Keywords
Anti-glomerular basement membrane disease, Kaposi sarcoma, vasculitis, immunosuppressive treatment
This article is included in the Rare diseases collection.
Anti-glomerular basement membrane (anti-GBM) disease is a rare and life-threatening autoimmune vasculitis characterized by rapidly progressive glomerulonephritis, with or without pulmonary hemorrhage. Its management requires aggressive immunosuppressive therapy, including corticosteroids, cyclophosphamide, and plasmapheresis. Kaposi sarcoma (KS) is a vascular neoplasm linked to human herpes virus 8 (HHV-8), most frequently seen in HIV-infected or iatrogenically immunosuppressed patients. We report the case of a 39-year-old man with anti-GBM vasculitis who developed cutaneous Kaposi sarcoma two months after initiation of immunosuppressive treatment. HHV-8–positive spindle cell proliferation was confirmed on skin biopsy. HIV testing was negative, and the lesions regressed following the tapering of corticosteroids and withdrawal of cyclophosphamide. This case highlights the importance of considering KS as a differential diagnosis in patients developing skin lesions under immunosuppressive therapy for autoimmune vasculitis.
Anti-glomerular basement membrane disease, Kaposi sarcoma, vasculitis, immunosuppressive treatment
Anti-glomerular basement membrane (anti-GBM) disease, also known as Goodpasture’s disease, is a rare but severe autoimmune vasculitis characterized by autoantibodies targeting the non-collagenous domain of the alpha-3 chain of type IV collagen (α3[IV]NC1) in glomerular and alveolar basement membranes.1 It typically presents as rapidly progressive glomerulonephritis (RPGN), often accompanied by pulmonary hemorrhage, constituting the classic pulmonary–renal syndrome.2 The condition is life-threatening without prompt diagnosis and aggressive treatment. Standard therapy combines high-dose corticosteroids, cytotoxic agents such as cyclophosphamide, and plasmapheresis to remove circulating antibodies.3
Kaposi sarcoma (KS) is an angioproliferative neoplasm associated with latent infection by human herpesvirus 8 (HHV-8). It most commonly affects individuals with acquired immunodeficiency, such as people living with HIV/AIDS or organ transplant recipients under long-term immunosuppression.4 Iatrogenic KS can also emerge in patients receiving immunosuppressive therapy for autoimmune diseases, although this presentation remains uncommon. The cutaneous form of KS typically manifests as violaceous macules, papules, or plaques, predominantly on the lower limbs. Histologically, KS is characterized by spindle cell proliferation with neoangiogenesis and erythrocyte extravasation, and HHV-8 immunohistochemistry is diagnostic.5
We report a rare case of iatrogenic Kaposi sarcoma in a patient treated for anti-GBM vasculitis. This case illustrates the potential for opportunistic neoplasms to develop in non-transplant autoimmune settings, underscoring the need for dermatologic vigilance in patients undergoing intensive immunosuppressive therapy.
A 39-year-old man with no significant past medical history presented with gross hematuria that appeared four days after an episode of acute tonsillitis. Physical examination was unremarkable. Laboratory tests revealed acute kidney injury (serum creatinine 196 μmol/L), mild normocytic anemia (hemoglobin 11.9 g/dL), leukocytosis (11,800/mm3), and elevated C-reactive protein (CRP) at 110 mg/L. Urinalysis showed a urinary albumin/creatinine ratio of 0.5 g/g and macroscopic hematuria (600,000 red blood cells/mL), with a sterile urine culture. Abdominal ultrasound was normal.
Given the presentation of glomerulonephritis without extrarenal manifestations, an immunologic workup was performed. Antinuclear antibodies and antineutrophil cytoplasmic antibodies were negative, while anti-glomerular basement membrane (anti-GBM) antibodies were strongly positive (+++). Kidney biopsy revealed extracapillary proliferation (crescent formation) in over 60% of glomeruli (9 out of 14) and acute tubular necrosis with early signs of regeneration. A chest computed tomography scan ruled out alveolar hemorrhage.
The diagnosis of anti-GBM vasculitis was established, and treatment was initiated with intravenous steroid pulses followed by high-dose oral prednisone, daily oral cyclophosphamide, and a course of plasmapheresis. Despite treatment, the patient’s renal function deteriorated, progressing to anuria and requiring initiation of hemodialysis. Anti-GBM antibodies remained positive, requiring a total of 29 plasmapheresis sessions. During hospitalization, the patient developed multiple infections, including pyelonephritis, infectious diarrhea, and esophageal candidiasis, all of which were treated with broad-spectrum antimicrobials.
Approximately two months after starting immunosuppressive therapy, the patient developed violaceous skin lesions on the lower extremities ( Figure 1). At that time, he was on oral cyclophosphamide and prednisone 40 mg/day. He remained otherwise asymptomatic. A skin biopsy from the left foot revealed HHV-8–positive spindle cell vascular proliferation without vasculitis, consistent with Kaposi sarcoma ( Figures 2, 3, 4). HIV testing was negative.
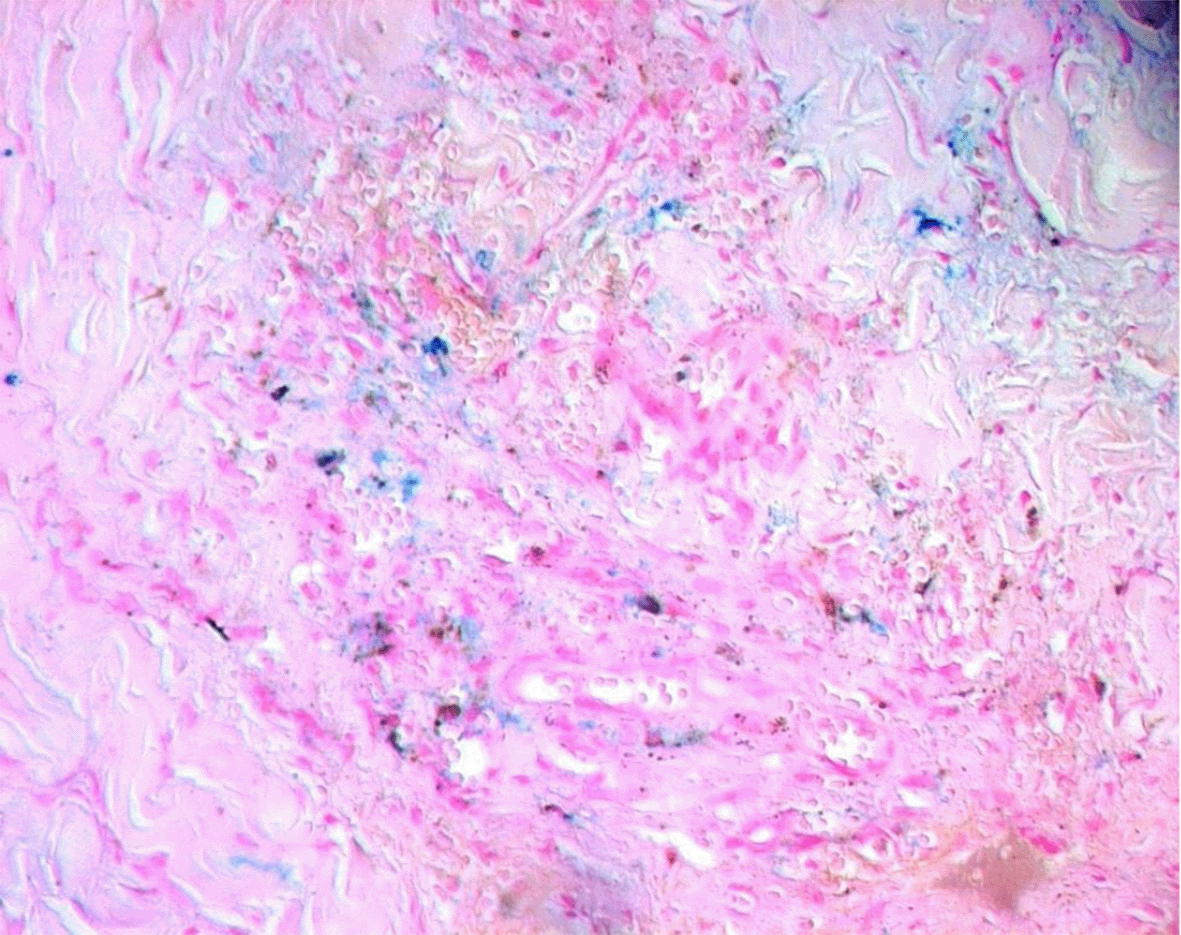
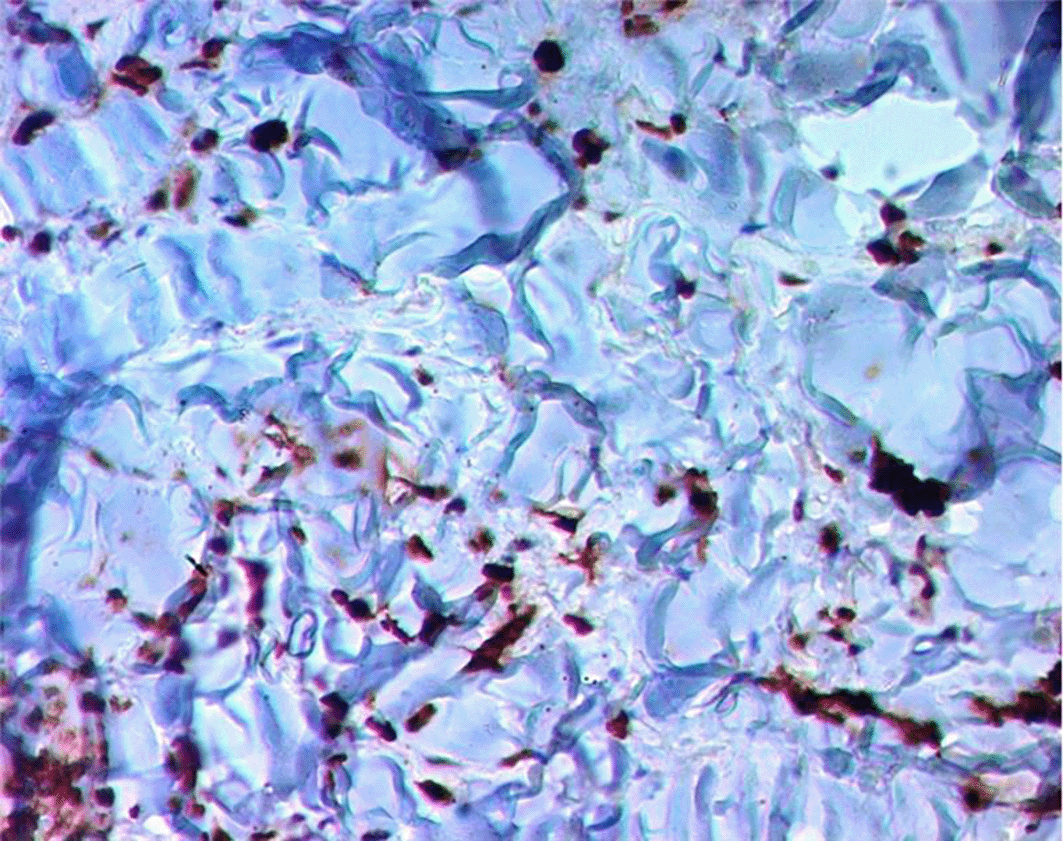
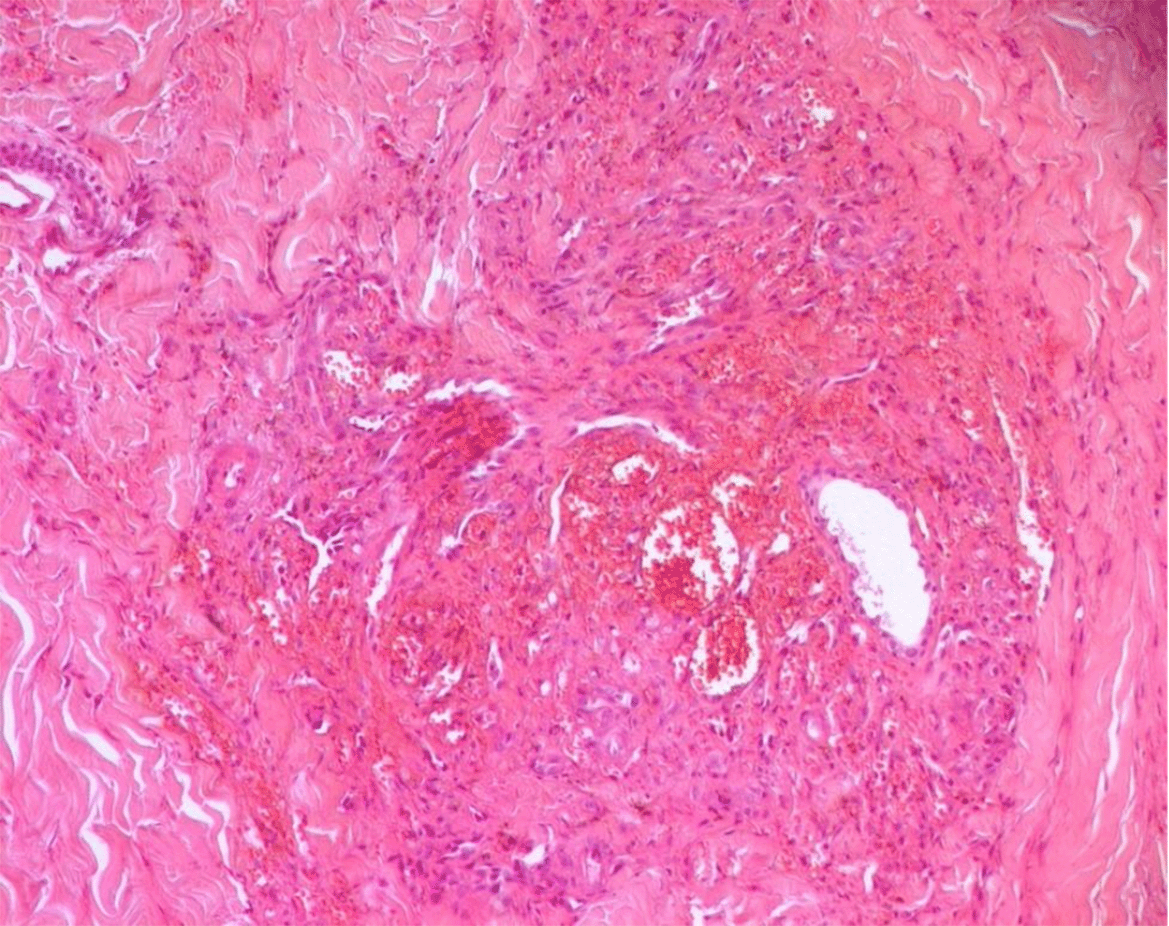
Shortly afterward, the patient was admitted for upper gastrointestinal bleeding. Gastroscopy revealed actively bleeding lesions, but biopsy excluded gastrointestinal Kaposi sarcoma. The bleeding was attributed to direct oral anticoagulant, which was prescribed after arteriovenous fistula placement, and resolved with octreotide and proton pump inhibitors.
In light of the Kaposi sarcoma diagnosis and absence of renal recovery, cyclophosphamide was discontinued and prednisone was rapidly tapered to 10 mg/day. The patient was referred to dermatology for follow-up but he declined further evaluation. His skin lesions regressed spontaneously over the following weeks.
This case illustrates a rare but significant complication of immunosuppressive therapy in a patient with anti-glomerular basement membrane (anti-GBM) vasculitis. Anti-GBM disease is classically associated with rapidly progressive glomerulonephritis and pulmonary hemorrhage. Therefore, its management requires aggressive immunosuppression that may predispose patients to opportunistic infections and, more rarely, virus-associated malignancies.1,2
Kaposi sarcoma (KS) is a vascular tumor caused by human herpesvirus 8 (HHV-8), which can persist in a latent form and reactivates under immunosuppressive conditions.4 Iatrogenic KS is typically reported in organ transplant recipients, but it may also occur in patients treated for autoimmune diseases such as systemic lupus erythematosus or vasculitis.6 In the context of anti-GBM vasculitis, such presentations remain exceptional.
Our patient developed KS two months after initiation of immunosuppressive therapy combining corticosteroids and cyclophosphamide. The diagnosis was confirmed histologically by the presence of HHV-8–positive spindle cell vascular proliferation. The regression of skin lesions following reduction of immunosuppression supports the iatrogenic origin of the disease. He had no other recognized risk factors such as HIV infection.
While the majority of iatrogenic KS cases occur in transplant medicine, the literature describes rare occurrences in autoimmune vasculitis. Tiong et al. reviewed several such cases, mostly associated with ANCA-associated vasculitis, treated with cyclophosphamide and corticosteroids.7 The proposed mechanism involves a combination of impaired T-cell–mediated immunity and HHV-8 reactivation.
In the absence of systemic involvement, reducing or discontinuing immunosuppression is often sufficient for KS regression.4,6 More advanced or visceral forms may require chemotherapy or antiviral therapy. In our case, the spontaneous regression after drug tapering and the patient’s refusal of further dermatologic management guided a conservative approach.
This case underlines the need to include KS among the differential diagnosis of skin lesions in immunosuppressed patients, even outside the context of HIV or organ transplantation. Early recognition allows timely therapeutic adjustment and may avoid unnecessary interventions.
This case highlights a rare but clinically important complication of immunosuppressive therapy in anti-GBM vasculitis. Although Kaposi sarcoma is typically associated with HIV infection or solid organ transplantation, it may also occur in patients treated for autoimmune diseases. In this context, the development of cutaneous Kaposi sarcoma should prompt consideration of iatrogenic immunosuppression as a precipitating factor. Early dermatologic evaluation and timely tapering of immunosuppressive therapy can lead to spontaneous regression of lesions, as illustrated in this case. Vigilance for unusual cutaneous manifestations is therefore essential during follow-up of patients receiving potent immunosuppressive regimens.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: autoimmune kidney diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 08 Sep 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)