Keywords
Pharmacogenomics, Polypharmacy, Geriatric Pharmacotherapy, Adverse Drug Reactions, Genetic Variations, Personalized Medicine
This article is included in the Genomics and Genetics gateway.
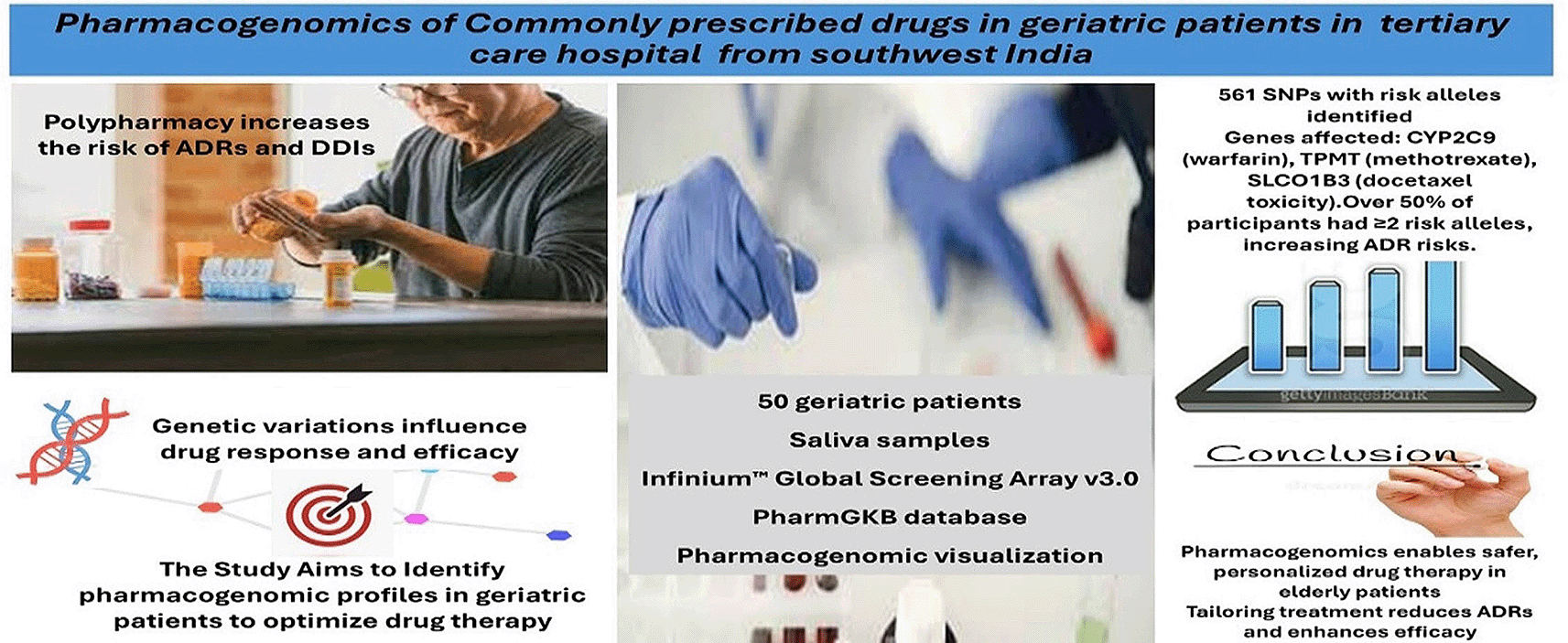
Polypharmacy, or taking multiple medications simultaneously, is a common occurrence in the elderly population, increasing the risk of adverse drug reactions (ADRs) and drug-drug interactions (DDIs) requiring the intricate management of multiple comorbid conditions. Pharmacogenomics, the study of genetic differences affecting drug metabolism and response, can offer hope of more tailored medication regimens and enhanced therapeutic response.
The aim of this study is to create pharmacogenomic profiles of elderly patients in order to determine patient-specific genetic variation contributing to drug response, ultimately influencing the efficacy of drug therapy and increasing the potential risk of ADRs.
A cross-sectional study was conducted involving 50 geriatric patients aged 60 years or older receiving multi-drug therapy. Saliva samples were collected and genotyped using the Infinium™ Global Screening Array v3.0 BeadChip. Single nucleotide polymorphisms (SNPs) associated with drug response were identified using the PharmGKB database. SNPs with significant clinical relevance were analysed, and pharmacogenomic profiles were visualized to assess risks for ADRs and altered drug efficacy.
Out of 1,243 SNPs analysed per individual, 561 SNPs with two risk alleles were identified. Fifteen SNPs were present in over 90% of participants, with significant variants observed in genes such as CYP2C9 (associated with warfarin metabolism), TPMT (linked to methotrexate dosing), and SLCO1B3 (associated with docetaxel toxicity). More than 50% of participants had two or more risk alleles, highlighting their predisposition to ADRs and altered drug metabolism.
The findings of this study emphasize the transformative potential of pharmacogenomics for rationalizing drug therapy among elderly patients, especially when they are susceptible to polypharmacy. Through identification of major genetic differences such as CYP2C9, TPMT and other pharmacogenes the study underlines the requirement for individualized pharmacotherapy based on distinct genetic profiles.
Pharmacogenomics, Polypharmacy, Geriatric Pharmacotherapy, Adverse Drug Reactions, Genetic Variations, Personalized Medicine
Polypharmacy, or taking multiple medications simultaneously, is a common occurrence in the elderly population increasing the risk of adverse drug reactions (ADRs) and drug-drug interactions (DDIs) requiring the intricate management of multiple comorbid conditions. This phenomenon will thus be associated with benefits and risks simultaneously.1 Although it may often be necessary for polypharmacy to address the various health concerns, there are several serious challenges as well, such as the heightened risk of ADRs, drug-drug interactions, and medication non-adherence.2 The presence of polypharmacy in elderly individuals acts as an indication of an emergent need for better methods of management in their medication regimen.3
Pharmacogenomics is an up-and-coming methodology that considers individual genetic variation in different responses to drugs. It conveys one of the promising ways to improve the management of polypharmacy.4 Pharmacogenetic analysis allows for the personalization of drug therapies regarding a patient’s genetic background, in view of enhancing the efficacy of drugs and reducing the risk of side effects.5 Genetic variations in drug-metabolizing enzymes, which include the majority of the significant ones encoded by the cytochrome P450 genes, serve as one example.
Such variants in genes could therefore alter the levels and responses of drugs in individuals and hence are an important guide to pharmacogenomic insights for the optimization of the medication regimen.6 The integration of pharmacogenomic analysis into clinical practice is a multistep process. First, genetic testing will provide information on specific gene variants that may influence drug metabolism or its response.7 Pharmacogenomics provides new insights into the metabolism and accurate targeting of both newly created targeted therapies and frequently used medications. By exploiting patients’ genetic information to predict drug responses and reduce ADRs, they have demonstrated favorable outcomes.8 With its innovative use of genetics in precision medicine, pharmacogenomics-informed pharmacotherapy holds the potential to transform traditional medical practice by promising therapeutic efficacy and individualization through the careful selection of the best medications and dosages.9
Pharmacogenomic-guided therapy has considerable advantages in polypharmacy management. If a healthcare provider applied pharmacogenomics in the selection and dosing of medications, there would be improved drug efficacy and less incidence of ADRs and DDIs. This personalized medicine would further enhance patient safety and contribute to better use of healthcare resources. For example, genetic information utilized to optimize doses can prevent cases of underdosing and overdosing; this will, in turn, result in better therapeutic outcomes that might reduce the cases of hospitalization attributed to drug errors.10
On the other hand, pharmacogenomics has also got its own set of challenges in application to geriatric care, including highly specialized clinical knowledge for the interpretation of genetic data and possible cost implications related to genetic testing.11 Integration of pharmacogenomic data into routine clinical practice also involves ethical and logistic issues, such as ensuring patient consent and data privacy.12
With all these challenges, the addition of pharmacogenomics into the management of polypharmacy really does mean a quantum leap in personalized medicine, with the potential to optimally drive therapeutic outcomes and quality of life in older adults, thus representing a particularly useful tool in the rapidly developing field of geriatric pharmacotherapy. It goes without saying that as pharmacogenomics continues to advance, its application in polypharmacy will no doubt be integrated further into the personalized healthcare approach for the elderly.13 The aim of this study is to create pharmacogenomic profiles of elderly patients in order to determine patient-specific genetic variation contributing to drug response, ultimately influencing the efficacy of drug therapy and increasing the potential risk of ADRs.
A cross-sectional study was conducted for patients receiving multiple drug therapy who were older than 60. A practical sampling technique was used to ascertain whether each patient’s genetic profile was associated with the effectiveness of the medication therapy.
This cross-sectional study was carried out in the Indian state of Karnataka, at the tertiary care teaching hospital, Yenepoya Medical College and Hospital, Deralakatte. This hospital, which serves a variety of patients from urban, peri-urban and rural areas, was an ideal location to study the effects of poly-pharmacy in the elderly.
The study individuals were age 60 years or older and were receiving multi-drug treatment. This population was chosen due to its genetic constitution and vulnerability to polypharmacy-induced issues.
This genetic study included a combination of both in-patients (IP) and out-patients (OP) and resulted in 50 patients in total.
Inclusion criteria: The study included elderly patients of 60 years and older and receiving multi-drug therapy (taking 5 or more and 9 or more drugs for OP and IP, respectively).
Exclusion criteria: Patients with critical condition in need of intensive care, patients with mental retardation or cognitive disorders, and patients who refused to be included were all excluded.
The study was carried out according to the ethical standards of the institutional ethics committee and the Declaration of Helsinki. Ethical approval was obtained from the Yenepoya ethics committee 1 (Approval number: YEC1/2022/041) dated 01-06-22. Written consent form was taken from all the geriatric patients based on inclusion criteria for the study.
Medication data were collected from patient records, focusing on those aged 60 years or older who were undergoing multi-drug therapy. The study defined multi-drug therapy as a regimen involving at least five medications for outpatients and nine medications for inpatients. Saliva samples were collected from participants to analyse their genetic profiles and identify associations between pharmacogenomic (PGx) markers and medication response.
Pharmacogenomic profiling was conducted to evaluate whether the recruited geriatric patients could metabolize their prescribed medications or were at risk of ADRs, such as drug toxicity. Pharmacogenetic loci referenced in PharmGKB14 (https://www.pharmgkb.org) were examined for drugs commonly involved in polypharmacy. A comprehensive list of associated SNP markers (N = 648465) linked to drug response was compiled, focusing on medications frequently prescribed in polypharmacy settings. Saliva samples (2 ml) were collected from each participant, stored under appropriate conditions, and later processed for DNA extraction and genotyping using the Infinium™ Global Screening Array-24 v3.0 (GSA v3) BeadChip by Illumina Inc. Quality control (QC) of the genotype data was performed using PLINK v.1.9, excluding Single nucleotide polymorphisms (SNPs) with more than 5% missing data, those that failed the Hardy-Weinberg equilibrium test (P < 0.0001), and SNPs with a minor allele frequency (MAF) below 5% or more than 10% missing genotypes.
The curated SNP markers from PharmGKB were compared against the bim file containing SNPs present in the GSA v3 array to identify overlapping markers resulting in a final list of (561) SNPs. The presence of these SNPs, potentially associated with altered drug metabolism or increased toxicity, was evaluated in the patients’ genotype data, with each individual assessed for the presence of such variants. We evaluated antidiabetic, CNS, GI acting, anticancer, hmg COA reductase inhibitors, ARBs, antivirals and antibiotic drugs that are commonly prescribed to the geriatric patients. Pharmacogenomic profiling for each individual was visualized using a bubble plot created in R v4.4.1, where the size of each bubble corresponds to the number of risk alleles present, and a dot represents the complete absence of any risk alleles. Different coloured drugs represented different drug categories.
Drug targets, ligands, and genetic variants related to drug response were the main subjects of the pharmacogenomic analysis. The SNPs for the PharmGKB database was queried. These SNPs are established biomarkers which determine the metabolism, efficacy and toxicity of drugs that are most commonly used in elderly patients. The targeted genes were variants responsible for cytochrome P450 enzymes, which are critical in the metabolism of drugs.
The drugs investigated in the study were categorised according to their pharmacogenomic relevance. These drugs that could have varying effects on the metabolism, efficacy, or toxicity on the study participants. Selected drugs were also analysed for particular risk alleles in participants’ genomic data to gain an understanding of their potential pharmacogenomic interactions.
The pharmacogenomic analysis was performed in multiple steps, aimed at obtaining, accurate and relevant interpretation of the genetic profiles of geriatric patients. Genetic data from the participants was obtained by genotyping saliva samples with Infinium™ Global Screening Array v3. 0 (GSA v3); Illumina Inc., San Diego, CA, USA, which has allowed for the identification of genetic variation that contributes to drug response. Quality assurance steps were taken to ensure the credibility of the data. SNPs with >5% missing data, that deviated from Hardy-Weinberg equilibrium (P < 0.0001), and with minor allele frequency (MAF)<5% were excluded. Using PLINK v1. 9, a package of bioinformatic tools for genome-wide association studies, SNPs from the curated list in PharmGKB was compared to the genotyped data. Cross-mapping the SNP markers with genetic data allowed for a final selection of pharmacogenomic SNPs for the most used drugs in the study population. The pharmacogenomic profiles of the studied cohort were plotted in R software (v4. 4. 1). Bubble plots were generated to illustrate the results with the size of the bubble reflecting the count of the risk alleles for subjects in their genetic profile. Drug categories were color-coded, and dots to the left were shaded signifying absence of risk alleles for a given drug.
The pharmacogenomic profiles of 50 elderly polypharmacy patients were analysed in this study. Figure 1 depicts the pictorial representation of the results of the pharmacogenomics study of the commonly prescribed drugs in the Intensive Care Hospital from Southwest India. Genotyping was performed to detect SNPs known to be related to ADRs, altered drug metabolism or toxicity using saliva samples. Less than 50% of the individuals in the 60-year-old and older age group were carriers of at least one risk allele, while more than 50% were carriers of two or more risk alleles for the development of ADRs. A total of 1,243 SNPs was analysed in each participant, and 561 SNPs were determined to have two risk alleles in one or more of the individuals. SNP markers also act as important genetic metrics for evaluation of the individual drug responses. This study identified numerous SNPs that influence enzyme activity, drug metabolism, and toxicity, underscoring their clinical relevance in pharmacogenomics. Figures 2 and 3 illustrate the prevalence of SNPs with two risk alleles across participants. SNPs present in more than 50% of the study population were classified as higher-risk variants, which could significantly impact drug efficacy and safety. Notably, the presence of these SNPs was associated with modified enzyme activity, leading to enhanced or reduced drug metabolism and an increased risk of toxicity.
The presence of certain SNPs can affect enzyme activity, potentially enhancing or reducing drug efficacy and increasing the risk of toxicity. We identified 15 SNPs that have two risk alleles in >90% recruited patients ( Table 1). These SNPs are associated with various drug classes, prominently anti-cancer and immunosuppressive drugs. For instance, individuals with specific SNPs, such as rs1142345 in the TPMT gene, may require a decreased dose of methotrexate, a well-known anti-cancer drug. Similarly, certain SNPs in the CYP2B6 gene are linked to the metabolism of methadone and efavirenz. The SNP rs10455872 has been associated with the efficacy of HMG-CoA reductase inhibitors. Furthermore, SNP rs11045585 is associated with docetaxel toxicity.
SNPs rs72558187 in the CYP2C9 gene are connected to the metabolism of drugs such as diclofenac and losartan, the clearance of zafirlukast, and the dosage of warfarin. Moreover, SNPs in CYP2C9 and OPRM1 related to rs28371685 affect the doses and metabolisms of hydroxy phenytoin, losartan, and warfarin. Lastly, the SNPs rs17216177 and rs3213619 in the ABCB1 gene are correlated with tacrolimus and cyclosporine metabolism as well as imatinib response. Clinically relevant findings were discovered in all individuals, indicating the possibility of personalized health care. The research underscores the importance of reanalysing genomic data as new informatics tools and disease associations develop, which could enhance risk predictions. Many individuals were found to carry high-risk alleles in pharmacogenes, emphasizing the necessity for systematic pharmacogenetic testing. The study also calls for the validation of next-generation sequencing (NGS) platforms for identifying pharmacogenetic variants and suggests conducting economic evaluations for the implementation of pharmacogenomics (PGx).
Variants in the TPMT gene (rs1142345) were linked to the need for reduced methotrexate doses in both immunosuppressive and anti-cancer therapies, a finding consistent with earlier studies demonstrating that TPMT polymorphisms can lead to reduced enzyme activity and increased toxicity.15 Similarly, the SLCO1B3 gene (rs11045585) was associated with elevated toxicity of docetaxel, a chemotherapeutic agent, aligning with prior evidence of SLCO1B3’s role in drug transport and toxicity.16 SNPs in the CYP2B6 gene (rs28399499) were shown to influence the metabolism of methadone (increased) and efavirenz (decreased). These findings corroborate earlier research indicating that CYP2B6 polymorphisms significantly affect the pharmacokinetics of CNS and antiviral drugs.17 Variants in CYP2C9 (rs72558187 and rs28371685) were linked to reduced metabolism of diclofenac, losartan, and warfarin, consistent with existing literature that identifies CYP2C9 polymorphisms as major contributors to altered drug metabolism and the risk of adverse drug reactions, particularly for anticoagulants.18
The LPA gene (rs10455872) was associated with decreased efficacy of HMG-CoA reductase inhibitors, such as rosuvastatin. Previous studies have demonstrated that LPA polymorphisms not only influence statin efficacy but also modulate lipid levels, further supporting the need for pharmacogenomic considerations in managing cardiovascular disease.19 Other notable findings included SNPs in the CYP3A4 gene (rs2687116), which were linked to the efficacy of anticonvulsants like carbamazepine and valproic acid. These findings are supported by earlier studies showing that CYP3A4 polymorphisms play a critical role in the metabolism of a broad range of medications.20 Variants in ABCB1 gene (rs3213619) were also observed to modulate the metabolism of tacrolimus and cyclosporine as well as response to imatinib. These findings are consistent with others, where ABCB1 polymorphisms modify drug transport and clinical response, especially of immunosuppressants.21
Our results highlight the crucial application of pharmacogenomics for the optimal treatment of elderly patients. Most noteworthy, was the recognition of 15 SNPs with at least two risk alleles represented in more than 90% of participants, largely predisposing subjects to ARDs and compromised drug efficiency. Variants in genes like CYP2C9 and TPMT illustrate the requirement for dose alteration and close monitoring of the drug treatment to avoid risks. The study also reflects the possibilities of personalized medicine by use of the standardized pharmacogenomic testing. Pharmacogenomics personalized medicine is advocated to increase efficacy and decrease the occurrence of ADRs and subsequently patient safety by individually tuning the drug regimen to the corresponding genetic profile. Incorporating pharmacogenomics into standard clinical care could help decrease healthcare costs by preventing hospitalizations and other adverse outcomes due to medication errors. And lastly, the results to highlight and underscore the importance of more efficient technological platforms (i.e., NGS) to uncover pharmacogenomic variants. These tools may be at the forefront of precision medicine efforts. However, additional investigations are required to confirm these findings in larger and more diverse patient populations. Moreover, establishing the economic viability of routine pharmacogenomic testing is essential to its successful broader adoption into the clinical practice workflows.
The findings of this study emphasize the transformative potential of pharmacogenomics for rationalizing drug therapy among elderly patients, especially when they are susceptible to polypharmacy. Through identification of major genetic differences such as CYP2C9, TPMT and other pharmacogenes the study underlines the requirement for individualized pharmacotherapy based on distinct genetic profiles. Pharmacogenomic testing can also be applied to reduce the adverse drug reactions, drug effectiveness, and overall patient safety on a systematic basis. Although these results highlight the potential of personalized medicine, additional large-scale validation and cost-effectiveness assessments are needed for wider clinical use of pharmacogenomics targeting elderly patients.
Umaima Farheen Khaiser: Conceptualization, Investigation, Data curation, Formal analysis, Resources, Writing – original draft; Rokeya Sultana: Conceptualization, Methodology, Investigation, Data curation, Supervision, Validation, Resources, Writing-review & editing; Ranajit Das: Conceptualization, Investigation, Methodology, Data curation, Resources, Data curation, Supervision, Writing – original draft, Writing-review & editing; Dr Juveriya Farooq: Methodology, Investigation, Formal analysis; Haleema Shahin DH: Data curation, Investigation, Formal analysis, Methodology, Resources, Writing – original draft, Writing-review & editing; Mohammed Gulzar Ahmed: Data curation, Software, Investigation, Formal analysis, Methodology, Resources, Writing – original draft. Chetan Ashok: Methodology, Data curation, Data curation, Writing-review & editing: Srikanth Jeyabalan: Methodology, Investigation, Data curation, Writing-review & editing; Ling Shing Wong: Conceptualization, Data curation, Formal analysis, Writing-review & editing; Vetriselvan Subramaniyan: Conceptualization, Data curation, Formal analysis, Writing-review & editing.
The authors did not use generative AI or AI-assisted technologies in the development of this manuscript.
The authors confirm that all data supporting the findings of this study are contained within the manuscript and supplementary materials. No additional datasets were generated or analysed during the current study. Due to ethical and privacy considerations related to patient genetic information, the underlying data cannot be made publicly available. Data can be made available upon reasonable request to the corresponding author (rokeya009ster@gmail.com, drrokeyasultana@yenepoya.edu.in), subject to institutional and ethical approvals.
Umaima Farhin Khaiser, Rokeya Sultana, Ranajit Das, Juveriya Farooq, Haleema Shahin, and Mohammed Gulzar Ahmed, would like to acknowledge Yenepoya (Deemed to be University) for providing the research facilities to conduct the experiments. The Graphical abstract was drawn by Biorender.com
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. John S, Klumsathian S, Own‐eium P, Eu‐ahsunthornwattana J, et al.: A comprehensive Thai pharmacogenomics database (TPGxD ‐1): Phenotype prediction and variants identification in 942 whole‐genome sequencing data. Clinical and Translational Science. 2024; 17 (6). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacogenetic, clinical pharmacology, cardiovascular
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 10 Sep 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)