Keywords
Carcasses, Decomposition, Insects, Colonization, Flunitrazepam, Succession
Determining the post-mortem interval (PMI) through examination of insect activity on deceased bodies is essential in forensic science. Establishing the time of death in cases involving drug ingestion can present difficulties for law enforcement, complicating evidence collection. In unnatural death investigations, a forensic pathology approach is commonly employed, focusing exclusively on samples obtained from the body, which can lead to biases and errors, particularly beyond 72 hours after death. Notably, no research on insect colonization and succession on cadavers has been conducted in Kenya, despite a growing number of unidentified deaths. This research aimed to identify and assess forensically significant insects and to establish the effect of flunitrazepam (Rohypnol®) on carrion-insect successional patterns on pig carcasses.
Four domestic pigs, averaging 24.8 kg, were used, with one designated as the control and three assigned as the experimental group. The experimental pigs received oral drug administration mixed with 250ml of vodka to simulate drink spiking in a bar. Subsequently, the pigs were euthanized, and their carcasses were placed in cages. Adult insects and flightless adult invertebrates were sampled daily until the dry remains stage of decomposition. Existing insect identification keys were utilized for species identification.
The results revealed consistent insect succession patterns across the four carcasses. The mean number of insects across developmental stages decreased as flunitrazepam dosage increased. However, no significant variation among the carcass groups was observed in insect genera. Decomposition was categorized into five stages: fresh, bloated, active decay, advanced decay, and dry, consistent with prior studies. Insect succession included Diptera (e.g., Chrysomya spp., Lucilia sericata), Coleoptera (e.g., Dermestes maculatus), and Hymenoptera (e.g., Camponotus sericeus), with Calliphoridae being most abundant. The flunitrazepam-ingested carcasses showed prolonged decomposition stages compared to control.
These findings highlight the need to consider drugs like flunitrazepam in forensic entomology analyses.
Carcasses, Decomposition, Insects, Colonization, Flunitrazepam, Succession
Forensic entomology can be defined as using insects and other arthropods to process criminal cases.1 In crime scene investigations globally, it helps the practitioner establish the sequence of events leading to death.2 Forensic entomologists can be helpful as they analyse the insect succession on any remains, helping to reconstruct factors such as the time of death, drug presence, and possible post-mortem body displacement.3 After death, autolysis commences, and the cells start degrading as the enzymes inside them come into action. This results in the process of decomposition, where bacteria in the gastrointestinal system emit fluids and gases such as hydrogen sulphide, carbon dioxide, methane, ammonia, sulphur dioxide, and hydrogen.4
Insects and arthropods are attracted to volatile compounds emitted from a decaying body to feed on the decomposing vertebrate remains known as carrion.5 These remains host four primary types of insects6: necrophagous species, predators and parasites, omnivores, and other species, including springtails and spiders. In forensic entomology, the first two groups, mainly from the Diptera (flies) and Coleoptera (beetles) orders, are significant.7 The pattern and frequency of arthropod arrival depend on the specific stage of carrion decomposition.8
True flies, or Diptera, are crucial in forensic investigations, with the most prevalent species being Calliphoridae (blow flies), Sarcophagidae (flesh flies), and Muscidae (house flies). Calliphoridae and Sarcophagidae usually arrive shortly after death, while Muscidae generally start to colonize during the bloating stage of decomposition.9 Insect colonizers play a vital role in forensic investigations by helping to estimate the time of death, trace the movement of the corpse, clarify the circumstances and cause of death, link suspects to the crime scene, and determine the post-mortem interval (PMI), which is the period between death and the discovery of the body.10 However, despite its widespread use globally, forensic entomology is still lagging in Kenya, despite the rising cases of unknown deaths over the years. The estimation of time elapsed since death can be deduced through knowledge of the insect colonization and succession on dead bodies.11 No research has been conducted on insect colonization and succession on cadavers in Kenya. Furthermore, the presence of drugs in the body significantly impacts insect development and succession.12 In some cases, the effects of drugs on these insects are determined by their concentration, while in others, their mere presence is enough to influence the insects.13 Therefore, this study sought to identify the effect of flunitrazepam, describe the baseline insect fauna, and develop insect successional patterns in the upper Kabete region of Kiambu County.
This study utilized an experimental case-control research design. Four domestic pigs (Sus Scrofa domesticus) weighing 24.6 kg, 24.7 kg, 24.9 kg, and 25.0 kg were used. The pigs were housed in an adequate, well-ventilated pen two weeks before the study commenced. During that period, they were provided fresh food and water and excluded from any medication. One pig was used as a control, and the other three were used as the experimental group. The pigs were selected because they have similar gastrointestinal fauna and skin features to human beings.14 Also, their size is similar to that of an average human torso. Such experiments are conducted using pigs because of their controlled environment and the fact that they provide a less controversial alternative to human cadavers.15
The pigs utilized in this research were sourced from the veterinary farm of the University of Nairobi’s Faculty of Veterinary Medicine, a facility dedicated to breeding and maintaining animals for academic and research activities. Therefore, the animals were not privately owned, and their use was approved by the institution in line with established ethical and animal welfare guidelines.
To mimic the effects of drink spiking, the experimental pigs were orally dosed with different doses of flunitrazepam dissolved in 250 ml of vodka (40 per cent ethanol strength) on the evening of June 30, 2021.16 Particularly, Experimental Group 1 (EXP GR1) was given 1 mg, Experimental Group 2 (EXP GR2) was given 2 mg, and Experimental Group 3 (EXP GR3) was given 3 mg of flunitrazepam. All pigs were euthanised at 5.00 a.m., using the electric stunning method that involves applying electric current through the brain.
The electric stunning method was performed as follows; electrodes were placed on both sides of the pig’s head to deliver a current that passes through the brain, causing a rapid loss of sensibility and insensibility to pain. The electrocution was done with sufficient current of 1.3 A at 250V for 20 seconds followed by a second electrocution at 300V on the chest for 5 seconds to fibrillate the heart causing death.17
The Directorate of Veterinary Services in Kenya, as well as the American Veterinary Medical Association, approves electric stunning as a humane and fast way of euthanizing the animals in commercial slaughter facilities, and it is thus in compliance with animal welfare regulations.
The pig carcasses were promptly transported to the veterinary farm at Upper Kabete Campus. At the site, the carcasses were left in field conditions to decompose naturally until they reached the final stage of decay. The decomposition and insect colonization were monitored for approximately 3 months. Each pig was on open ground and secured against predators with metal cages ( Figure 1). These 92 x 92 x 153 cm cages were made of steel-welded frames using 2.5 cm tubing and enclosed with 1.27 cm mesh hardware cloth. They were positioned approximately 100 meters apart at the transition zone between a dense wooded area and an open pasture on the farm.

The experimental groups had been administered with 1mg/250 ml Vodka (EXP GR1 = Experimental Group 1), 2 mg/250 ml Vodka (EXP GR2 = Experimental Group 2), and 3 mg/250 ml Vodka (EXP GR3 = Experimental Group 3).
Ethical approval for animal use was provided by the Biosafety, Animal Use and Ethics Committee of the Department of Veterinary Anatomy and Physiology, Faculty of Veterinary Medicine, University of Nairobi. REF: FVM BAUEC/2019/203.16
The study was conducted at Kanyariri in the upper Kabete region of Kiambu County within the veterinary farm of the University of Nairobi (Kenya).16 The site was situated in a softwood tree forest. The soil of the site is black, and the tree cover is low, and as a result, the air was humid near the forest floor. The altitude of the study site is 1820M (Latitude -1.2492350 E and Longitude 36.7420570 S), which is a typical montane region. The study site contained remnants of potential natural vegetation, secondary forest species, and other tree species common in evergreen upland forests ( Figure 2). The site is also characterized by disturbance and colonized by native invasive tree species. Furthermore, exotic invasive species were also common.

The soil of the site is black, and the tree cover is low, and as a result, the air was humid near the forest floor. The altitude of the study site is 1820M (Latitude -1.2492350 E and Longitude 36.7420570 S), which is a typical montane region.
Insect sampling encompassing adult insects and non-flying invertebrates was performed daily until the carcasses reached the dry remains stage of decomposition. The sampling schedule was as follows: three times daily (7:00 AM, 1:00 PM, and 7:00 PM) for the initial eight days, twice daily (11:00 AM and 5:00 PM) for the subsequent eight days, and once daily (12:00 PM) until the dry remains stage was achieved, which occurred 60 days after death. Each sampling involved capturing arthropods flying near or resting on the carcass using an entomological net. Insects were also collected from natural body cavities (eyes, nose, mouth, and anus) as well as from the cardiac puncture wound. This study concentrated on Sarcophagidae (flesh flies), Calliphoridae (blow flies), Muscidae (house flies), and skin beetles. Adult insects were captured using aerial net sweeps above the carcasses and in the surrounding environment. Flightless insects were removed from the carcasses with forceps. Pitfall traps and plastic cups filled with soapy water were used to capture crawling insects.
The insects were immersed in boiling water for 30 seconds and then preserved in 75% ethyl alcohol (sup: Scharlau, Cat No: ET00052500). This method halts further development, allowing for the assessment of the insects’ developmental stages. Each carcass had two pitfall traps placed approximately 8 cm away from the abdomen; each trap consisted of a plastic cup of soapy water (12 mm diameter and 7 mm deep). All the samples taken were carefully labelled with details such as the geographical location, case number, date and time of collection, name of the collector, and environmental conditions.
The insects were identified using a microscope (Model: Leica zoom 2000, Serial No: 1408CX) and standard identification keys. Data analysis was carried out using Microsoft Excel Office 2010 and Statistical Package for the Social Sciences (SPSS) version 25. Correlations between the insect orders that were collected, the number of insects on the decomposing carcasses, the levels of decomposition, and the corresponding periods were depicted in tables. Species diversity, abundance, and distribution in the study were depicted in graphs.
The successional pattern of major forensically important insects and arthropods was analyzed only to identify species’ occurrence and density trends. The major emphasis was on the Diptera species that colonized the remains and their succession. It is important to note that only the Diptera species that colonized the remains are represented in the results with a view to their outlook on their application in forensic investigations in Kenya. The findings are presented in chronological sequence for each habitat, and the succession pattern is exhibited as a tabulation. While it is unlikely that the list of species found with the remains is exhaustive, it does indicate the succession patterns of Hymenoptera and Coleoptera.
Generally, three different arthropod orders were isolated from carrion at different stages of decomposition. This study obtained 27 insect species, 20 families, and three orders. The insect species were the same in the four groups; however, the number of insects reduced with the introduction and increased concentration of flunitrazepam. The control had the highest number of insects at 1,259, while EXP GR1, EXP GR2, and EXP GR3 had 1,089, 932, and 852, respectively ( Table 1).
In all four groups (Control, EXP GR1, EXP GR2, and EXP GR3), Calliphoridae was the most abundant, comprising 229 (19.30%), 191 (17.54%), 174 (18.67%), and 148 (17.37%) individuals, respectively ( Figure 2). Muscidae followed this in all four groups with 119 (12.63%), 141 (12.95%), 90 (9.66%), and 87 (10.21%) individuals, respectively ( Figure 3). The least abundant family in all four groups was the family Stratiomyidae. These results provide insight into the relative abundance of different insect families within each experimental group, highlighting the consistent dominance of Calliphoridae across all groups.
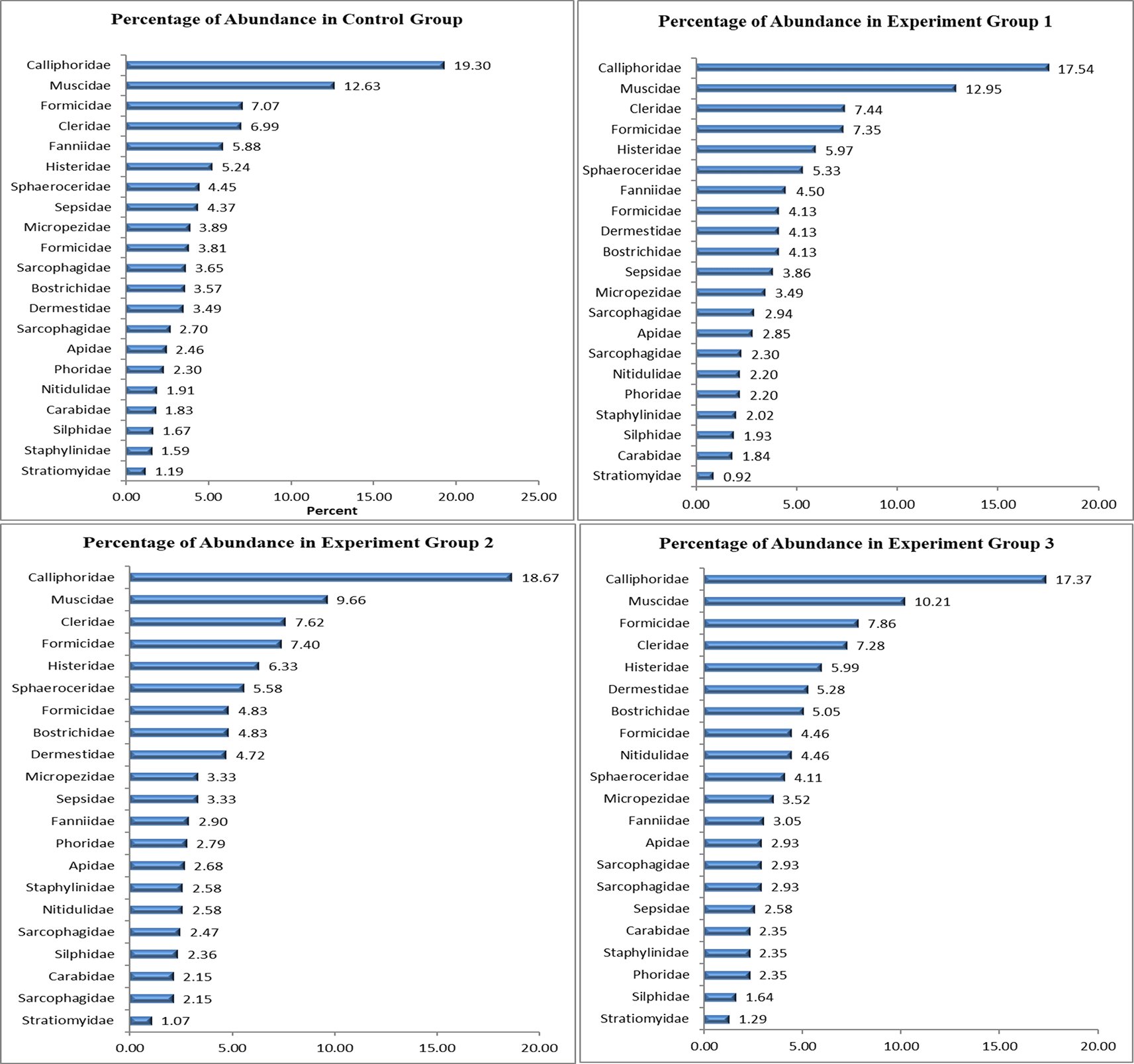
In all four groups (Control, EXP GR1, EXP GR2, and EXP GR3), Calliphoridae was the most abundant, comprising 229 (19.30%), 191 (17.54%), 174 (18.67%), and 148 (17.37%) individuals, respectively. Muscidae followed this in all four groups with 119 (12.63%), 141 (12.95%), 90 (9.66%), and 87 (10.21%) individuals.
Different species of arthropods were associated with various stages of pig decay. However, the number of insects decreased with the introduction of flunitrazepam and its concentration.
Fresh stage: At the fresh stage of decay, the recovered insects included Pheidole megacephala, Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Protophormia terraenovae, Parasarcophaga ruficornis, Musca domestica, Stomoxys evanida, and Sarcophaga Inzi. There were also a few specimens of Leptocera sp.
Bloat stage: The bloat stage was characterized by the presence of Pheidole megacephala, Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Protophormia terraenovae, Parasarcophaga ruficornis, Musca domestica, Stomoxys evanida, Sarcophaga Inzi, Leptocera sp., Bostrichidae sp., Staphylinidae violaceous, Angionychus lividus, Necrobia rufipes, and Trigona carbonaria.
Active decay stage: Insects included Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Parasarcophaga ruficornis, Musca domestica, Stomoxys evanida, Sarcophaga Inzi, Leptocera sp., Allosepsis indica, Mimegralla albimana, Megaselia scalaris, Fannia canicularis, Pheidole megacephala, Nitidulidae sp., Staphylinidae violaceous, Angionychus lividus, Hister monitor, Dermestes maculatus, Necrobia rufipes, Camponotus sericeus, and Trigona carbonaria.
Advance decay stage: Was characterized by the presence of Stomoxys evanida, Leptocera sp., Allosepsis indica, Mimegralla albimana, Ptecticus melanurus, Megaselia scalaris, Fannia canicularis, Pheidole megacephala, Nitidulidae sp., Staphylinidae violaceous, Bostrichidae sp., Hister monitor, Thanatophilus sinuatus, Dermestes maculatus, Necrobia rufipes. Additionally, other species like Musca domestica, Calliphora vicina, Chrysomya bezziana, Lucilia sericata, Chrysomya chloropyga, Chrysomya vomitoria, and Chrysomya megacephala were also present, although their numbers had significantly reduced.
Dry stage: The main species included Dermestes maculatus, Bostrichidae sp., Hister monitor, Pheidole megacephala, Necrobia rufipes, Nitidulidae sp., Chrysomya chloropyga, and Allosepsis indica. However, there were only a few specimens of Musca domestica, Mimegralla albimana, Ptecticus melanurus, Calliphora vicina, Megaselia scalaris, and Thanatophilus sinuatus.
The succession of insects in different stages of decomposition among the four groups of carcasses (Control, EXP GR1, EXP GR2, and EXP GR3) was consistent.
Fresh stage: In the Diptera order, species collected during the fresh stage of carcasses in all four groups included Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Lucilia sericata, Protophormia terraenovae, Parasarcophaga ruficornis, Musca domestica, and Stomoxys evanida. In the Coleoptera order, the species found in the fresh stage included Pheidole megacephala, while in the Hymenoptera order, Camponotus sericeus was found.
Bloating stage: In the bloating stage, species in the Diptera order found in all four groups (control, experiment group one, experiment group two, and experiment group three) included Chrysomya bezziana, Chrysomya megacephala, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Protophormia terraenovae, Parasarcophaga ruficornis, Sarcophaga Inzi, Musca domestica, Stomoxys evanida, Fannia canicularis, and Ptecticus melanurus. In the Coleoptera order, species found included Pheidole megacephala, Staphylinidae violaceous, Angionychus lividus, Bostrichidae sp., Camponotus sericeus, Necrobia rufipes, and Trigona carbonaria.
Active decay stage: In the Control, EXP GR1, EXP GR2, and EXP GR3 groups, most of the categorized insect species were found, except for a few. For instance, Ptecticus melanurus, Bostrichidae sp., and Thanatophilus sinuatus were not found in this stage in any of the four groups of pigs. Additionally, Allosepsis indica and Mimegralla albimana species were present in very low numbers. Abundant species in this stage included Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Parasarcophaga ruficornis, Sarcophaga Inzi, Musca domestica, Stomoxys evanida, Leptocera sp., Fannia canicularis, Pheidole megacephala, Nitidulidae sp., Staphylinidae violaceous, Angionychus lividus, Hister monitor, Dermestes maculatus, Necrobia rufipes, Camponotus sericeus, and Trigona carbonaria.
Advanced decay stage: New insects began to appear while others disappeared at this stage. For instance, Chrysomya bezziana, Chrysomya megacephala, Parasarcophaga ruficornis, Sarcophaga Inzi, Angionychus lividus, and Camponotus sericeus disappeared in this stage. Additionally, the Megaselia scalaris species had very few insects during this stage. Therefore, species in the advanced stage included Trigona carbonaria, Musca domestica, Stomoxys evanida, Leptocera sp., Allosepsis indica, Mimegralla albimana, Ptecticus melanurus, Megaselia scalaris, Fannia canicularis, Pheidole megacephala, Nitidulidae sp., Staphylinidae violaceous, Bostrichidae sp., Hister monitor, Thanatophilus sinuatus, Dermestes maculatus, Necrobia rufipes, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, and Lucilia sericata.
Dry stage: Species from the Diptera and Hymenoptera orders significantly decreased in all four groups of decomposing pigs (control, experiment group one, experiment group two, and experiment group three), leaving species from the Coleoptera order. The remaining Diptera order species included Chrysomya chloropyga, Musca domestica, Allosepsis indica, Mimegralla albimana, Ptecticus melanurus, and Megaselia scalaris. The remaining Coleoptera order species included Pheidole megacephala, Nitidulidae sp., Bostrichidae sp., Hister monitor, Dermestes maculatus, and Necrobia rufipes. This information provides insights into the arthropod species’ changing composition at different pig decomposition stages.
An independent sample t-test was used to assess these differences. Table 2 compares the mean insect numbers between the control and experimental groups. The results reveal no significant difference (p>0.05) in insect numbers between these two groups.
Table 3 compares mean insect numbers between the control and experimental group two. The results indicate no significant difference (p>0.05) in insect populations between these groups.
Table 4 compares the mean insect populations between the control and experimental group three. The results suggest no significant difference (p>0.05) in insect numbers between these groups.
Pig carcass decomposition was classified into five stages: fresh, bloated, active decay, advanced decay, and dry. These stages correspond with the five primary decomposition stages described by Eberhardt and Elliot18 fresh, bloated, active decay, post-decay, and skeletal.19 They also match the classification by Moura,20 which includes fresh, bloated, decaying (combining active and advanced decay), and dry stages.
The current study results differ from Odo’s findings,21 which reported the fresh stage lasting from day 0 to day 1, the bloated stage occurring from day 2 to day 3, the active decay stage spanning from day 3 to day 6, the advanced decay phase starting on day 7 and continuing until day 15, and the dry decay stage beginning on day 16 and ending on day 60.22 These differences in decomposition rates may be attributed to temperature variations. Temperature is one of the extrinsic factors crucial for bacterial development, affecting decomposition rates. It plays a pivotal role in decomposition, and its fluctuations can significantly affect the observed decomposition stages.23 It is important to mention that temperature was not measured directly in this study, but in future studies, it may be useful to add temperature data as a variable.24 This would give a better idea of the role of temperature changes, daily and seasonal, in the course of decomposition. Additional factors that affect decomposition are age, constitution, cause of death, ventilation, and humidity.21
The four carcass groups contained insects of the orders Diptera, Coleoptera and Hymenoptera. This observation is consistent with that made by Vitta25 on the decomposition of pig carcasses in which two insect orders were observed. However, they differ from Moura’s findings,20 which included three orders of insects: Diptera, Coleoptera, and Hymenoptera. Throughout all decomposition stages, the observed insect species included Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Protophormia terraenovae, Parasarcophaga ruficornis, Sarcophaga Inzi, Musca domestica, Stomoxys evanida, Leptocera sp., Allosepsis indica, Mimegralla albimana, Ptecticus melanurus, Megaselia scalaris, Fannia canicularis, Pheidole megacephala, Nitidulidae sp., Staphylinidae violaceous, Angionychus lividus, Bostrichidae sp., Hister monitor, Thanatophilus sinuatus, Dermestes maculatus, Necrobia rufipes, Camponotus sericeus, and Trigona carbonaria. These findings support Vitta25 observation of species such as Chrysomya rufifacies, Chrysomya megacephala, Musca domestica, Fannia canicularis, Parasarcophaga ruficornis, Piophila casei, Dermestes maculatus, and Necrobia rufipes during pig carcass decomposition.
Among all the collected insects, Calliphoridae were the most abundant, followed by families Muscidae, Formicidae, Cleridae, Sarcophagidae, Fanniidae, Histeridae, Sphaeroceridae, Sepsidae, Micropezidae, Formicidae, Bostrichidae, Dermestidae, Apidae, Phoridae, Nitidulidae, Carabidae, Silphidae, Staphylinidae, and Stratiomyidae. These findings are consistent with Eberhardt and Elliot’s observations18 of the primary colonizers being Calliphoridae, Muscidae, Formicidae, Cleridae, Sarcophagidae, Fanniidae, Histeridae, Micropezidae, and Formicidae. They also align with discovery of adult specimens from eight Diptera families: Calliphoridae, Muscidae, Sarcophagidae, Phoridae, Piophilidae, Fanniidae, Sphaeroceridae, and Anthomyiidae during five stages of decomposition.26
The insect succession during different decomposition stages among the four carcass groups (control, experiment group one, experiment group two, and experiment group three) was consistent.27 In the fresh stage, Diptera species such as Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Lucilia sericata, Protophormia terraenovae, Parasarcophaga ruficornis, Musca domestica, and Stomoxys evanida were observed. Coleoptera species, including Pheidole megacephala, and Hymenoptera species like Camponotus sericeus, were also found.28 These findings agree with Odo’s observation21 of main species during the fresh stage, which included Chrysomya albiceps, Lucilia sericata, Musca domestica, Stomoxys evanida, Sarcophaga inzi, and Camponotus sericeus. They also align with Heo identification of species29 like Chrysomya rufifacies, Chrysomya megacephala, Parasarcophaga ruficornis, and Musca domestica during the fresh stage.
During the bloating stage, Diptera species such as Chrysomya bezziana, Chrysomya megacephala, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Protophormia terraenovae, Parasarcophaga ruficornis, Sarcophaga Inzi, Musca domestica, Stomoxys evanida, Fannia canicularis, and Ptecticus melanurus were observed. Coleoptera species like Pheidole megacephala, Staphylinidae violaceous, Angionychus lividus, Bostrichidae sp., Camponotus sericeus, Necrobia rufipes, and Trigona carbonaria were also found. These findings correspond with Odo’s observation of species21 during the bloated stage, including Chrysomya chloropyga, L. sericata, Chrysomya vomitoria, Musca domestica, Stomoxys evanida, Trigona carbonaria, Melipona beecheii, Camponotus sericeus, Camponotus perrisii, Monomorium minimum, Crematogaster sp, S. violaceous, and Necrobia rufipes. They also align with Vitta identification of species25 like Fannia canicularis, Chrysomya rufifacies, Chrysomya megacephala, Parasarcophaga ruficornis, and Musca domestica during the bloated stage.
Most insect species from the four groups were present in the active decay stage, except for Ptecticus melanurus, Bostrichidae sp., and Thanatophilus sinuatus.
Additionally, Allosepsis indica and Mimegralla albimana were scarce. Abundant species during this stage included Chrysomya bezziana, Chrysomya megacephala, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, Lucilia sericata, Parasarcophaga ruficornis, Sarcophaga Inzi, Musca domestica, Stomoxys evanida, Leptocera sp., Fannia canicularis, Pheidole megacephala, Nitidulidae sp., Staphylinidae violaceous, Angionychus lividus, Hister monitor, Dermestes maculatus, Necrobia rufipes, Camponotus sericeus, and Trigona carbonaria. Odo also observed insects during the active decay stage of pig carrion decomposition, including L. sericata, Chrysomya chloropyga, Chrysomya vomitoria, Musca domestica, Stomoxys evanida, Sarcophaga inzi, Necrobia rufipes, Coelus ciliata, Hister monitor, and D. maculatus, among others.21 These findings are consistent with Abd El-Gawad et al.30 identification of species like Chrysomya megacephala, Parasarcophaga ruficornis, and Piophila casei during the active decay stage.
In the advanced decay stage, new insect species appeared while others disappeared. Species like Chrysomya bezziana, Chrysomya megacephala, Parasarcophaga ruficornis, Sarcophaga Inzi, Angionychus lividus, and Camponotus sericeus disappeared in this stage. Additionally, the Megaselia scalaris species were scarce during this stage. The species present in the advanced stage included Trigona carbonaria, Musca domestica, Stomoxys evanida, Leptocera sp., Allosepsis indica, Mimegralla albimana, Ptecticus melanurus, Megaselia scalaris, Fannia canicularis, Pheidole megacephala, Nitidulidae sp., Staphylinidae violaceous, Bostrichidae sp., Hister monitor, Thanatophilus sinuatus, Dermestes maculatus, Necrobia rufipes, Chrysomya chloropyga, Chrysomya vomitoria, Calliphora vicina, and Lucilia sericata. These findings are like Odo’s observations of species during the advanced decay stage of pig carrion decomposition, including C. vomitoria, Chrysomya chloropyga, Chrysomya albiceps, Lucilia sericata, Stomoxys evanida, Musca domestica, Thanatophilus sinuatus, H. monitor, N. rufipes, Coelus ciliatus, Harmonia axyridis, Messor galla, and Melipona beecheii.21 They also align with Vitta identification of species like Parasarcophaga ruficornis, Dermestes maculatus, Hister sp., and Necrobia rufipes during the advanced decay stage.25
In the dry stage, species from the Diptera and Hymenoptera orders significantly decreased in all four groups of decomposing pigs (control, experiment group one, experiment group two, and experiment group three), leaving species from the Coleoptera order.31 Remaining species from the Diptera order included Chrysomya chloropyga, Musca domestica, Allosepsis indica, Mimegralla albimana, Ptecticus melanurus, and Megaselia scalaris. Species from the Coleoptera order that remained included Pheidole megacephala, Nitidulidae sp., Bostrichidae sp., Hister monitor, Dermestes maculatus, and Necrobia rufipes. These findings correspond with Odo’s observation of species during the dry decay stage of pig carrion decomposition, including M. domestica, Chrysomya chloropyga, L. sericata, Monomonium minimum, Solenopsis molesta, N. rufipes, H. monitor, and Zootermopsis augusticollis.21 They also align with Packard and Dabbs’ identification of species like Dermestes maculatus and Trox sp. during the dry stage.32
Compared to the control, the experimental pig carcasses showed extended durations in each of the five decomposition stages (fresh, bloated, active, advanced, and dry).22 The extension increased with the concentration of flunitrazepam in experimental groups one, two, and three. These findings align with a study by the National Institute of Justice,33 which found that drugs, especially cancer drugs, tend to lower the decomposition rate of human corpse. The insect species were the same in all four groups, including the control. However, the number of insects decreased with the introduction and concentration of flunitrazepam for EXP GR1, EXP GR2, and EXP GR3, respectively. The control had the highest number of insects at 1,259, followed by EXP GR1 with 1,089 insects, then EXP GR2 with 932 insects, and the least was EXP GR3, which had 852 insects. This suggests that the introduction and concentration of flunitrazepam affects the abundance of insects colonizing the corpse.
Nonetheless, it is important to mention this study’s limitations to ensure readers do not misunderstand the results. Climatic differences constituted one of the major constraints, with conditions regulating the speed of decomposition. Moreover, although the sample size of this research is large, it might not be sufficient to capture the diversity of conditions that exist in a setting where forensic investigators work.34 Such limitations must be considered during the analysis of the findings and should be improved in the future.
Temperature is one of the elements that defines decomposition and affects the phases observed.35 Temperature was not controlled in this study or even measured as a variable, but treating it as a potential variable in future studies would be helpful. This would assist in knowing how temperature changes, either daily or seasonally, affect decomposition.
Other than temperature, other factors such as air movement and populations of microbes in the context of decomposition can also affect the decomposition rate and the process’s various phases. For example, the oxygen and bacterial activity during the process may vary widely depending on the burial depth and soil type.36 The moderating effect of these factors on the relationship between decomposition stages and these factors can also be determined through further research.37
The presence and activity of specific insect species have been known to influence decomposition rates. For instance, in the fresh stage, the predominance of particular species, like Chrysomya bezziana and Chrysomya megacephala, suggests their significant role as early colonizers.38 Their feeding activities accelerate decomposition during this phase. Future studies could delve deeper into the ecological dynamics of insect species during decomposition, shedding light on their distinct contributions to the process.
Although this study was done on flunitrazepam, it is important to note that other drugs can also react with the process of decomposition. Future studies ought to broaden the search by exploring an extended array of substances typically connected with forensic cases. Such a thorough approach will allow forensic scientists to make better judgments in instances where drugs are suspected.
This paper shows that increasing concentrations of flunitrazepam led to an elongation of the five decomposition phases. Further, the insect succession trends during these periods were similar among all four groups of carcasses (control, EXP GR1, EXP GR2, and EXP GR3). The total population of insects in each stage, however, decreased as the concentration of flunitrazepam increased. Despite these observations, no statistically significant differences in insect numbers were detected among the four carcass groups.
While this study focused on flunitrazepam, it is crucial to acknowledge that other drugs may also interact with decomposition processes. Future research should expand the scope to investigate a broader range of substances commonly associated with forensic cases. This comprehensive approach will enable forensic scientists to make more informed assessments when drugs are suspected to be involved. Further research should also consider using human corpses.
The Biosafety, Animal Use and Ethics Committee of the Department of Veterinary Anatomy and Physiology, Faculty of Veterinary Medicine, University of Nairobi, provided ethical approval for animal use. REF: FVM BAUEC/2019/203 on 12/03/2019.16
Mendeley Data: ARRIVE 2.0 checklist for Effect of Flunitrazepam on Decomposition and Forensically Important Insects Colonization of Pig Carcasses: An Entomology Perspective. https://doi.org/10.17632/mnt66z9tks.1.40
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Mendeley Data: Effect of Flunitrazepam on Decomposition and Forensically Important Insects Colonization of Pig Carcasses with Corresponding Meteorological Records: https://doi.org/10.17632/4r4sp2vzn9.1.39
This project contains the following underlying data
• Data file 1. Insect Colonization.xlsx
• Data file 2. Kabete Climate Data.xlsx
• Data file 3. Figures and Tables for the Decomposition of Pig Carcasses Data.docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank Mr. Caleb Musango, Mr. Richard Ochieng, and Mrs. Phyllis Mwai for their unwavering support and the Department of Human Anatomy, Department of Biochemistry, and Department of Biology (Entomology section), University of Nairobi, for providing the study site and all the required equipment and reagents for this research. The authors would like to extend their appreciation to Phoebe and Richard Ochieng for the identification of insects. The above-mentioned individuals and departments have granted permission to be acknowledged for their contribution to this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Forensic Entomology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Forensic entomology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 08 Sep 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)