the study focused solely on S. mutans, while other cariogenic and commensal microorganisms play significant roles in oral biofilm ecology and caries development. Third, although different types and concentrations of coconut and frankincense oils were tested, variability in oil composition due to sourcing, processing, and brand differences could affect reproducibility and generalizability. Additionally, the mechanism of action was not directly investigated, limiting insights into how the oils exert their antibacterial effects. Future studies incorporating multi-species biofilm models, and advanced imaging or molecular techniques would strengthen the evidence for the therapeutic potential of these natural oils.
This study offered a comparative analysis of different types of coconut and frankincense oils against S. mutans, particularly in its biofilm form, which is clinically relevant for caries development. By highlighting the superior performance of organic extra virgin coconut oil and uplifting frankincense essential oil, the study identifies promising natural alternatives to conventional antimicrobials in oral care. Future research should build upon these findings through in vivo studies and clinical trials to assess real-world efficacy, safety, and patient compliance. Additionally, mechanistic studies exploring the specific bioactive compounds responsible for antimicrobial activity, such as lauric acid in coconut oil and boswellic acids in frankincense, would offer deeper insights. Expanding investigations to include multi-species biofilms and interactions within the oral microbiome could also better simulate clinical conditions. Finally, formulating standardized, stable delivery systems (e.g., mouthwashes, gels, or varnishes) will be critical for translating these natural products into practical oral health solutions.
Certain coconut and frankincense oils, particularly organic refined, organic virgin, and extra virgin coconut oils, and uplifting frankincense essential oil, effectively reduced S. mutans biofilms in vitro. The highest amount of biofilm reduction was associated with uplifting frankincense essential oil, which reduced the biofilm growth by 2.5-fold. These findings support their potential use as natural agents for caries prevention and oral health care. Opposingly, cocos nucifera coconut oil and frankincense pure essential oil did not induce antibiofilm effects against S. mutans biofilms.
N.A., L.A., and S.A.: contributed to design, resources, acquisition and analysis, data curation, and drafted the manuscript, and critically revised the manuscript. J.F.A., T.A., N.N.A., and A.A.B.: contributed to the concept and design, acquisition, supervision, drafted the manuscript, and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Figshare. Coconut oil.xlsx https://doi.org/10.6084/m9.figshare.29875172.v238
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank Imam Abdulrahman bin Faisal University (IAU) for the use of equipment.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology; Pediatric Dentistry
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: cariology, ecological caries, dental public health
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomaterials, Bioestatistics, Microbiololgy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 01 Sep 25 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)