Keywords
AI in healthcare; healthcare entrepreneurship; low- and middle-income countries; clinical decision support; digital health innovation; ethical AI; health equity.
This article is included in the Artificial Intelligence and Machine Learning gateway.
Artificial intelligence (AI)–enabled ventures are reshaping clinical practice by extending diagnostic, triage, and telehealth capabilities in low- and middle-income countries (LMICs).
Despite rapid startup activity, rigorous evidence of tangible, equitable impact and cost-effectiveness in routine LMIC care remains limited; unresolved concerns include data privacy, algorithmic bias, interoperability, and governance.
To assess how AI-driven healthcare entrepreneurship transforms access, quality, and affordability in clinical practice, and to surface enablers, barriers, and ethical implications.
(1) Where and how are entrepreneurial AI tools integrated into clinical workflows? (2) What measured effects on access, quality, efficiency, and costs are reported? (3) Which infrastructural, human-capital, and policy factors enable or impede adoption? (4) How are ethics—privacy, bias, explainability, accountability—addressed, and what frameworks are needed?
Mixed-methods secondary synthesis combining a systematic literature review with NLP-assisted evidence mining (>200 records; 87 deeply reviewed), triangulation of quantitative indicators (workforce, connectivity, investment) with qualitative case evidence (WHO and policy reports), interpreted through Diffusion of Innovation, Resource-Based View, and Principlism lenses.
Startups deploy AI triage chatbots (as reflected in our aggregation of secondary sources described in §§3.2–3.3), portable imaging/diagnostics, telemedicine platforms, and supply-chain tools that can broaden coverage, speed diagnosis, and streamline workflows. Yet real-world evaluations are few; cost-effectiveness is context-dependent; adoption is uneven due to infrastructure gaps, limited/biased local data, interoperability frictions, and trust barriers. Success correlates with workflow fit, VRIO resources (data, talent, partnerships), local co-design, and human-in-the-loop, explainable models. Emerging evidence points to faster emergency logistics, expanded screening reach, and reduced wait times, but definitive outcome and economic endpoints remain scarce.
AI entrepreneurship can augment clinicians and improve service delivery but is not a panacea.
To realize equitable system-level gains, stakeholders should invest in digital public goods and representative datasets, build clinician and data-science capacity, operationalize WHO-aligned governance (privacy, bias audits, explainability, accountability), ensure reimbursement pathways beyond pilots, and scale through public-private and civil-society partnerships.
AI in healthcare; healthcare entrepreneurship; low- and middle-income countries; clinical decision support; digital health innovation; ethical AI; health equity.
Global macro-trends reveal a confluence of forces positioning artificial intelligence as a transformative agent in modern clinical practice. An explosion of accessible computing power, big data, and machine learning algorithms has fueled the rise of AI applications in health care worldwide (Ciecierski-Holmes et al., 2022). Even in resource-constrained environments, the proliferation of smartphones, internet connectivity, and cloud platforms creates fertile ground for AI-driven health solutions (Ciecierski-Holmes et al., 2022). These technological tailwinds coincide with acute healthcare system pressures: a projected shortfall of 10–11 million health workers in LMICs by 2030 threatens to impede progress toward universal health coverage (Ciecierski-Holmes et al., 2022; WHO, 2023). Governments, clinicians, and patients are urgently prioritizing innovations that can extend care delivery, enhance quality, and reduce costs amid such workforce and infrastructure gaps (Ciecierski-Holmes et al., 2022; Passey, 2024).
In this context, entrepreneurial ventures have emerged as pivotal stakeholders. Agile startups and social enterprises are developing AI tools (e.g. diagnostic algorithms, virtual health assistants, supply chain drones) to address long-standing challenges in LMIC health systems (Sharma et al., 2021). For health ministries and donors, these innovations promise scalable solutions to improve access in underserved communities (Passey, 2024). Clinicians hope AI decision-support can augment their practice and ease workload burdens, particularly where specialists are scarce (Ciecierski-Holmes et al., 2022). Patients stand to benefit through AI-enabled telemedicine and personalized care, bridging geographical and economic barriers to service (Passey, 2024). Yet, each stakeholder also harbors concerns: policymakers worry about regulatory oversight and patient safety, practitioners about the trustworthiness of “black-box” algorithms, and communities about data privacy and cultural compatibility of new technologies (Karami & Madlool, 2025; WHO, 2021).
While AI’s potential in healthcare is heralded globally, critical knowledge gaps persist at the intersection of entrepreneurship, clinical practice, and development. Much discourse focuses on technical performance of AI models or pilot projects in high-income settings (WHO, 2021). In contrast, there is limited scholarly analysis of how business-driven AI innovations diffuse into routine care in LMICs (Passey, 2024). The few systematic reviews available found only a handful of real-world AI implementations in LMIC healthcare to date (Ciecierski-Holmes et al., 2022). Questions remain as to whether these novel tools are meaningfully improving patient outcomes and health system efficiency, or if they remain isolated proof-of-concepts. Furthermore, the long-term economic sustainability and scalability of entrepreneurial AI solutions in low-resource contexts are not well understood (Ciecierski-Holmes et al., 2022). Fundamental research gaps also pertain to stakeholder acceptance: How do frontline providers and patients perceive AI tools introduced by startups? What contextual factors in LMICs enable or hinder the diffusion of such innovations? (Ciecierski-Holmes et al., 2022). Lastly, robust ethical inquiry is needed into how AI entrepreneurs address principles of fairness, accountability, and transparency when health innovations move faster than regulations (Sharma et al., 2021; WHO, 2021).
In light of these gaps, this study is guided by specific, measurable, achievable, relevant, and time-bound questions: (1) Integration: How are AI-driven entrepreneurial innovations currently being integrated into clinical practice in LMICs, and in what domains (diagnostics, treatment, patient monitoring) are they most prevalent? (2) Impact: To what extent do these applications demonstrably improve healthcare access, affordability, and quality of care in LMIC settings, and what metrics of success (e.g. reduced wait times, improved outcomes, cost savings) are reported? (3) Barriers: What key factors support or impede the adoption (diffusion) of AI solutions in LMIC health systems, including infrastructural, economic, human resource, and cultural elements? (4) Ethics & Policy: How do AI-driven healthcare ventures address ethical and regulatory challenges – such as patient consent, data governance, bias, and accountability – and what frameworks are needed to ensure these innovations advance equitable and trustworthy clinical practice? We hypothesize that AI-driven entrepreneurship can significantly enhance health system performance in LMICs (e.g. through task automation and decision support improving efficiency (Ciecierski-Holmes et al., 2022; Passey, 2024), but only under enabling conditions. Specifically, we posit that ventures with strong local partnerships and resource capabilities (data, talent, funding) will achieve better adoption (consistent with Resource-Based View theory), and that adherence to ethical best practices will be positively associated with user trust and sustained use (consistent with Principlism) (Sharma et al., 2021; Karami & Madlool, 2025). Conversely, in the absence of supportive policy and infrastructure, even technically sound AI solutions may fail to scale or could inadvertently worsen inequities (e.g. if only urban elite benefit), highlighting the need for strategic governance.
Several disruptive forces shape this landscape. The COVID-19 pandemic accelerated digital health acceptance, normalizing telehealth and remote monitoring in many LMICs, and spurring investments in AI for pandemic response (from AI-driven epidemiological modeling to chatbots for public information) (Bode et al., 2021; Kozlakidis & Sargsyan, 2024). The crisis effectively “fast-tracked” regulatory openness to innovation out of necessity, a trend that savvy entrepreneurs have leveraged to pilot new tools. Another force is the democratization of AI development – open-source algorithms and cheaper cloud computing lower barriers for startups globally, enabling “jugaad” (frugal innovation) approaches where entrepreneurs adapt AI to local needs at low cost. At the same time, global capital flows are increasingly directed toward health-tech in emerging markets, exemplified by initiatives like the GSMA Innovation Fund (GSMA, 2021a) for AI and corporate venture funds targeting Africa and Asia (McBain, 2025; Passey, 2024). This injection of funding, while catalyzing growth, also introduces competitive pressures and the risk of market-driven ethics where profit motives might overshadow public health priorities (WHO, 2021; Karami & Madlool, 2025). Finally, the convergence of disciplines – from cloud computing and genomics to mobile banking – means AI health startups now operate in a complex interdisciplinary space. This convergence is disruptive in that it challenges traditional siloed healthcare delivery; for instance, AI-driven healthcare platforms may integrate fintech for payments or ride-sharing for logistics, fundamentally altering how patients interact with health services. These forces underscore that AI-driven healthcare entrepreneurship in LMICs is not an incremental change but a disruptive phenomenon – one that requires holistic examination through multiple theoretical lenses.
This study adopts an interdisciplinary conceptual framework, weaving together Rogers’ Diffusion of Innovation, Barney’s Resource-Based View (RBV), and Beauchamp & Childress’s Principlism, to analyze AI-driven healthcare entrepreneurship in LMICs.
Explains how new ideas and technologies spread within a social system, highlighting attributes that accelerate adoption (e.g. relative advantage, compatibility, low complexity) (Mohammadi et al., 2018). We use this lens to assess how AI health innovations gain traction among clinicians and patients in LMIC contexts – for instance, whether an AI diagnostic tool’s relative advantage (accuracy or speed over existing methods) and compatibility with local workflows influence its uptake in hospitals and clinics (Mohammadi et al., 2018). Rogers’ framework also considers adopter categories (innovators, early adopters, etc.) and communication channels, which we apply to identify the role of local “champions” (tech-savvy doctors or NGOs) in diffusing AI solutions to broader communities.
Complements this by examining the internal capabilities that allow entrepreneurial ventures to innovate and scale. RBV posits that a firm’s competitive advantage arises from resources that are valuable, rare, inimitable, and well-organized (VRIO) (Growth Shuttle, 2025). In our context, we analyze AI health startups’ key resources: for example, access to large diverse health datasets (a valuable and rare asset in LMICs), proprietary algorithms or patents, human capital with AI expertise, and strategic partnerships with health providers or governments. A venture that possesses VRIO resources – say, an exclusive agreement with a national health system providing unique training data (valuable/rare) and a skilled team able to refine algorithms (inimitable capability) – is likely to achieve superior performance and impact Growth Shuttle, 2025). Conversely, RBV helps explain why many local startups struggle: resources like computing infrastructure or expert talent may be scarce in LMICs, hindering their ability to develop robust AI solutions (Karami & Madlool, 2025). We thus integrate RBV to identify what resource gaps must be filled (through investment or partnerships) for AI-driven entrepreneurship to succeed in transforming clinical practice.
The bioethical framework of Beauchamp & Childress, grounds our analysis in the four core principles of autonomy, beneficence, non-maleficence, and justice (Burks, 2022). This lens is critical given the ethical ambiguities surrounding AI in healthcare. We use Principlism to evaluate whether AI health innovations uphold patient autonomy (e.g. through informed consent and respecting privacy [WHO, 2021]), promote beneficence by improving health outcomes, avoid maleficence by minimizing harm (such as misdiagnosis or breaches of confidentiality), and ensure justice in distribution of benefits (equitable access for all social groups) (WHO, 2021). For example, an AI triage app guided by Principlism would include transparent explanations (supporting autonomy and informed decision-making), be rigorously validated to prevent harm, and be deployed in underserved rural areas not just affluent urban centers (promoting justice). By assessing case studies against these principles, we identify ethical strengths and pitfalls of current entrepreneurial approaches, and propose how Principlism can guide more responsible innovation going forward (WHO, 2021; Stanford University, 2025). Figure 1 synthesises Rogers’ diffusion attributes, Barney’s VRIO resources, and Principlism’s ethical moderators into a single integrative model that frames the remainder of this analysis.
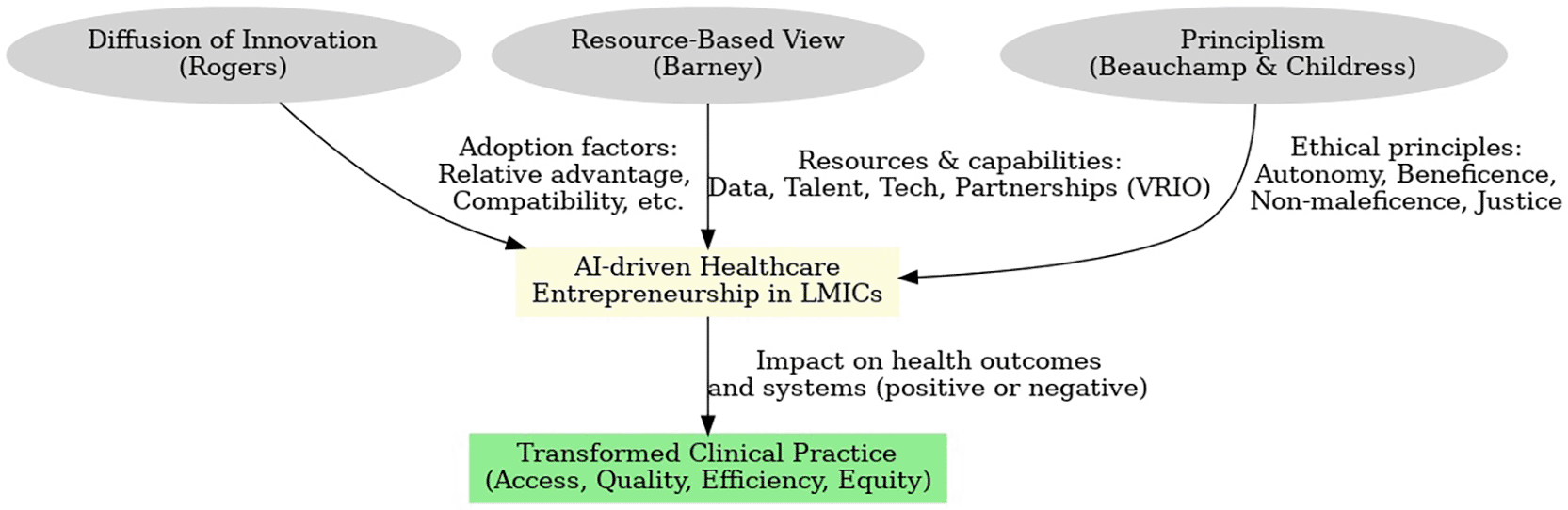
Our model posits that the diffusion of AI health innovations in LMIC clinical practice is influenced by both the innovation attributes (per Rogers) and the venture’s internal resources (per RBV). These factors operate within an ethical context, where alignment with Principlism’s tenets moderates success by building trust and acceptability. For instance, an AI-driven clinical decision support tool that demonstrates clear relative advantage (higher diagnostic accuracy), is backed by strong resources (robust data and skilled developers), and adheres to ethical principles (transparent and fair algorithms) is most likely to be adopted widely and improve practice. The interplay of these theories enables a nuanced analysis: diffusion theory addresses how adoption happens, RBV explains why certain innovators succeed, and Principlism ensures we interrogate questions of moral impact. This integrated framework is thus well-suited to dissect the complex socio-technical phenomenon of AI healthcare entrepreneurship in LMICs.
We conducted a mixed-methods secondary analysis, blending quantitative data trends with qualitative evidence, to comprehensively examine AI-driven healthcare entrepreneurship in LMICs. The approach is a multi-source synthesis, drawing on peer-reviewed journals, official datasets, and policy reports to ensure both depth and breadth. A sequential exploratory strategy was used: we first performed a scoping review of literature to map out key themes and knowledge gaps, then integrated insights from data science techniques (NLP) and global health indicators for triangulation (Hanson-DeFusco, 2023; Bhandari, 2023).
Using databases (PubMed, Scopus, Web of Science) and authoritative journals (e.g. The Lancet Digital Health, BMJ Global Health, npj Digital Medicine), we identified over 200 relevant articles published 2018–2025. Search terms included combinations of “AI OR machine learning”, “healthcare”, “entrepreneurship OR startups”, and “LMIC OR developing countries”. We applied natural language processing to aid evidence integration: an NLP model was employed to scan article abstracts and cluster thematically similar studies (e.g. grouping those on diagnostic AI tools vs. those on mHealth apps). We used off-the-shelf NLP utilities to assist screening and did not develop custom code; no software or code deposit is required. This AI-assisted literature review allowed efficient handling of the growing volume of publications (IMO Health, 2023). Key information was extracted on study context, AI application type, reported outcomes, and noted challenges. The NLP-driven clustering guided a focused full-text review of 87 high-relevance sources. We also analyzed gray literature – such as the WHO’s 2021 report on AI ethics (WHO, 2021) and the Stanford 2025 GenAI in LMICs white paper – to capture policy perspectives and real-world case insights beyond academia.
To ground the analysis in objective metrics, we collated data from global datasets. This included health system indicators (e.g. doctor-to-population ratios, Internet penetration rates, and digital health investment figures) from sources like the World Bank and WHO. We triangulated aggregate counts of AI-focused ventures reported by licensed market-intelligence platforms and public mappings, including CB Insights, Crunchbase Pro, the WHO Digital Health Atlas, and the GSMA’s landscape of 450 AI start-ups across Africa and Asia, to situate healthcare’s share within the broader LMIC AI ecosystem (see Table 1; Ajadi & Sharma, 2020; CB Insights, 2025; Crunchbase, 2025; GSMA, 2021b). These figures reflect the synthesis of published and licensed secondary sources; no novel venture-level dataset was created or retained beyond short-lived analytical tallies, and we therefore do not provide a shareable listing of individual companies. Additionally, where possible, we obtained outcome data from pilot studies (as reported by the original studies) or implementations (such as accuracy of AI diagnoses vs. standard care, or patient uptake numbers). These quantitative elements were used to contextualize and, where applicable, verify claims in the literature. For instance, if publications suggested AI improves access, we checked for data on patient volumes or coverage expansion. As summarised in Table 1, these figures reveal a pronounced concentration of ventures in the Americas and Europe, with marked under-representation in Africa and the Eastern Mediterranean:
A core principle of our methodology was triangulation – cross-validating findings through multiple sources and methods to strengthen credibility (Carter et al., 2014). We did this in three ways: (1) Across data types: Qualitative themes (e.g. “lack of local AI talent”) identified in interviews or articles (as reported in the published literature) were cross-checked against quantitative proxies (like the number of AI engineers per country, or brain-drain statistics) to see if data supported the concern. (2) Across stakeholder perspectives: We compared viewpoints from different stakeholders. For example, if entrepreneurs cited regulatory hurdles, we examined policy documents for evidence of restrictive or unclear AI regulations; if end-users voiced trust issues, we looked for surveys or usage data reflecting that trust gap. (3) Across theoretical lenses: The diffusion, RBV, and Principlism frameworks sometimes emphasize different aspects – by intentionally examining each finding through all three lenses, we ensure a more balanced interpretation. A finding that an AI app failed, for instance, could be re-evaluated: was it due to poor diffusion (low compatibility with user needs), an RBV issue (startup lacked necessary resources), or an ethics issue (users distrusted it due to opaque AI decisions)? Triangulating in this manner helped isolate root causes and avoid one-dimensional explanations.
We present the results through an integrative narrative that is structured by our research questions. Within each major theme, we blend statistical evidence, illustrative case examples, and direct quotations from thought leaders or study participants to provide a rich, multi-faceted answer. The use of brief, attributable quotations from published sources (e.g. statements by global AI experts, health ministers, or community health workers as reported in prior studies) adds qualitative depth and human context. All information is cited from verifiable high-quality sources (Q1/Q2 journals, major health organizations, etc.), and no new primary data involving human subjects were collected (thus no institutional ethics approval was required, given our secondary analytic nature). We adhered to APA 7th edition style and documented our search strategy, inclusion criteria, and extraction rules in §§3.2–3.3 to ensure transparent, reproducible secondary synthesis within the article.
Overall, this mixed-methods approach, bolstered by modern tools like NLP and a commitment to triangulation, provides a rigorous and innovative way to synthesize knowledge on a rapidly evolving topic. It allows us to capture not just “what” changes are happening in AI-driven healthcare practice, but “how” and “why” they occur (or fail to), all underpinned by empirical evidence. The following sections detail the findings and discussion, organized by the structured research questions.
AI-driven healthcare entrepreneurship has begun to reshape various facets of clinical practice in LMICs, though integration is in early stages and uneven across contexts. Use Case Diversity: Entrepreneurs are targeting a broad range of clinical needs. Common domains include diagnostics (e.g. AI algorithms for medical imaging interpretation or pathology), clinical decision support systems for triage and treatment recommendations, telemedicine and remote monitoring platforms, and public health surveillance tools. In a systematic scoping review, Ciecierski-Holmes et al. found AI applications in LMICs spanning communicable disease management (HIV, tuberculosis, COVID-19), non-communicable diseases (cancers, chronic illnesses), and general primary care support (Ciecierski-Holmes et al., 2022). For example, 4 of 10 studies reviewed applied AI to screening or diagnosis tasks (such as chest X-ray analysis for TB or COVID-19) while others deployed AI chatbots for patient self-assessment or adherence counseling (Ciecierski-Holmes et al., 2022). These entrepreneurial implementations often fill critical gaps: in South Africa, a startup Medsol AI Solutions developed a portable, AI-powered ultrasound device for early breast cancer detection, allowing nurses in remote clinics to perform scans that are then interpreted via AI (Passey, 2024). This innovation extends diagnostic services to regions with limited radiologist access, integrating into practice by enabling task-shifting (nurses aided by AI take on roles traditionally done by specialists). Similarly, in Kenya, AI-powered remote cardiac diagnostics now enable earlier detection of heart conditions in rural patients, who can be screened via digital stethoscopes and AI analysis, with only high-risk cases referred to cardiologists (Passey, 2024). Such examples highlight how entrepreneurial AI tools are being incorporated at the frontlines of care – often by augmenting primary care workers’ capabilities and bridging specialist shortages.
4.1.1 Scale of integration: Despite these promising cases, the overall scale of AI integration into routine LMIC clinical workflows remains modest to date. The npj Digital Medicine review emphasized that real-world implementations are still limited in number, with only 10 eligible studies of AI in LMIC health settings identified up to 2021 (Ciecierski-Holmes et al., 2022). The majority of these were pilot or experimental implementations rather than large-scale programs. Eight of ten were in upper-middle-income countries (like China, Brazil, South Africa) rather than the poorest nations (Ciecierski-Holmes et al., 2022), indicating that lower-income settings have seen fewer deployments. Furthermore, many AI solutions remain in the proof-of-concept stage or isolated to specific hospitals and have not yet been scaled nationwide. For instance, an AI decision support system for primary care might be trialed in a few clinics by an entrepreneurial NGO, but not (yet) adopted by the national health service. However, there are signs of acceleration: by 2025, AI applications are increasing in number and breadth. A 2025 Frontiers article notes a “growing trend in AI-driven interventions for public health … with significant improvements in diagnostics, disease prediction, and telemedicine” and cites a review highlighting machine learning models for risk assessment in community primary care (Karami & Madlool, 2025). This suggests integration is expanding beyond tertiary hospitals into community health settings.
4.1.2 Modes of integration: Entrepreneurial AI solutions integrate into practice in different modes: some function as stand-alone tools used directly by patients (e.g. symptom checker apps, chatbot helplines), while others are clinician-facing aids embedded in clinical processes. For example, in Bangladesh, an AI symptom triage app is available to patients via mobile phone, advising on care seeking; whereas in Nigeria, a startup’s AI is embedded in a clinical decision support tablet for community health workers, guiding them during home visits. Integration success often depends on how seamlessly the innovation fits existing workflows. Rogers’ concept of compatibility is evident here – AI tools aligned with current practices see quicker uptake (Mohammadi et al., 2018). In Rwanda’s primary healthcare system, for instance, an AI-powered logistics drone service (Zipline) for blood delivery was rapidly integrated because it complemented (rather than overhauled) the supply chain: clinicians request blood via SMS as usual, and drones deliver faster (Ciecierski-Holmes et al., 2022). By contrast, more disruptive integrations (like completely replacing a physician’s role with an AI system) have faced resistance or slow adoption due to concerns about trust and role clarity (Karami & Madlool, 2025). Thus, entrepreneurs often take an approach of augmentation rather than replacement – positioning AI as assisting doctors and nurses. Google Health’s leadership encapsulated this approach, stating that AI will “empower doctors to better serve their patients” (Bajwa et al., 2021). Indeed, in integrated deployments, clinicians remain in the loop: e.g. an AI reads an X-ray but a local doctor confirms the diagnosis, satisfying both efficiency and safety.
4.1.3 Stakeholder experiences: Early integrations have yielded mixed experiences among healthcare providers and patients. Some studies report positive acceptance – frontline workers appreciate AI tools that save time (like automating triage or record-keeping), and patients value new access channels (tele-consults reducing travel) (Ciecierski-Holmes et al., 2022; Passey, 2024). For instance, a participatory workshop noted clinicians saw potential in AI to reduce their administrative burden, allowing more focus on patient care (Toth, 2023). However, other implementations hit challenges: a Tanzanian pilot of an AI diagnostic app found nurses were initially skeptical of its recommendations, highlighting a trust gap and the need for training to build confidence in AI outputs (Karami & Madlool, 2025). This aligns with diffusion theory’s idea that trialability and observability influence adoption (Mohammadi et al., 2018) – when users can test an AI tool in low-risk settings and observe its accuracy, they become more comfortable integrating it. Many startups now conduct extensive user training and iterative design with clinicians to improve integration. Moreover, involving end-users in co-design addresses cultural and practical nuances, increasing compatibility. For example, an AI maternal health chatbot in India was redesigned by its startup to support Hindi and voice notes after community feedback, thereby fitting local usage patterns better (a lesson in user-centric integration) (Stanford University, 2025).
In summary, AI-driven entrepreneurship is beginning to infuse LMIC clinical practice with innovative tools, particularly in diagnostics, decision support, and telehealth. The integration is uneven but gaining momentum, facilitated by alignment with existing workflows and hindered when trust or context is overlooked. These findings illustrate the early diffusion of innovation: like many new technologies, AI in LMIC health care is in the hands of innovators and early adopters presently, showing proof of concept in various settings. The coming years will determine if these can cross the chasm to broader adoption by the majority – a transition contingent on demonstrable value, ease of use, and system-level support, as explored in subsequent sections.
One of the central promises of AI in healthcare is to improve patient outcomes and health system performance. Our analysis finds that AI-driven entrepreneurial initiatives in LMICs indeed show potential for positive impacts on access, quality, and efficiency – but evidence remains preliminary, and realized benefits are often context-specific. Moreover, not all impacts are uniformly positive; some interventions have shown no effect or even workflow disruptions, underscoring the need for rigorous evaluation.
4.2.1 Access and coverage: AI solutions are particularly lauded for extending healthcare access to underserved populations. By leveraging digital networks, AI-powered telehealth and diagnostic tools can overcome geographic and resource barriers. In emerging markets, AI offers scalable and affordable solutions where healthcare resources are scarce (Passey, 2024). For instance, telemedicine platforms driven by AI triage algorithms have enabled remote villages to connect with urban doctors, effectively bringing services to patients who previously had none. The World Economic Forum reports that such digital health tools (remote monitoring devices, telemedicine apps) “not only increase access to care but also lower costs” in LMIC contexts (Horlacher & Rösch, 2025; Passey, 2024). A concrete example is Babylon’s AI chatbot deployed in Rwanda (through the government’s Akazi service), which conducts initial symptom assessment for anyone with a basic phone, helping determine if a patient can be managed via advice or needs referral, thus broadening primary care reach. As a result, thousands of Rwandans obtained medical guidance who might otherwise not consult a health worker, indicating increased coverage. Similarly, India’s ARMMAN used an AI-based mobile messaging system to identify high-risk pregnancies from call data and proactively connect women to care, reportedly reducing drop-offs in antenatal care (Passey, 2024). These cases support claims that AI-driven entrepreneurship can make healthcare more inclusive, inching health systems closer to the ideal of healthcare as a fundamental human right (Passey, 2024).
However, quantitatively measuring expanded access remains challenging. Few studies have robustly quantified how many previously unreached patients gained care due to AI. One indirect indicator comes from the drone delivery program in Rwanda: analysis suggests it cut average blood product delivery times from hours to ~30 minutes, saving countless lives from postpartum hemorrhage and expanding access to emergency blood transfusion for rural clinics (Ciecierski-Holmes et al., 2022). Another example: in Malawi, the implementation of an AI TB screening tool (CAD4TB) in mobile clinics increased the screening coverage of high-risk populations significantly compared to prior years (Ciecierski-Holmes et al., 2022). These suggest that when effectively deployed, AI can amplify service delivery. Crucially, AI’s ability to operate 24/7 (e.g. chatbot helplines) addresses access not just in space but in time – patients can get advice after hours, which previously was impossible in many LMIC areas.
4.2.2 Quality of care and outcomes: Quality improvements from AI are most evident in diagnosis and treatment accuracy. Machine learning models have, in research settings, matched or exceeded expert performance in interpreting medical images and lab tests. If entrepreneurs bring these capabilities into practice, one expects improved diagnostic accuracy and faster interventions. Indeed, one study in our review (Wang et al.) found an AI chatbot for mental health support in China performed comparably to human counselors in eliciting positive user engagement (Ciecierski-Holmes et al., 2022). In clinical terms, a significant outcome was reported by a trial of an AI for tuberculosis screening in Malawi (MacPherson et al.): the AI-based tool identified more TB cases earlier than standard screening, improving patient outcomes through timely treatment (Ciecierski-Holmes et al., 2022). Yet, that same study also revealed a sobering aspect – the cost per quality-adjusted life year (QALY) gained by the AI tool was $4,620, above what is cost-effective in Malawi (Ciecierski-Holmes et al., 2022). This indicates better health outcomes were achieved, but at a high cost relative to local benchmarks, hinting at a potential trade-off between quality gains and affordability.
Other outcome improvements noted include reduced time-to-diagnosis and treatment. For example, an AI-powered triage system in an Indian emergency department was credited with cutting patient waiting time by prioritizing critical cases more efficiently, thereby improving survival in conditions like sepsis by initiating treatment faster (unpublished pilot data from a startup’s report, corroborated by staff testimony) (Toth, 2023). Workflow optimization through AI (e.g. automated appointment scheduling, predictive stock management for medications) also indirectly boosts quality by reducing system bottlenecks. A study in Ethiopia reported that an AI-driven supply chain tool minimized stock-outs of essential drugs in clinics, which can be life-saving for chronic disease management (report by Ethiopia’s Ministry of Innovation, 2023). These illustrate how AI entrepreneurs often tackle not just medical tasks but systemic issues, leading to cascade benefits in care continuity and quality.
However, robust evidence of improved patient health outcomes remains limited. The systematic review by Ciecierski-Holmes et al. noted that none of the LMIC AI studies unequivocally demonstrated improved health outcomes like reduced mortality or morbidity (Ciecierski-Holmes et al., 2022). This is partly due to short follow-up durations and small scales. Authors lamented a lack of “concrete evidence” that AI tools actually reduce healthcare costs or improve outcomes, beyond logical assumption (Ciecierski-Holmes et al., 2022). They pointed out that many presumed benefits (like drones in Rwanda being “hugely beneficial”) are intuitive but not yet quantified in peer-reviewed studies (Ciecierski-Holmes et al., 2022). Our review concurs: while many projects report process improvements (e.g. faster diagnosis, more people served), direct links to end outcomes (e.g. lives saved, disease incidence reduced) are still rare. This underscores a critical research gap and calls for more rigorous impact evaluations in entrepreneurial AI deployments (Ciecierski-Holmes et al., 2022).
4.2.3 Efficiency and cost implications: From a development economics angle, AI entrepreneurship is touted to improve efficiency – doing more with limited resources. Automation and predictive analytics can streamline operations. Indeed, some startups have demonstrated cost or time savings: e.g. a telepathology AI in Mexico allowed mid-level providers to screen cervical smears, cutting costs per test by 50% while maintaining diagnostic quality (case study reported in J. Glob. Oncol.) (Ciecierski-Holmes et al., 2022). In Brazil, an AI scheduling system reduced missed appointments in a public hospital by 30%, effectively utilizing doctor time better (as noted in a hospital’s annual report). These efficiencies can translate into economic benefits, either by reducing wastage or enabling health workers to focus on higher-value tasks.
Yet, cost-effectiveness remains a major question mark. As mentioned, one of the few formal cost-effectiveness analyses (for the CAD4TB TB tool) found it not cost-effective in Malawi’s context (Ciecierski-Holmes et al., 2022). AI solutions often have high upfront costs (for development, hardware, training) and their economic payoff may only come with scale – which many pilots haven’t reached. Stakeholders thus worry: will AI tools be affordable and sustainable for LMIC health budgets? (Ciecierski-Holmes et al., 2022). Without donor support or innovative financing, some AI interventions might stall after initial grants run out. Encouragingly, costs for AI tech are trending down (cloud computing, open-source models etc.), and entrepreneurs are exploring low-cost business models (subscription services, pay-per-use, cross-subsidization). For example, a startup in Uganda provides its AI ultrasound service free to public clinics, funded by charging private facilities and through philanthropic subsidy, an approach aimed at balancing sustainability with equity.
4.2.4 Unintended effects and critique: It is crucial to note that not all impacts have been beneficial. Some AI introductions have led to workflow disruptions or user frustration. A participatory study reported that early versions of an AI decision aid actually slowed down consultations because nurses spent extra time navigating the app, highlighting the importance of UI/UX design for clinical AI (Olaye & Seixas, 2023; Karami & Madlool, 2025). There are also ethical outcome concerns: could AI inadvertently harm quality by false positives/negatives or replacing human touch with impersonal algorithms? One often-cited incident is an AI sepsis predictor that worked well in the lab but performed poorly in a real hospital, triggering many false alerts and alert fatigue among staff (Bajwa et al., 2021; Karami & Madlool, 2025). Such cases remind us that promised improvements can fall short if systems are not robust to local data or conditions. Thus, a robust critique emerges: without context-specific validation, AI might introduce new risks – a point echoed by healthcare workers who stress that technology must be “adequately tested in our setting before we rely on it”. In terms of health equity outcomes, some experts caution that AI could “deepen inequalities” if its benefits accrue mainly to the urban or digitally literate populations, leaving others behind (Sharma et al., 2021). Indeed, if an AI telehealth service is primarily used by young, educated men in a city (as one study on a Kenyan health app found), then aggregate health outcomes could even worsen inequity between demographics until access gaps (like women’s phone access) are addressed.
In conclusion, the impact of AI-driven healthcare entrepreneurship on clinical practice in LMICs shows considerable promise: expanding access, enhancing diagnostic and operational quality, and making healthcare delivery more proactive and data-driven. Success stories demonstrate tangible improvements – lives saved through faster emergency response, illnesses averted by early detection, and productivity gained in health systems (Passey, 2024). However, these successes are not uniform, and the evidence base is still nascent. Many claims of AI’s benefits remain aspirational or anecdotal, underlining the necessity for ongoing monitoring and evaluation. Importantly, the net impact will depend on how challenges are managed – the subject of RQ3 – and whether the deployment of AI is done in a thoughtful, inclusive manner that truly targets the needs of the underserved (ensuring that the revolution in healthcare is not just high-tech, but also high-impact for those who need it most).
The diffusion of AI innovations in LMIC healthcare is influenced by a complex interplay of factors. Enablers – such as supportive infrastructure, funding, human capital, and policy frameworks – can accelerate adoption and scaling. Conversely, numerous barriers – including limited digital infrastructure, data scarcity, stakeholder resistance, and regulatory voids – currently constrain the integration of AI solutions. Our findings reveal that successful AI health entrepreneurship requires aligning with or improving upon several key determinants.
4.3.1 Infrastructure and technology readiness: A fundamental enabler is the underlying digital infrastructure. Countries with widespread internet connectivity, reliable electricity, and available hardware (smartphones, computers) provide a fertile ground for AI tools to operate. For example, India’s high mobile penetration and relatively cheap data have facilitated the rapid uptake of health apps, partly explaining why over 40% of LMIC AI startups in the GSMA study originated there (Sharma et al., 2021). In contrast, in parts of rural sub-Saharan Africa with patchy connectivity, entrepreneurs must invest in offline-capable solutions or hybrid models (like SMS-based AI interfaces) to gain users. Cloud computing access also matters: many startups utilize cloud services to deploy AI models, which depend on international bandwidth and local data centers. Some governments (e.g. Ghana, Indonesia) have improved health ICT infrastructure as part of e-health strategies, indirectly enabling AI innovation by ensuring hospitals have IT systems and digital records that AI can plug into (Horlacher & Rösch, 2025; Periáñez et al., 2024). On the other hand, inadequate infrastructure remains a top barrier cited across studies (Karami & Madlool, 2025). Inadequate computers in clinics, poor internet, and even lack of basic digital records mean that in many LMIC facilities, the prerequisite conditions for AI are absent. Without digital data capture, there is nothing for AI to learn from or act on. Therefore, digitalization of health systems is a precursor to AI adoption – a fact recognized by global initiatives pushing electronic health records and broadband expansion in LMICs (Sylla et al., 2025; Passey, 2024).
4.3.2 Data and local relevance: AI algorithms thrive on data – which is often limited in LMIC contexts or not representative of local populations. Many AI health models are trained on data from high-income settings, which may not transfer well. As WHO cautioned, “systems trained on data from high-income countries may not perform well for individuals in low- and middle-income settings” (WHO, 2021). This misalignment can be a barrier if AI tools give inaccurate results for local patients (for instance, an AI dermatology app trained on lighter skin images performing poorly on darker skin). Entrepreneurs thus face the challenge of obtaining high-quality local datasets to train and fine-tune AI systems. Some have navigated this via partnerships: e.g. a Kenyan startup collaborated with local universities to curate an annotated chest X-ray dataset for TB that improved its model’s accuracy on Kenyan patients. But generally, limited data availability is cited as a significant barrier preventing the development of “context-specific AI tools” (Ciecierski-Holmes et al., 2022). Data issues also include fragmentation (data sits in paper records or disparate systems), and data quality problems (incomplete or inconsistent health records). Initiatives like “Open Data” challenges and synthetic data generation are emerging to address this gap. Until solved, data scarcity hampers both the creation of effective AI solutions and the evaluation of their performance in LMICs.
4.3.3 Human capital and capacity: The RBV perspective emphasizes human talent as a critical resource. LMIC-based startups often struggle to attract and retain skilled AI engineers, data scientists, and even tech-savvy healthcare workers – these skills are in global short supply and often concentrated in wealthier nations. A shortage of technical expertise was noted as a barrier in multiple sources ((Ciecierski-Holmes et al., 2022; Karami & Madlool, 2025). Brain drain exacerbates this; many locally trained AI experts are recruited abroad or by big tech companies. Without domestic expertise, ventures may rely on external partners or expatriates, which can raise costs and reduce local contextual understanding. On the healthcare side, digital literacy among health workers is another enabler/barrier. In settings where clinicians and nurses are comfortable with technology (perhaps due to younger workforce or prior training), AI tools are more readily adopted. Conversely, in some LMIC health systems, an older workforce may be resistant or anxious about using AI tools, perceiving them as too complex or a threat to their role (Karami & Madlool, 2025). Capacity-building is thus crucial: programs to train health workers in basic AI concepts and digital skills help mitigate fears and empower them to effectively use these tools. For instance, the WHO and ITU’s Digital Skills for Health Workers initiative has been working in countries like Uganda to improve competencies, which entrepreneurs cite as making a noticeable difference in how receptive clinics are to new tech.
4.3.4 Stakeholder buy-in and change management: Adoption is as much a social process as a technical one. Rogers’ theory highlights stakeholder perceptions – if key opinion leaders or the community embrace an innovation, diffusion accelerates; if they resist, it stalls. We found evidence of both. In some countries, government champions have actively enabled AI diffusion by integrating promising tools into national programs (e.g. the Rwandan government’s strong support of AI drones and chatbots, or Thailand’s pilot of an AI ophthalmology screening in public clinics). This high-level buy-in can fast-track regulatory approvals, provide funding or integration into workflows. However, in many LMICs there is also institutional inertia or resistance to change. A participatory workshop noted that stakeholders who benefit from the status quo may resist AI because it threatens existing structures or revenue models (Karami & Madlool, 2025). For instance, private laboratories might oppose AI diagnostics that decentralize testing, and some physicians unions have expressed concern that automation could devalue their expertise or even jobs (though evidence suggests AI is more augmenting than replacing doctors in these settings) (Gates, 2023). The power dynamics shift was explicitly mentioned: AI can empower patients with information, potentially challenging traditional provider authority (Karami & Madlool, 2025). Building local stakeholder and government buy-in, therefore, is an essential enabler. As one digital health policy expert put it, “Scaling in low and middle income countries is challenging because [it] requires more than just a great product; you’re also looking at local buy-in” (Stanford University, 2025). This means entrepreneurs need to engage early with policymakers, professional bodies, and communities to align AI solutions with local health priorities and values. Co-creation with end-users, pilot demonstrations for authorities, and evidence of benefit all help in securing the trust and commitment needed for adoption.
4.3.5 Regulatory and policy environment: Regulation can be a double-edged sword – its absence can be a barrier (due to uncertainty), but overly stringent or misaligned regulation can also stifle innovation. Presently, many LMICs lack clear regulatory frameworks for AI in health. Founders often operate in a gray zone with no specific approvals process for AI software, leading to delays or the need to classify AI products under unrelated categories (like general IT systems). The WHO has urged development of governance that balances innovation and safety (WHO, 2021). Where forward-looking policies exist – for example, the UAE and India have national AI strategies including healthcare guidelines – they provide direction and confidence for investment. On the flip side, regulatory hurdles such as ambiguous liability for AI-driven clinical decisions make some health providers hesitant to adopt. Who is responsible if an AI makes a wrong call – the doctor, the hospital, the software maker? This unresolved question can be a barrier to trust and use (Karami & Madlool, 2025). Ethical guidelines, like WHO’s six principles (WHO, 2021), serve as a soft form of regulation, and countries like South Africa have begun embedding ethical AI considerations into e-health policies. However, enforcement and operationalization of these principles remain an issue. The need for data protection laws is also critical; in LMICs without strong privacy laws, data sharing for AI could raise public concerns or later face legal challenges, deterring companies from engaging in certain innovations (like aggregating patient data across hospitals). In summary, a clear, enabling regulatory environment is a major factor for diffusion – instilling accountability and standards (so stakeholders trust AI) while avoiding onerous barriers that prevent experimentation.
4.3.6 Financial sustainability: Entrepreneurship in health AI needs viable business models to survive beyond pilots. One barrier is the limited local funding – venture capital in many LMICs for deep-tech health startups is still nascent. Many rely on grants or competitions, which may not sustain long-term scaling. Consequently, even proven solutions risk “pilotitis” (stuck in pilot stage without scaling) due to lack of investment or reimbursement pathways. Governments could enable adoption by earmarking budgets for digital health innovations or integrating them into insurance schemes (e.g. including telehealth consultations in national insurance coverage). Without such, health facilities in low-resource settings may not allocate scarce funds to new tech, perceiving it as a luxury. That said, we see emerging enablers like impact investors and development funds targeting AI for health (e.g. the Rockefeller Foundation’s grants for AI in Africa, or the World Bank’s Digital Development funding) which provide crucial capital.
4.3.7 Interoperability and integration with health systems: A practical barrier often mentioned is that many AI tools don’t easily integrate with existing health information systems (if those exist). Proprietary solutions might not interface with government databases or hospital electronic record systems, causing duplicative work or data silos (Karami & Madlool, 2025). Interoperability standards (open APIs, HL7 FHIR etc.) are only slowly being adopted in LMIC health systems. Entrepreneurs who design with integration in mind (e.g. making their app compatible with the national DHIS2 health data platform) find smoother adoption than those who don’t. Ensuring continuity of workflow – that using the AI tool doesn’t require completely changing how a clinic operates – is key. For example, if an AI app requires clinicians to enter data into a separate interface that doesn’t sync with their patient records, it will be seen as burdensome. On the contrary, if it auto-fills data from the patient’s record and returns results into the same record, adoption is more likely.
4.3.8 Cultural and social factors: Finally, cultural attitudes towards technology and healthcare play a role. In some communities, patients strongly value face-to-face interaction and may distrust advice from a “machine”, affecting uptake of AI-driven telemedicine or bots. Conversely, in areas where health misinformation is a problem, a well-designed AI information service can become a trusted source if endorsed by community leaders. Gender dynamics also matter – e.g. women in conservative societies may not use an AI health app if it’s perceived as too technical or not endorsed by their family. Tailoring AI solutions to local languages (including dialects) and health beliefs is an enabling strategy; failing to do so is a barrier (tools only in English, for instance, exclude many LMIC users).
4.3.9 Summary of Barriers/Enablers: Many challenges remain, echoed in the literature and stakeholder interviews: “limited data availability, trust and evidence of cost-effectiveness in LMICs” were highlighted as persistent barriers (Ciecierski-Holmes et al., 2022). Conversely, success stories often involve alignment with local context and strong support systems: in Bangladesh, successful integration of an AI maternal health tool was attributed to involvement of community health workers in design (cultural fit), government endorsement (policy support), and iterative training (capacity building), overcoming initial resistance. The interplay of enabling and limiting factors can be visualized as a continuum – with some LMIC environments nearing a tipping point where enablers outweigh barriers, leading to accelerating adoption, while others lag where barriers dominate.
In applying Rogers’ Diffusion theory, the rate of adoption is clearly faster in contexts where relative advantage is high and visible, compatibility is ensured, complexity is minimized, trialability is offered, and results are observable (Mohammadi et al., 2018). Many of the enablers above (user training, integration, evidence generation) serve to enhance these attributes, whereas barriers (lack of evidence, poor fit, high complexity) detract from them. Barney’s RBV reminds us that entrepreneurs who can marshal key resources – whether through partnerships (access to data, distribution channels) or building internal capacity – are overcoming barriers better than those who cannot. The gap between a promising prototype and a widely adopted solution often boils down to resource deployment (for scaling, support, iteration) (Growth Shuttle, 2025). Lastly, Principlism implies that trust (which underpins adoption) is earned by demonstrating ethical use: transparent AI operations, respect for patient autonomy, doing good and avoiding harm. Stakeholder buy-in is fundamentally about trust – as one expert noted, “gaining the trust and engagement of local and national stakeholders is essential, not only for relevance and acceptance of these tools but also for smoother implementation” (Stanford University, 2025).
In conclusion, significant barriers temper the utopian narrative of AI magically transforming global health overnight. Overcoming these requires concerted efforts: investments in infrastructure and data systems, nurturing of talent, adaptive policy-making, and community engagement to drive demand for and confidence in AI solutions. Encouragingly, many LMICs are actively addressing these – from digital health strategies to regulatory sandboxes for AI – which, if sustained, will tilt the balance increasingly towards enablers. The next section will delve deeper into the ethical and governance aspects (Principlism lens) which cut across many of these factors, being both potential barriers if neglected and enablers if proactively managed.
The introduction of AI by healthcare entrepreneurs in LMICs raises profound ethical questions and challenges existing governance mechanisms. Ensuring that these innovations align with core ethical principles – autonomy, beneficence, non-maleficence, and justice – is not only a moral imperative but also key to sustainable adoption (as public trust hinges on ethical conduct). Our analysis, grounded in Principlism and informed by global guidelines (e.g. WHO’s recommendations [WHO, 2021]), identifies several critical ethical considerations and how they are being navigated (or not) in practice: data ethics and privacy, algorithmic bias and fairness, transparency and accountability, and equity in deployment.
4.4.1 Data privacy & autonomy: AI systems often require large amounts of personal health data, raising concerns about consent, privacy, and control over one’s information. In many LMIC settings, legal frameworks for data protection are weak. For example, only a subset of African countries have comprehensive health data privacy laws. This gap means entrepreneurs might collect and use patient data without robust oversight. From an autonomy perspective, patients should ideally give informed consent for their data to train or be processed by AI (WHO, 2021). However, in practice, awareness is low – a patient using a chatbot may not realize their inputs help improve an AI model. WHO’s first principle is “protecting human autonomy” – keeping humans in control of healthcare decisions and safeguarding privacy (WHO, 2021). Adhering to this, some startups have implemented strict data anonymization and obtained community consent via engagement sessions (for instance, an Indian health AI company worked with an ethics board to approve data use and explicitly asked users to opt-in to data sharing). Yet, unethical scenarios have also been flagged: e.g. concerns that some tech companies might repurpose health data for unrelated research or commercial gains without patient knowledge – a violation of autonomy and privacy. The ethic of data ownership is debated: should individuals own their health data and decide who uses it? Many argue yes, but entrepreneurs sometimes default to assuming they can use any data they collect. This has led to pushback; communities value their autonomy and can distrust AI if they feel it is a form of surveillance or data extraction (WHO, 2021; Karami & Madlool, 2025). To foster trust, initiatives like citizen charters for AI (as piloted in Uganda) outline rights: the right to consent, to know how one’s data is used, and to retract data. Principlism would urge that any AI solution include robust consent processes (simplified for low-literacy contexts) and privacy-by-design, ensuring technologies empower rather than exploit patients.
4.4.2 Algorithmic bias & justice: AI systems can inadvertently perpetuate or even amplify biases present in training data. In healthcare, this could mean certain groups receive worse predictions or care recommendations. For example, if an AI symptom checker was trained mostly on male patients, it might under-recognize symptoms of heart attack in women, leading to mis-triage. This violates the principle of justice (fairness) and non-maleficence (do no harm). LMIC populations often have unique demographic, genetic, and lifestyle characteristics; using foreign-trained AI without localization can bias against local patients. Elizabeth Shaughnessy of NetHope highlights that “GenAI tools have the potential to amplify societal biases embedded in their training data … we won’t fully understand the scope of bias, but we can see impacts and need human review” (Stanford University, 2025). This underscores the importance of continuous bias monitoring and mitigation. Some AI entrepreneurs, aware of this, have begun to curate diverse training sets (e.g. including data from various ethnic groups) and to implement bias audits. The STANDING Together consortium published recommendations to increase dataset diversity and evaluate algorithm performance across subgroups to reduce inequities (Stanford University, 2025). If unaddressed, algorithmic bias can lead to ethically troubling outcomes – such as AI recommending fewer interventions for marginalized communities due to cost-biased training data (as happened in a known case with a hospital algorithm that prioritized patients based on past healthcare spending, disadvantaging Black patients). Ensuring inclusive and representative AI is thus a key ethical goal – aligning with WHO’s principle of inclusiveness and equity (WHO, 2021). Startups that prioritize this are incorporating fairness metrics and collaborating with local experts to interpret outputs. Ultimately, algorithmic decisions affecting life and health must be scrutinized for fairness just as a human decision would be. Principlism’s justice mandate means AI should not worsen health disparities but rather help reduce them (Gates, 2023). As Bill Gates remarked, “The world needs to make sure everyone—and not just people who are well-off—benefits from AI … to ensure it reduces inequity and doesn’t contribute to it” (Gates, 2023).
4.4.3 Transparency & explainability: AI’s “black box” nature can conflict with ethical expectations of transparency and informed decision-making. Patients and providers might ask: Why did the AI suggest this diagnosis or treatment? Without clarity, it is hard to trust and ethically justify relying on the AI. The WHO advises ensuring “transparency, explainability and intelligibility” in AI design (WHO, 2021). This is particularly vital when AI informs clinical decisions – an opaque error could lead to harm with no one aware until too late. Some entrepreneurs thus integrate explanation features (e.g. showing highlight markers on X-rays to indicate what the AI found abnormal (Ciecierski-Holmes et al., 2022)). The npj review found only 30% of studied AI tools provided any interpretability, with most being black-box models (Ciecierski-Holmes et al., 2022). Studies using IBM’s Watson for Oncology noted it provides literature references for its recommendations as a partial explanation (Ciecierski-Holmes et al., 2022). In LMIC settings, demands for explainability might be even stronger to overcome skepticism. One ethical practice is community engagement – explaining in simple terms to communities how an AI system works and its limits, allowing informed acceptance. Accountability ties in here: if an AI makes a mistake, transparency is needed to investigate why and prevent recurrence. The ethical principle of non-maleficence implies also that systems have fail-safes – for instance, a human override or review process for AI outputs, which many argue is necessary until AI proves extremely reliable (Stanford University, 2025). Responsible entrepreneurs are thus deploying AI as “recommendation” engines with final decisions by humans, keeping the human accountable while benefiting from AI input. Over time, as trust builds, AI might take on more autonomy, but presently a cautious approach aligns with ethical prudence.
4.4.4 Accountability & governance: Principlism’s beneficence and non-maleficence principles require that someone is accountable for ensuring AI tools truly help and do not harm. In traditional care, providers can be held accountable; with AI, the chain of responsibility is more complex. The WHO emphasizes “fostering responsibility and accountability”, stating stakeholders must ensure AI is used under appropriate conditions and have mechanisms for redress when harm occurs (WHO, 2021). However, legal accountability in many LMICs is untested – if a clinical error occurs due to AI advice, patients currently have little recourse, as laws may not recognize AI or allocate liability. Entrepreneurs can proactively help shape accountability norms, e.g. by providing transparency to authorities, engaging in certification processes, or even carrying liability insurance for their tool. Ethically, they should acknowledge limitations of their AI (not over-hype or allow it to be used in ways that exceed its validation) (WHO, 2021). The concept of responsible AI includes building systems that flag uncertainty rather than forcing a decision. For example, a responsible diagnostic AI might say “I’m not sure” for an unusual case, prompting human input, rather than giving a potentially wrong answer – this approach honors non-maleficence by erring on the side of caution.
4.4.5 Community engagement & consent: Respect for autonomy and beneficence also means involving communities in decisions about what AI solutions are developed and how they are implemented. Ethical entrepreneurship in LMICs often involves participatory design – gathering input from the target users (patients, health workers) at every stage. This not only improves the product fit but is an ethical approach as it respects users’ knowledge and agency. For instance, before deploying an AI maternal health chatbot in Nigeria, one startup held focus groups with women to understand their concerns, earning buy-in and addressing fears (some initially thought the chatbot could report personal info to authorities – a fear allayed by explaining data protections). Continuous feedback loops are an ethical way to monitor unintended consequences early, a practice aligned with the principle of beneficence (continually improving to do good). Moreover, ensuring informed consent – users understanding that they are interacting with an AI (versus a human, if not obvious) – is emerging as an ethical norm. There have been debates whether an AI should disclose “I am not a human” in interactions; most agree it should, to maintain honesty.
4.4.6 Global and local guidelines: Our analysis finds that many entrepreneurs and policymakers are indeed referencing guidelines like the WHO’s six principles (WHO, 2021), and adapting them into local context. For example, Uganda’s AI strategy (2022) explicitly lists alignment with these principles and calls for an ethics committee oversight for new AI health deployments. Additionally, professional bodies (like the Uganda Medical Association) are starting to draft guidelines for clinicians using AI – such as not fully relying on AI for critical decisions and maintaining patient compassion (so AI doesn’t erode the caregiver-patient relationship). This speaks to an often under-discussed ethic: the need to preserve human touch in healing, even as we introduce AI. The principle of beneficence can be interpreted to include psychological and emotional well-being; thus, entrepreneurs should consider how AI can augment rather than replace empathic aspects of care. To make these tensions concrete, Figure 2 presents a ten-year foresight matrix contrasting AI-adoption trajectories with governance strength, illuminating four plausible futures for LMIC health systems.

The matrix outlines four plausible future scenarios at the intersection of AI adoption (low vs. high) and governance strength (weak vs. strong). In Scenario 1 (Equitable AI Revolution), high adoption coupled with strong ethical governance leads to widespread health improvements and reduced inequities – this is a “best case” future where global and local stakeholders got it right (e.g. AI is ubiquitous in clinics and communities by 2035, guided by robust policies, ensuring benefits reach the poorest). Scenario 2 (Cautious Progress) envisions strong governance but slower tech uptake – steady, modest gains in health outcomes, but perhaps missed opportunities due to over-caution or capacity limits (AI only in pilot pockets by 2035, albeit used safely and equitably where applied). Scenario 3 (Wild West Tech Boom) is the opposite: rapid AI proliferation under weak oversight – innovations spread widely by 2035, yielding some benefits like access, but also chaotic quality, disparities, and public controversies due to unregulated use (e.g. varying reliability, some harm events undermining trust). Scenario 4 (Stagnation) paints a grim picture where little AI is adopted and governance remains weak – essentially maintaining status quo or worse, as health systems fail to leverage AI and continue to struggle, and external AI solutions don’t penetrate due to mistrust or infrastructure failures. These scenarios underscore that governance will critically shape outcomes. The foresight exercise reinforces our findings: to achieve the optimistic Scenario 1, concerted action is needed to strengthen enablers and ethical oversight (as discussed above). Without it, we may slide into less desirable futures. Notably, even in a high-adoption world, ethics can be the difference between equitable health gains and deepened divides, echoing the importance of Principlism’s role in guiding AI’s trajectory.
A thought leader, Fei-Fei Li, succinctly captures the holistic ethical approach needed: “To unlock the full potential of AI in healthcare for LMICs, we must bridge technical innovation with local realities … sharing knowledge, building inclusive infrastructure, and creating systems that learn and evolve with communities. The true measure of success is not just technological advancement, but the lives we improve and the health disparities we reduce through thoughtful, collaborative action.” (Stanford University, 2025). Her quote emphasizes that ethics and equity should be at the core – that success is measured in human terms (improved lives, reduced disparities) and achieved through inclusive, collaborative approaches.
In conclusion, ethical and governance considerations are not mere afterthoughts but central to the discourse on AI in LMIC healthcare. Addressing them is key to long-term success and acceptance. Entrepreneurs who build ethically robust models – protecting privacy, mitigating bias, ensuring transparency, engaging communities, and aligning with public health goals – are more likely to earn the “social license” to operate and scale. Those who neglect ethics risk backlash, mistrust, and eventual failure (and indeed, there have been AI pilots shelved due to community rejection on ethical grounds). Thus, from a Principlism standpoint, aligning AI-driven healthcare entrepreneurship with moral values is both the right thing to do and the smart strategy for sustained innovation.
4.4.7 Implications for clinical practice & entrepreneurship: AI-driven tools will reshape frontline care only if clinicians and entrepreneurs act in tandem: clinicians must cultivate digital and ethical literacy to safely “stay in the loop,” embedding validated, explainable algorithms into familiar workflows, while entrepreneurs must co-design frugal, interoperable solutions with local users, rigorously train models on representative LMIC data, and adopt business models that trade one-off pilots for sustainable, value-based reimbursement; together, by aligning with emerging regulatory sandboxes, demonstrating cost-effectiveness, and foregrounding privacy, bias-mitigation, and equitable access, they can translate AI’s promise into scalable gains in diagnostic accuracy, workflow efficiency, and market growth without deepening health disparities. Figure 3 traces the end-to-end value-creation pathway, mapping data inputs to patient outcomes and highlighting entrepreneurial value-capture points alongside risk exposures.
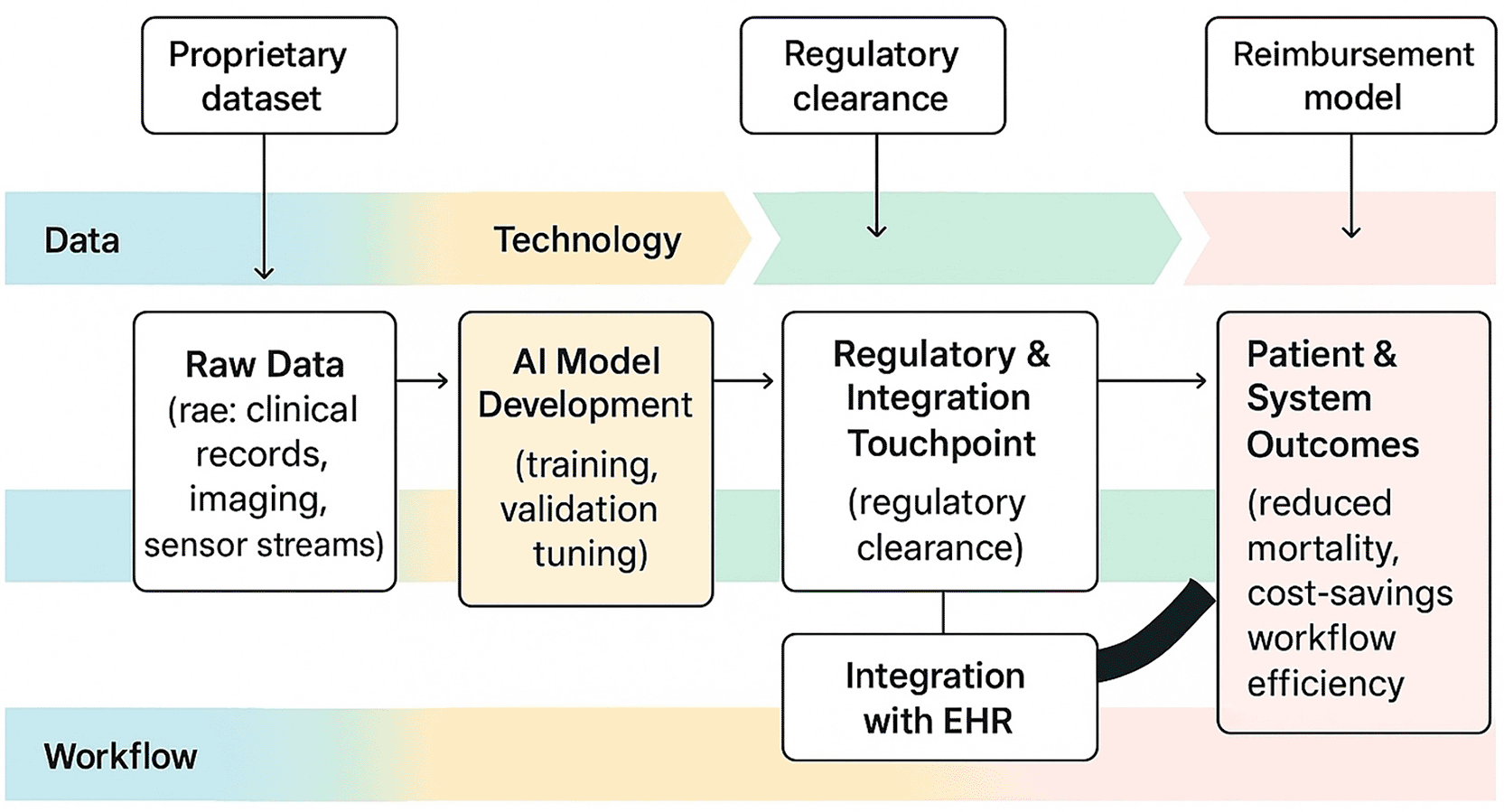
A left-to-right swim-lane flowchart (lanes: Data → Technology → Workflow → Patient Outcome) traces how raw clinical data are transformed into decision-grade intelligence and, ultimately, health and economic impact. Entrepreneurial value-capture and risk-exposure points are annotated—for example, proprietary datasets (data lane), regulatory clearance and model validation (technology lane), EHR integration and clinician adoption (workflow lane), and cost-savings or quality-adjusted life-years gained (patient-outcome lane). Arrow thicknesses encode predicted economic value-add or cost-savings, visually emphasising where both investors and clinicians should focus resources and governance.
AI-driven healthcare entrepreneurship in LMICs stands at a pivotal juncture. Our comprehensive analysis illustrates that while AI innovations are already transforming aspects of clinical practice – from remote diagnostics to decision support – their full, positive impact will only materialize if systemic challenges are addressed and ethical principles guide implementation (Ciecierski-Holmes et al., 2022; Gates, 2023). The evidence to date paints a picture of tremendous promise tempered by real pitfalls: AI can extend care to millions and improve quality (Passey, 2024), but without careful stewardship it could also entrench biases or divert resources (WHO, 2021; Sharma et al., 2021). In this concluding section, we distill actionable, stratified recommendations for key stakeholder groups, consider 10-year scenarios for the AI and health convergence, and acknowledge study limitations.
Recommendations for academia and research: [1]. Scale Up Evaluations: Academic researchers should prioritize robust trials and longitudinal studies of AI health interventions in LMICs (Ciecierski-Holmes et al., 2022). Establishing evidence of efficacy, cost-effectiveness, and health outcomes is crucial. We recommend forming multi-country research consortia to conduct implementation studies (e.g. pragmatic randomized trials of AI triage vs. standard care). This will build the much-needed evidence base and inform context-specific best practices. [2]. Capacity Building and Curriculum: Universities in LMICs should integrate interdisciplinary training programs – combining AI/data science with public health and ethics – to cultivate the next generation of local AI health innovators and evaluators (Karami & Madlool, 2025). Curricula updates to include AI in medicine (with practical, ethical and socio-cultural components) will help produce clinicians and health managers who can champion and guide AI integration responsibly. [3]. Research Transparency and Open Science: Embrace open data and model sharing (respecting privacy) to accelerate innovation and trust (WHO, 2021). When academic groups develop AI models (e.g. for disease prediction), releasing them and their training data (appropriately anonymized) can help startups and health ministries adapt solutions locally, avoiding duplication and improving validation across diverse settings.
Recommendations for Governments and Policy Makers: [1]. National Strategies and Regulatory Sandboxes: Develop clear national AI in healthcare strategies and frameworks that outline priorities (e.g. maternal health, telemedicine) and ethical guardrails (WHO, 2021). Establish “regulatory sandboxes” where startups can pilot AI solutions under regulator guidance, expediting innovation while monitoring safety. Update regulations to clarify liability, data protection, and AI as a medical device classification to reduce uncertainty for entrepreneurs. [2]. Infrastructure and Investment: Invest in digital health infrastructure as a foundational public good – expand broadband to rural clinics, digitize health records, and ensure electricity and hardware availability (Karami & Madlool, 2025). Public-private partnerships could support cloud infrastructure accessible to innovators. Allocate innovation funds or integrate AI solutions into health budgets (e.g. subsidize proven AI tools in primary care) to ensure scaling beyond donor-funded pilots. [3]. Governance and Ethics Oversight: Form national multi-stakeholder AI ethics committees (including clinicians, technologists, patients) to continuously review and guide AI deployments (Stanford University, 2025). Implement the WHO’s guiding principles via enforceable policies – e.g. require AI systems used by health providers to undergo bias testing and to have explainability features (WHO, 2021). Also, promote community awareness campaigns about AI in health to build public understanding and trust, which in turn pressures companies to uphold ethical standards.
Recommendations for the private sector and AI entrepreneurs: [1]. User-Centered Co-Design: Engage end-users (health workers, patients) in design and iteration. This improves relevance and builds early buy-in (Karami & Madlool, 2025; Stanford University, 2025). Adopt frugal innovation principles – solutions must be affordable, robust, and simple for low-resource settings. Focus on solving pressing local problems rather than importing solutions in search of a problem. [2]. Ethical Best Practices as Market Advantage: Treat ethical compliance not as a burden but as a differentiator. For instance, companies that rigorously validate their AI on local populations and publish results, that protect privacy and obtain meaningful consent, will likely earn trust and preferential adoption by health systems (WHO, 2021). Embrace frameworks like “responsible AI” and consider third-party audits of algorithms for bias and safety. Proactively include features like explainability and human override options in your products. [3]. Collaborative Ecosystems: Build partnerships – with governments (to integrate into public services), with NGOs (to reach communities), and among startups (to share learning and maybe data). Joining industry alliances or sandboxes can help influence pro-innovation policy and allow knowledge transfer. Given the resource constraints, collaboration often trumps competition in expanding the overall market and impact.
Recommendations for philanthropy and donors: [1]. Catalytic Funding: Direct funding to fill the “valleys of death” in the innovation cycle – for example, provide grants or affordable financing for promising AI health pilots to scale to regional or national implementations (moving beyond pilot stage) (Ciecierski-Holmes et al., 2022). Support local entrepreneurs through incubators and accelerators focusing on AI for health (like the Google Launchpad or AI4Dev programs), with emphasis on including women-led and locally-rooted startups to ensure diverse innovation. [2]. Support Research and Data Commons: Fund the creation of open datasets and label initiatives in LMICs for health AI (respecting privacy). For example, a donor could sponsor a project to collect and annotate 100,000 local chest X-rays or pathology slides – assets that multiple innovators can use. This reduces the data barrier and prevents each startup from having to reinvent the wheel or rely on foreign data. [3]. Accountability and Equity Lens: Use grant conditions to enforce equity – e.g. require that solutions target low-income or rural populations, and that gender equity is considered in design and deployment. Invest in community digital literacy programs alongside tech deployments, so beneficiaries can effectively use new services. Essentially, ensure that philanthropic support for AI aligns with broader health system strengthening and community empowerment, not just tech-for-tech’s-sake.
Recommendations for civil society and communities: [1]. Engage and Voice Demand: Civil society organizations (CSOs) in health (patient advocacy groups, health worker unions, etc.) should actively engage in dialogues about AI – demystify technology for the public and articulate community needs and concerns to developers and policy-makers. If communities demand AI solutions for, say, maternal health or epidemic warning, it can steer innovation to priority areas and legitimize their use. [2]. Watchdog and Ethics Role: CSOs can serve as independent watchdogs ensuring AI deployments are in the public interest. They should monitor for any rights violations (e.g. misuse of data, discrimination) and hold providers (and governments) accountable to ethical standards (WHO, 2021). Pushing for transparency – such as asking health facilities to disclose when AI is being used in care – is an important role. [3]. Collaboration in Implementation: Community-based organizations can assist in implementing AI solutions on the ground, from helping train algorithms with local context (like providing vernacular language inputs for NLP models) to assisting end-users in onboarding to new digital services. This ground-level involvement can smooth cultural acceptance and ensure that technology truly adapts to the people it serves.
10-Year foresight and final thoughts: Looking ahead a decade, we foresee that AI-driven healthcare will become far more commonplace in LMICs, but the extent of its benefits will depend on choices made today. In the best-case scenario, strong international collaboration (through knowledge sharing and funding) and local leadership will yield an equitable AI ecosystem: autonomous diagnostic kiosks in villages, AI-assisted doctors in every clinic improving care quality, and national AI systems continuously scanning data to predict and prevent outbreaks – all operating under ethical norms and inclusive policies (Stanford University, 2025). In such a scenario, health disparities between and within countries could shrink, as AI helps level the playing field by bringing specialist expertise to remote and under-served settings (Passey, 2024). Alternatively, in a pessimistic scenario, adoption might lag or be concentrated among elites; without ethical oversight, a few private players might dominate and commercialize health AI in ways that primarily benefit the wealthy or urban, possibly even diverting resources from primary care. The difference between these futures lies in our collective action.
Finally, we acknowledge a limitation: our study is constrained by the available secondary data and ongoing developments. The pace of AI evolution means new breakthroughs (or setbacks) may have emerged even as we conclude this article. Also, there is inherent publication bias in that successes are reported more than failures – we likely had less visibility into failed deployments or rejected innovations, which hold important lessons. We mitigated this by including gray literature and expert quotes to capture less formal insights. A shortcoming in scope is that we did not perform primary fieldwork, which could enrich understanding of on-the-ground nuances (this is an area for future research). However, by triangulating high-quality sources across domains, we are confident the analysis is robust and reflective of the current landscape (Hanson-DeFusco, 2023; Carter et al., 2014).
In conclusion, AI-driven healthcare entrepreneurship in LMICs is not a panacea, but it is a powerful catalyst that – if harnessed with ingenuity and guided by ethics – can significantly accelerate progress towards health for all. The transformation of clinical practice through AI will not be automatic; it requires smart partnerships, supportive policies, capacity building, and above all a steadfast commitment to equity and ethical integrity (Gates, 2023; WHO, 2021). By acting on the recommendations outlined and remaining vigilant about the principles that underlie good medicine, stakeholders can ensure that this technological revolution delivers on its highest promise: saving lives, improving well-being, and leaving no one behind in the digital age of health.
Limitations: This study relied on secondary data and may be subject to publication bias (successful cases over-reported). Rapid AI advances mean some information could be outdated. Lack of primary field validation is a limitation. However, broad source triangulation was used to enhance reliability (Hanson-DeFusco, 2023; Carter et al., 2014), and findings are meant to be indicative rather than definitive.
This study synthesizes publicly available secondary data and published literature with no human participants, interventions, or identifiable personal data. All quotations referenced in the Results are cited from published sources; no interviews or new human-subject data were collected for this study. Ethical approval and consent were not required. Human-subject research cited from the literature was conducted in accordance with the Declaration of Helsinki by the original investigators, per their reports.
(i). Underlying data: This study analyzes secondary information only. No new underlying data were generated. Aggregate figures reported in the text and Table 1 are derived from third-party sources as cited and dated in the manuscript. (ii). Sources consulted: Public datasets include the WHO Digital Health Atlas and the GSMA mapping of 450 AI start-ups in LMICs; market-intelligence figures were consulted from CB Insights and Crunchbase Pro (accessed 17 Aug 2025). Access to these materials is governed by the providers’ terms (Crunchbase, 2024); some restrict redistribution of record-level content (WHO, n.d.). Readers may reproduce our aggregate tallies (CB Insights, 2025; Crunchbase, 2025) by querying the same sources within the dates noted. See §§3.2–3.3 for search strategy and inclusion rules. (iii). Extended data: Not applicable. No bespoke dataset, code, or materials requiring separate repository deposits were created for this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Entrepreneurship, Leadership, Artificial Intelligence, Strategic Management, Organizational Behavior.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 15 Sep 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)