Keywords
Prevalence; malaria; Schistosoma haematobium; coinfection; sub Saharan Africa
This article is included in the Pathogens gateway.
Malaria-schistosomiasis coinfection is common in Africa. Also, coinfection of these parasites can cause significant clinical signs and pathology compared to infection with a single parasite species. However, there is a shortage of pooled data on the prevalence of malaria and Schistosoma haematobium coinfection in Sub-Saharan Africa.
This review intended to determine the incidence of malaria and Schistosoma haematobium co-infection in Sub-Saharan Africa.
Relevant studies were identified using a systematic search of PubMed, Scopus, Google Scholar, and Science Direct, in accordance with review and meta-analysis criteria. This review’s initial searches began on August 30, 2024, and the protocol was registered on August 29, 2024. A total of twenty seven relevant articles on the prevalence of malaria and Schistosoma haematobium coinfection were identified for this review. STATA software version 17.0 was used to analyze the extracted data. The absence or presence of publication bias was assessed. Subgroup analysis was performed if the I2 value was ≥50%, indicating considerable heterogeneity. Sensitivity analysis was conducted.
This review includes a total of 27 papers. The pooled prevalence of Schistosoma haematobium coinfection with malaria was 13.36% (95% CI: 6.16–20.56). The pooled prevalence of malaria and Schistosoma haematobium coinfection varied significantly by country, diagnostic method, and year of publication. Sensitivity analysis indicated that no one study altered the overall prevalence of malaria and Schistosoma haematobium coinfection.
This comprehensive study highlights the prevalence of malaria and Schistosoma haematobium co-infection in Sub-Saharan Africa, underscoring significant challenges in managing these diseases. Effective management requires regular monitoring, identification, and reduction of co-infection incidence. Additionally, collaborative efforts at local, national, and global levels are essential to tackle the complex factors contributing to these infections. Health programs should be developed to prevent and manage both malaria and Schistosoma haematobium infections effectively.
Prevalence; malaria; Schistosoma haematobium; coinfection; sub Saharan Africa
Malaria is a life-threatening disease brought about by plasmodium species that spreads by an infected female anopheles mosquito bite.1 Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae are the primary causes of malaria, with P. falciparum accounting for most of malaria-related mortality and life-threatening diseases.2 It was just recently found that P. knowlesi, which causes malaria in macaque monkeys, might infect people in Southeast Asia.3 According to the report, which is an annual assessment of worldwide trends in malaria control and elimination, an anticipated 249 million cases of malaria were reported in 85 malaria-endemic countries in 2022, representing a case incidence of 58 per 1000 population risk. The World Health Organization African Region accounted for 233 million (about 94%) of the 249 million cases reported in 2022. Also, malaria is predicted to have killed 608,000 people worldwide in 2022, or 14.3 deaths per 100,000 people who were at risk.4
Schistosomiasis is one of the water-borne diseases known as water-based neglected tropical diseases, mostly affecting hundreds of millions of people in Sub-Saharan Africa.5 There are six species of schistosomes that infect humans globally. Schistosoma intercalatum, Schistosoma mekongi, Schistosoma japonicum, and Schistosoma guineensis are restricted to certain places, but Schistosoma haematobium and Schistosoma mansoni are global.6,7 The most widespread species in Sub-Saharan Africa are S. haematobium and S. mansoni, which cause urogenital and intestinal schistosomiasis, respectively.8 Schistosoma pathogenesis is mostly caused by the host’s immune response to the antigens on the eggs, which results in the formation of granulomas in the liver and gut where the eggs are lodged. This results in a cellular, granulomatous reaction that causes fibrosis and the most severe infection-related disease symptoms.9 Sub-Saharan Africa accounts for 90% of all S. haematobium infection on the cases of the globe. Schistosomiasis affects over 207 million people, with 85% living in Africa. An estimated 700 million people are at risk of infection in 76 countries where the disease is endemic due to exposure to infested water through agricultural work, domestic chores, and recreational activities.10
Moreover, several causes contribute to schistosomiasis’ ongoing and persistent spread in Sub-Saharan Africa. These include climate changes and global warming, closeness to water sources, irrigation and dam building, as well as socioeconomic concerns such as occupation and poverty.11
Malaria and schistosomiasis are prevalent in rural regions with inadequate water supply, poverty, ignorance, and poor hygiene habits.12 Coinfection of these parasites can cause significant clinical signs and pathology compared to infection with a single parasite species. In addition, coinfection of P. falciparum with schistosomes can worsen hepatosplenic, anemia, and malnutrition conditions.13 Furthermore, co-infection has a significant impact on the management of inflammatory variables associated with the course of these illnesses and their relative morbidity.14
Understanding the interactions between coendemic helminth infections, like those caused by Schistosoma, and malaria has become more important due to the difficulties in developing a highly effective malaria vaccine. These interactions may affect the effectiveness of the vaccine by altering host-immune responses to Plasmodium infection and treatment.15,16 As a result, a thorough understanding of malaria epidemiology during Schistosoma co-infection is essential to make informed decisions about effective schistosomiasis and malaria management techniques in Sub-Saharan Africa. Furthermore, knowledge on the prevalence and clinical effects of schistosomiasis co-infection with malaria is essential to improve clinical care and malaria prevention, particularly in schistosomiasis-endemic areas.17 Also, by identifying cases of coinfection, health organizations may develop and put into practice mitigation initiatives and strategies that reduce the likelihood of getting both diseases.18 To the best of our knowledge, not much study has been conducted on the pooled prevalence of malaria and S. haematobium coinfection in Sub-Saharan Africa. As a result, this systematic review and meta-analysis sought to assess the pooled prevalence of malaria and S. haematobium coinfection in Sub-Saharan African countries from 2014 to 2024.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) used as the guidelines for this systematic review and meta-analyses. Consequently, this review was carried out in accordance with the Preferred Reporting Item for Systematic Review and Meta-analysis Protocol (PRISMA-P 2020) guideline.19 The four stages of the PRISMA flow chart were documented in the findings, and they show the process of selecting studies from the first identified records to the studies that were included. In the International Prospective Register of Systematic Reviews (PROSPERO) database, the review protocol was registered under the registration number CRD42023486459 and was developed before a literature search was carried out. By defining inclusion/exclusion criteria and outcomes of interest, a protocol was established to answer the review questions.20
The protocol was filed on August 29, 2024, and the first searches for this systematic review started on August 30, 2024. English-language research conducted in sub-Saharan Africa between 2014 and August 30, 2024, were included in this systematic review and meta-analysis. An extensive literature search was conducted to find research on the prevalence of malaria and S. haematobium coinfection in a sub-Saharan African community with a variety of study themes. Systematic searches were conducted for both electronic and gray literature. PubMed, Science Direct, Scopus, and Google Scholar were used to obtain the data. Both individual and combined search phrases were utilized, along with Boolean operators like “OR” and “AND” [Prevalence AND malaria OR S. haematobuim OR coinfection “AND” P. falciparum, OR P. malariae OR, P. vivax OR P. ovale OR “AND” Sub Saharan Africa “2014-2024”] were the search terms used in Google Scholar to locate pertinent papers. The reference lists of the listed studies were also subjected to a snowball search. The strings or keywords were reorganized to yield terms that were relevant to the desired result. Original research articles and reviews, as well as articles from the reference section and citation lists of full texts, were obtained in order to enhance the likelihood of obtaining more data. For each electronic database, several combinations were developed to increase the number of pertinent studies while reducing the number of results obtained.20 Studies conducted from 2014 to August 30, 2024, were included by the researcher.
The articles sourced from the previously mentioned databases were imported into EndNote version 20 reference management software (Thomson Reuters, New York, NY). For this systematic review and meta-analysis, the selected studies included: (1) observational studies such as cross-sectional studies, cohort studies (both retrospective and prospective), and case-control studies that reported the prevalence of either malaria or S. haematobium co-infection in countries within sub-Saharan Africa; (2) articles published in peer-reviewed journals or grey literature; and (3) articles published in English from the inception of the databases until August 30, 2024. We excluded studies that (1) were not fully accessible; (2) received a poor quality score based on the specified criteria; (3) case series, letters, comments, and editorials; and/or (4) did not measure the intended outcome (i.e., the prevalence of malaria and S. haematobium co-infection).
The prevalence of malaria and S. haematobuim coinfection was the primary outcome of interest in sub Saharan Africa countries reported in the original paper both as a percentage and as the number of cases (n)/total number of participants (N).
Quality appraisal of the studies was conducted using Joanna Brigg’s Institute quality appraisal criteria (JBI) and Studies with 50% and above on the quality scale was considered to have good quality.21 The screened titles identified in the abovementioned databases. Following this, the two authors screened eligible studies for abstract (W.A. and A.K.G.). Finally, full-text screening was conducted by two authors (W.A. and A.K.G.).
A standardized data extraction form in Microsoft Excel 2010 was utilized to obtain and record relevant information from each selected study. The extraction method encompassed a broad variety of domains, including research information such as first author, year of publication, study population, study design, number of participants, and study area/region. Each study reported the prevalence of malaria and S. haematobium coinfection. The two authors checked the extracted data for accuracy and consistency (W.A., and A.K.G.).
The extracted data was imported into Microsoft Excel and analyzed with STATA version 17 (Stata Corp. Stata Statistical Software, College Station, TX: Stata Corp LP). The overall summary estimate of prevalence across studies was obtained using a random-effects model. The point estimate was used, with a 95% confidence interval. Visual inspection of funnel plots and Egger’s Test was used to identify the presence of publication bias. Trim and fill method studies were used to get a bias-adjusted effect estimate. The study’s heterogeneity was assessed using inverse of variance (I2) statistics. An I2 score of 50% or higher indicated significant heterogeneity. We performed subgroup analyses to investigate the reasons of heterogeneity in studies with significant differences (I2 ≥ 50%). A sensitivity analysis was also carried out to investigate the impact of each study on overall prevalence.
A total of 3,232 studies were identified across all electronic databases. After removing 132 duplicates, 3,100 studies remained. These studies were screened based on titles, abstracts, and full-text articles, resulting in 27 studies being retained after the screening and eligibility assessment. Ultimately, 27 studies were included for both qualitative and quantitative analyses (Figure 1).
This study encompasses participants of all ages and genders. Also, from a total of 3232 studies, 27 articles were assessed.15,17,22–46 All studies were done by cross-sectional study designs except two studies.15,30 From the total included studies, most studies were done in the Nigeria with a total of nine studies.22–26,34,41,42,44 Most studies were used only microscopy technique for investigation of parasites.15,22,24,26,27,29–31,34,37–44,46 The prevalence of malaria and S. heamatobium coinfection ranges from a minimum of 0.6 %39 to a maximum of 78.2%42 ( Table 1).
| Author | Year of publication | Country | Study design | Diagnostic Techniques | Sample size | Prevalence (%) of M & Sh Co | Quality score/9 |
|---|---|---|---|---|---|---|---|
| Ayodele A. et al22 | 2015 | Nigeria | CS | Microscopy | 159 | 35.2 | 9 |
| Odeh P. et al23 | 2022 | Nigeria | CS | Microscopy and PCR | 1037 | 1.25 | 9 |
| Hafizu M. et al24 | 2023 | Nigeria | CS | Microscopy | 300 | 3.3 | 9 |
| Ologunde A. et al25 | 2021 | Nigeria | CS | Microscopy and RDT | 306 | 2.3 | 9 |
| Olajumoke A et al26 | 2016 | Nigeria | CS | Microscopy | 322 | 9.3 | 9 |
| Christopher K.et al27 | 2020 | Tanzania | CS | Microscopy | 374 | 4.5 | 9 |
| Daisy L. et al28 | 2017 | KENYA | CS | Microscopy and RDT | 151 | 7.95 | 9 |
| Irene S. et al29 | 2024 | Cameroon | CS | Microscopy | 606 | 8.3 | 9 |
| Esum M. et al17 | 2023 | Cameroon | CS | Microscopy and PCR | 397 | 7.8 | 9 |
| Jean C. et al30 | 2018 | Gabon | PLS | Microscopy | 754 | 9 | 9 |
| Safiatou D. et al15 | 2014 | Mali | PCS | Microscopy | 616 | 6.3 | 9 |
| Safari M. et al31 | 2014 | Tanzania | CS | Microscopy | 1546 | 10.2 | 9 |
| Muhammed O. et al32 | 2023 | Senegal | CS | Microscopy and PCR | 910 | 1.1 | 9 |
| Francis N. et al33 | 2022 | Cameroon | CS | Microscopy, PCR, and RDT | 65 | 50.8 | 9 |
| Olajumoke A. et al34 | 2014 | Nigeria | CS | Microscopy | 202 | 28.2 | 9 |
| Judith K. et al35 | 2017 | Cameroon | CS | Microscopy & Urine filtration | 250 | 15.2 | 9 |
| Naa A. et al36 | 2023 | Ghana | CS | Microscopy and PCR | 662 | 0.7 | 9 |
| Ruth N. et al37 | 2018 | Ghana | CS | Microscopy | 404 | 0.9 | 9 |
| Irene U. et al38 | 2021 | Cameroon | CS | Microscopy | 638 | 7.8 | 9 |
| Victor T. et al39 | 2022 | Kenya | CS | Microscopy | 534 | 0.6 | 9 |
| Janet M. et al40 | 2024 | Kenya | CS | Microscopy | 474 | 0.8 | 9 |
| Olayinka P. et al41 | 2020 | Nigeria | CS | Microscopy | 447 | 2.5 | 9 |
| Olajumoke A. et al42 | 2016 | Nigeria | CS | Microscopy | 173 | 78.2 | 9 |
| Keptcheu T. et al43 | 2020 | Cameroon | CS | Microscopy | 228 | 13.60 | 9 |
| Okafor E. et al44 | 2014 | Nigeria | CS | Microscopy | 1060 | 55.1 | 9 |
| Blessings C. et al45 | 2024 | Malawi | CS | Microscopy and RDT | 1134 | 5.5 | 9 |
| Akosah B. et al46 | 2021 | Ghana | CS | Microscopy | 493 | 0.8 | 9 |
The heterogeneity was assessed for the prevalence of malaria and S. haematobium coinfection. There was high variability in the incidence of malaria and S. haematobium coinfection, with I2 statistical values of 99.88% at P = 0.00. A funnel plot was utilized to assess potential publication bias in the included papers. As a result, the funnel plot was lopsided, indicating publication bias across research. The Egger’s test was performed to assess potential publication bias in the included papers. Egger’s test yielded a p-value of 0.00, indicating publication bias. To decrease and account for the observed publication bias in the studies, a trim and fill analysis was used to identify potentially missing studies. After controlling for publication bias, the estimated pooled prevalence of malaria and S. haematobium coinfection was 16.996 (95% CI = 10.545-23.448), according to trim and fill analysis ( Figure 2 and Table 2).
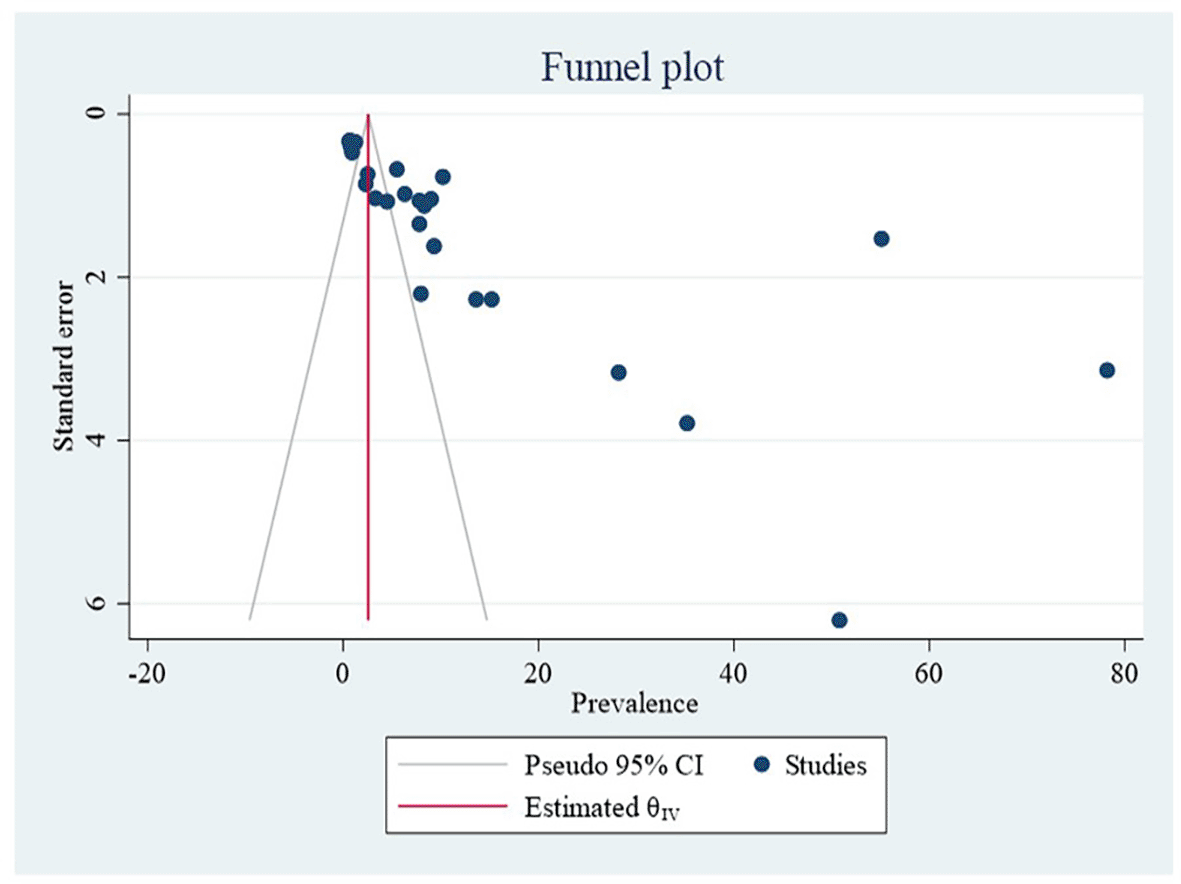
| Studie | Effect size | [95% CI] |
|---|---|---|
| Observed (27) | 13.358 | 6.158-20.558 |
| Observed + Imputed (27+6) | 16.996 | 10.545-23.448 |
Sensitivity: In sensitivity analyses using the leave-one-out technique, excluding no studies had a significant effect on pooled burden estimates and heterogeneity measures within primary studies. Therefore, sensitivity analysis using the random-effects model revealed that no single study impacted the overall incidence of malaria and S. haematobium coinfection ( Figure 3).
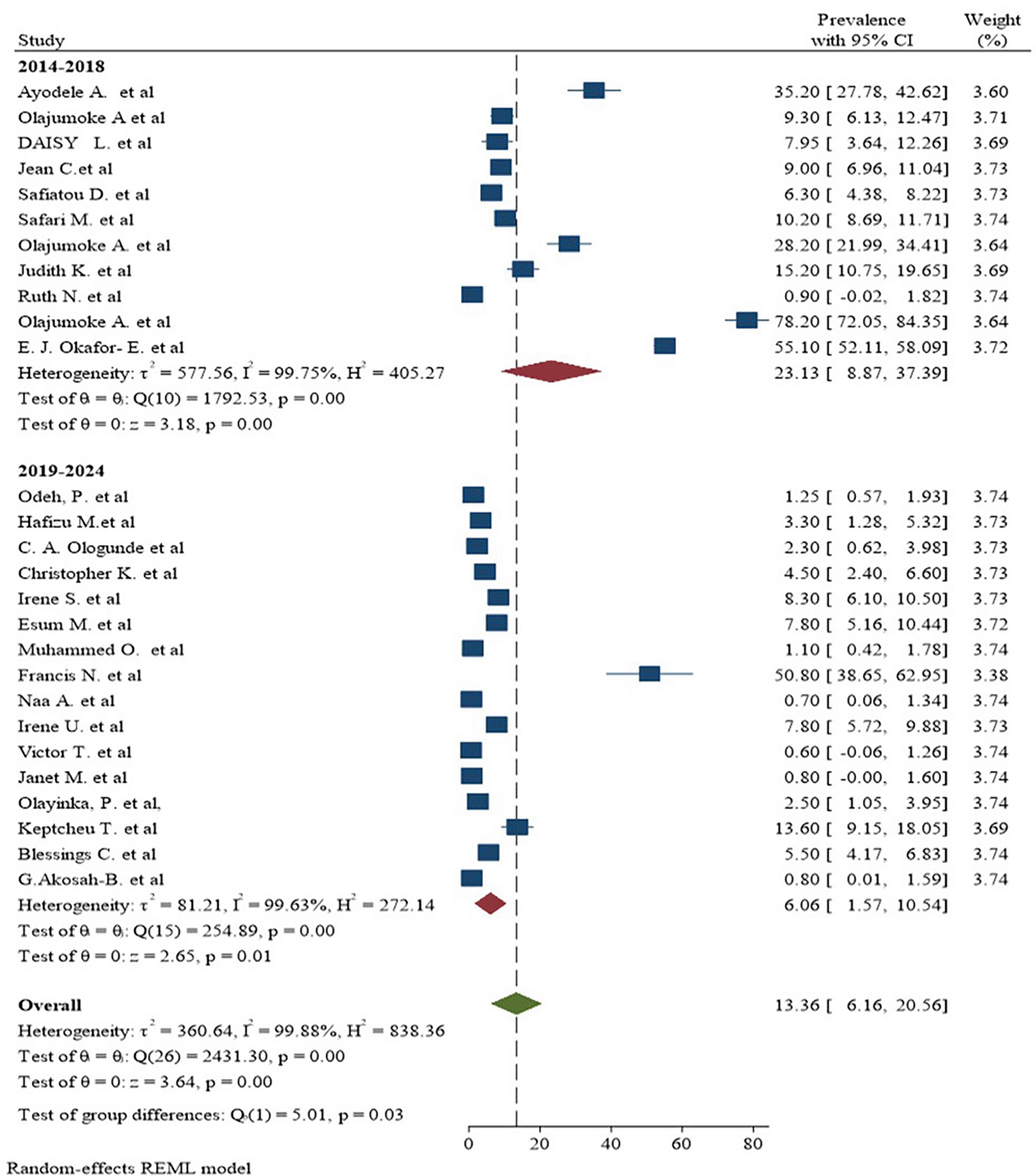
The pooled prevalence of malaria and S. haematobium coinfection was 13.36% (95% CI: 6.16–20.56). A random-effects model shows the presence of heterogeneity among the included studies with 95% CI (I2 = 99.88 % and P-value = 0.00). Due to the presence of significant heterogeneity between the included studies, subgroup analysis was carried out to know the prevalence of malaria and S. haematobium coinfection among articles ( Figure 4).
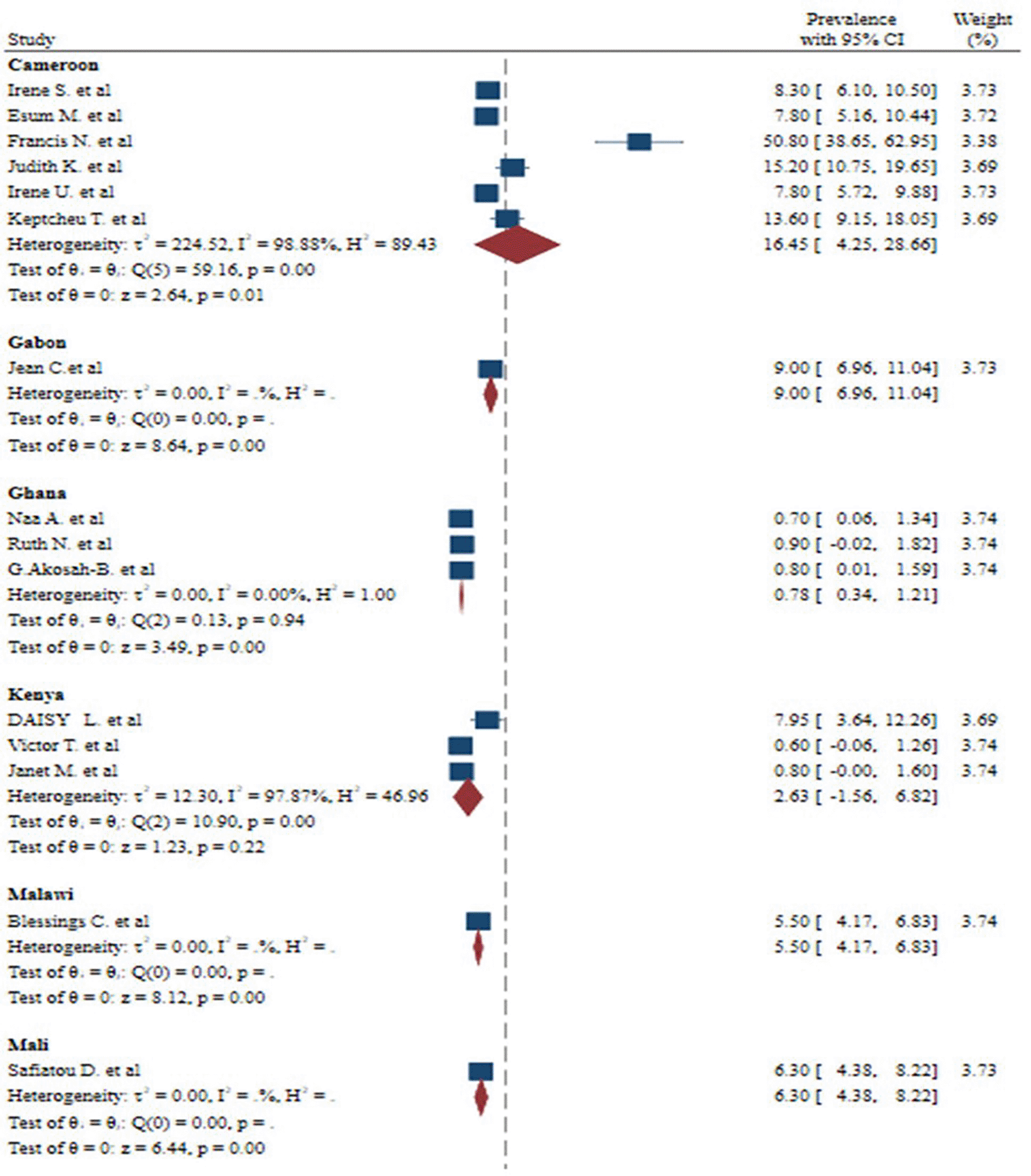
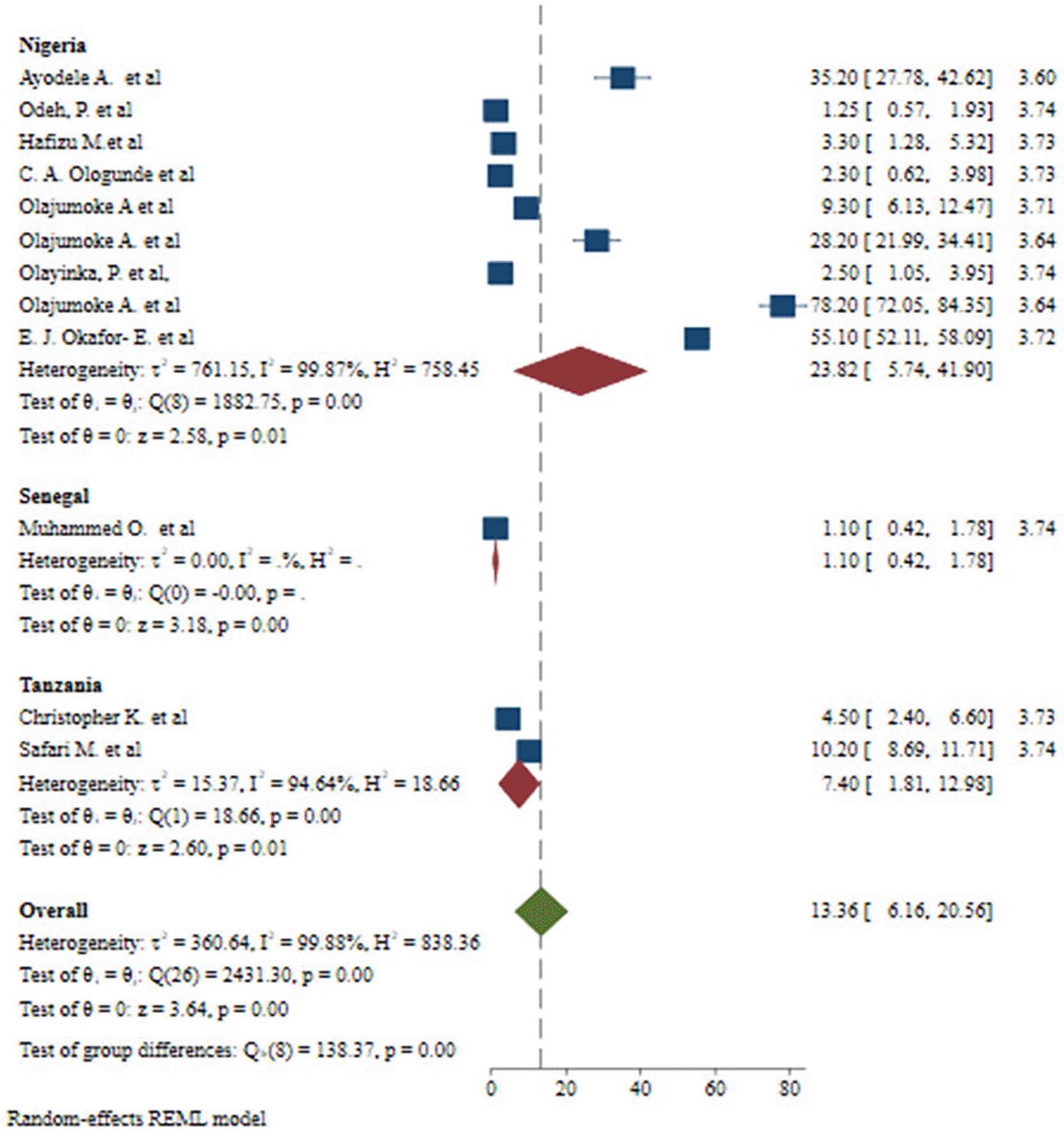
There was a large amount of variability across the included studies. Inverse of variance (I2) statistics revealed more than or equal to 99.88% heterogeneity among studies on the incidence of malaria and S. haematobium coinfection. To explore potential sources of heterogeneity, a subgroup analysis was done for the prevalence of malaria and S. haematobium coinfection based on publication year, country, and diagnostic procedures. As a result, the meta-analysis revealed a substantial variation in the incidence of malaria and S. haematobium coinfection between studies based on publication year ( Figure 5). Furthermore, the meta-analysis revealed a substantial variation in the incidence of malaria and S. haematobium coinfection between studies at the nation level ( Figure 6). Furthermore, the meta-analysis revealed a substantial variation in the prevalence of malaria and S. haematobium coinfection among diagnostic techniques ( Figure 7).
According to this study, the primary risk factors for malaria and S. haematobium coinfection were between the ages of 11 and 13 years, being a farmer, living in inadequate housing, not sleeping beneath an insecticide-treated net, and working in rice and sugarcane fields.27 In addition, the extent of coinfection was linked with age and gender level.22,33,44 Similarly, frequent water contact when swimming and residing in homes with cracks between the walls and roofs were statistically significant risk factors for malaria and S. haematobium coinfection.32 Correspondingly, less water contact (≤2 times/day) was linked to a lower infection incidence than greater water contact (>2 times/day). Furthermore, socioeconomic status, geography, and hygiene variables were some of the risk factors linked to malaria and S. haematobium coinfection.35,40
This review revealed the prevalence of malaria and S. haematobium coinfection over a period of eleven years in sub-Saharan Africa. In this review the pooled prevalence of malaria and S. haematobium coinfection was 13.36% (95% CI: 6.16–20.56). This finding was inline with that reported in Tanzania [10.9%]47 and Nigeria [15%].48 The correspondence in outcomes between malaria and S. haematobium co-infections might be attributed to a number of causes. This could be due to similarities in transmission dynamics, geographical overlap, environmental factors, socioeconomic factors, ecological settings, shared vector and host factors, age-related susceptibility. Additionally, the co-infection of malaria and S. haematobium may arise from their similar life cycles and environmental conditions that facilitate the transmission of both pathogens. For instance, malaria can be prevalent in populations near water sources where Anopheles species breed, which may also harbor helminth eggs and larvae. These water sources are often utilized by many individuals for consumption and domestic activities.49
However, this finding was higher than that reported in East Africa [1.0%].50 The high prevalence of co-infection between these two has been ascribed to comparable factors: inadequate sanitation, a lack of bathroom facilities, contaminated drinking water, and an insufficient public health education campaign. As a result, clean water, sanitation, and hygiene techniques remain effective against these extremely widespread tropical pathologies. The high incidence of co-infections with malaria and S. haematobium might be related to their epidemiological nexus, as both agents are dispersed similarly in the same tropical region.51
On the other hand, this finding was lower than that reported in malaria and helminth co-infection endemic countries (24.4%).52 The recent finding of a greater incidence of malaria and S. haematobium co-infections in certain endemic areas may be attributable to the availability of shared social or environmental variables enhancing persons’ susceptibility to infection with both parasite groups.13 This further strengthened the fact that the study population included people who were more likely to engage in risky behaviors, such as touching the ground, swimming or walking in freshwater bodies of water like rivers and lakes, consuming raw food, drinking untreated water, and being more likely to suffer from other illnesses like malnourishment, which cause for increasing of malaria and S. haematobium coinfection.53,54
Furthermore, this review indicated that significant difference in the prevalence of malaria and S. haematobium coinfection among studies on year of publication. The reported frequency of coinfection may change over time due to a variety of factors, including variations in diagnostic methods, public health initiatives like vaccination campaigns, vector control, and health education, environmental factors, epidemiological trends, and varying funding and research priorities.
Likewise, this systematic review and meta-analysis showed that significant difference in the prevalence of malaria and S. haematobium coinfection among studies on based on countries. Many factors, such as differences in geographic variability, the availability of preventive measures and treatments, socioeconomic status, variations in vaccination, vector control measures, population density, urbanization, and migration patterns, and local customs and practices pertaining to water use, agriculture, and health-seeking behavior, can affect the reported frequency of coinfection across different countries.
Furthermore, this systematic review and meta-analysis indicated that significant difference in the prevalence of malaria and S. haematobium coinfection among studies on diagnostic technique. There are several reasons for the significant variation in the prevalence of S. haematobium coinfection with malaria that was found in the systematic review and meta-analysis employing diagnostic methods. These include variations in the sensitivity and specificity of diagnostic techniques, seasonal variations, regional disparities, and the timing of sample collection in relation to infection dynamics.
Based on the review, there were statistically significant risk factors for the prevalence of malaria and S. haematobium co-infection, including age, socioeconomic status, geography, gender, occupation, frequent water contact, mosquito nets, living in homes with wall-to-roof cracks, and sanitation status. Data pertaining to co-infection between helminths and malaria indicates that individuals residing in rural regions with an agricultural economy are more susceptible to contracting the disease due to increased interaction with helminth vectors and infectious helminth forms. Because these regions are distinguished by higher levels of water stagnation, a lot of bushes surrounding homes, a lower level of education, poverty, and a lack of malaria preventive measures. Additionally, the majority of housing in rural areas is made of planks, has cracks, or has other features that make the malaria vector more comfortable.55,56
We adhered to a predetermined method for search strategy and data abstraction. We used widely accepted approaches to critically appraise and evaluate the quality of individual studies.
Language bias is probable because the included studies were all published in English. This review also includes studies from certain nations due to a lack of literature from other countries, which may affect the representativeness of the findings. Furthermore, only studies conducted from 2014 to 2024 were taken into account for inclusion.
The results of this systematic review and meta-analysis revealed that high prevalence of malaria and S. haematobium coinfection in sub-Saharan Africa. This indicated that managing infections brought on by this coinfection in healthcare settings is may cause a significant challenge. In order to stop the spread of malaria and S. haematobium coinfection in sub-Saharan Africa, improved infection control methods and better monitoring systems are crucial. Additionally, to address the complex mechanisms causing malaria and S. haematobium coinfection and reduce its impact on public health, cooperative actions at the local, national, and international levels are necessary. Similarly, enhance epidemiological investigations into the magnitude of coinfection, individuals at higher risk, and the creation and assessment of therapies focused at both health concerns in the study area. Also, health programs should be designed to prevent and control malaria and S. haematobium infection.
We declare that this manuscript is review and has not been submitted or published in other Journal.
The data analyzed and generated in this study are available within the manuscript itself, along with Extended data like PRISMA checklist at this link, https://zenodo.org/records/1694151657 and supplementary files accessible at this link, https://zenodo.org/records/16941670.58
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to acknowledge our colleagues who contribute for the preparation of this manuscript. Any AI software has not been used to prepare this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
No
Are the conclusions drawn adequately supported by the results presented in the review?
No
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: 10 years of research in malaria genetics and disease dynamics; 15 years of research experience.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
No
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases of public health importance, microbial and human genetics, and immunology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 02 Sep 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)