1.1 Historical background
Behavioral science has long sought formal laws to explain the emergence of behavior. Lewin (1936) introduced the field equation B = f(P, E), positing that behavior (B) arises from dynamic interactions between personal factors (P) and environmental influences (E). His formulation, modeled after Einstein’s field theories, underscored that neither internal nor external determinants alone suffice; instead, behavior emerges from their convergence.1 Tinbergen (1963) later articulated a framework that incorporates proximate mechanisms, ontogeny, function, and phylogeny, emphasizing that even reflexive responses are shaped by multiple, hierarchical constraints across biological systems.2
Although behavioral scientists have long recognized that multiple influences shape behavior, such convergence has usually been described only in qualitative terms. What has been missing is a formal analysis of convergence: the explicit claim that no single factor is sufficient, and that behavior emerges only when neural substrates, hormonal drive, and contextual inputs align above a functional threshold. Importantly, this formulation is not additive—where partial contributions from two strong factors might substitute for the absence of a third—but multiplicative, such that the lack of any one requisite factor abolishes the behavior (1 × 1 × 0 = 0).
By expressing this principle in a quantitative framework, convergence can be rendered predictive and falsifiable, rather than merely descriptive, opening the way toward a computational analysis of behavior. Establishing this is essential for three reasons. First, it unifies disparate findings across neuroendocrinology, ethology, and neuroscience into a coherent framework, allowing classic observations to be integrated rather than siloed. Second, it provides predictive and falsifiable hypotheses: if any one factor is absent, the behavior should fail, yielding a structure that can be tested experimentally and revised. Third, it offers the potential for generalization across behaviors and species, much like formal laws in physics or biology provide a common explanatory foundation. By reframing convergence as a testable equation, the present framework aims to move behavioral science toward principles that are not only descriptive, but predictive and unifying.
1.2 A multiplicative model
Recent theoretical advances in behavioral threat assessment have extended foundational concepts through the ARCH × Φ framework, which formalizes behavior as a multiplicative interaction of Archetype (A), Drive (D), Context (C), and a threshold field (Φ). According to this model, behavior occurs only when all components exceed a requisite threshold; if any one factor is absent or subthreshold, behavioral expression is abolished.3 This model is represented mathematically as:
This formulation offers one way to codify convergence: behavior does not emerge from additive contributions, but from the conjunctive alignment of all necessary factors, yielding falsifiable predictions. Although illustrated here with the lordosis reflex, the model provides a generalizable template for understanding other motivated behaviors where multiple systems must converge to cross a threshold.
1.3 Lordosis in female rodents
The lordosis reflex in female rodents is among the most extensively characterized behaviors in behavioral neuroendocrinology. Lordosis is a stereotyped, spinally mediated posture—ventral arching of the back with tail deflection—that facilitates copulation and is essential for sexual receptivity. Its expression depends on three well-established factors4–7:
•
Archetype (A): An intact ventromedial hypothalamic (VMH) circuits; bilateral lesions of the VMH abolish the reflex even in hormonally primed females.
•
Drive (D): Robust expression requires sequential estradiol priming followed by progesterone administration. Ovariectomized females do not exhibit lordosis unless these hormonal conditions are met.
•
Context (C): Immediate somatosensory input—specifically, pressure applied to the flanks and hindquarters—triggers the posture; without such stimulation, the reflex is absent.
• Threshold Field (Φ): Represents baseline arousal and neuromodulatory tone that scales the product of Archetype, Drive, and Context to determine if a behavior is expressed.
Because all three factors are indispensable, lordosis emerges only through their convergence. Decades of empirical work converge on the same principle: removal of any single component abolishes behavior, even when the others are intact. Lordosis reflects the integration of hormonal, neural, and sensory inputs within hypothalamic circuits. This integration has traditionally been described qualitatively, without a formal specification of how the components combine.7 The following research questions seek to formalize this principle within the ARCH × Φ framework, treating it as a conjunctive, multiplicative equation. This reframing specifies a candidate law of convergence: lordosis occurs only when all factors align above threshold and fails whenever any requisite factor is absent (1 × 1 × 0 = 0). This convergence is highlighted in
Figure 1.
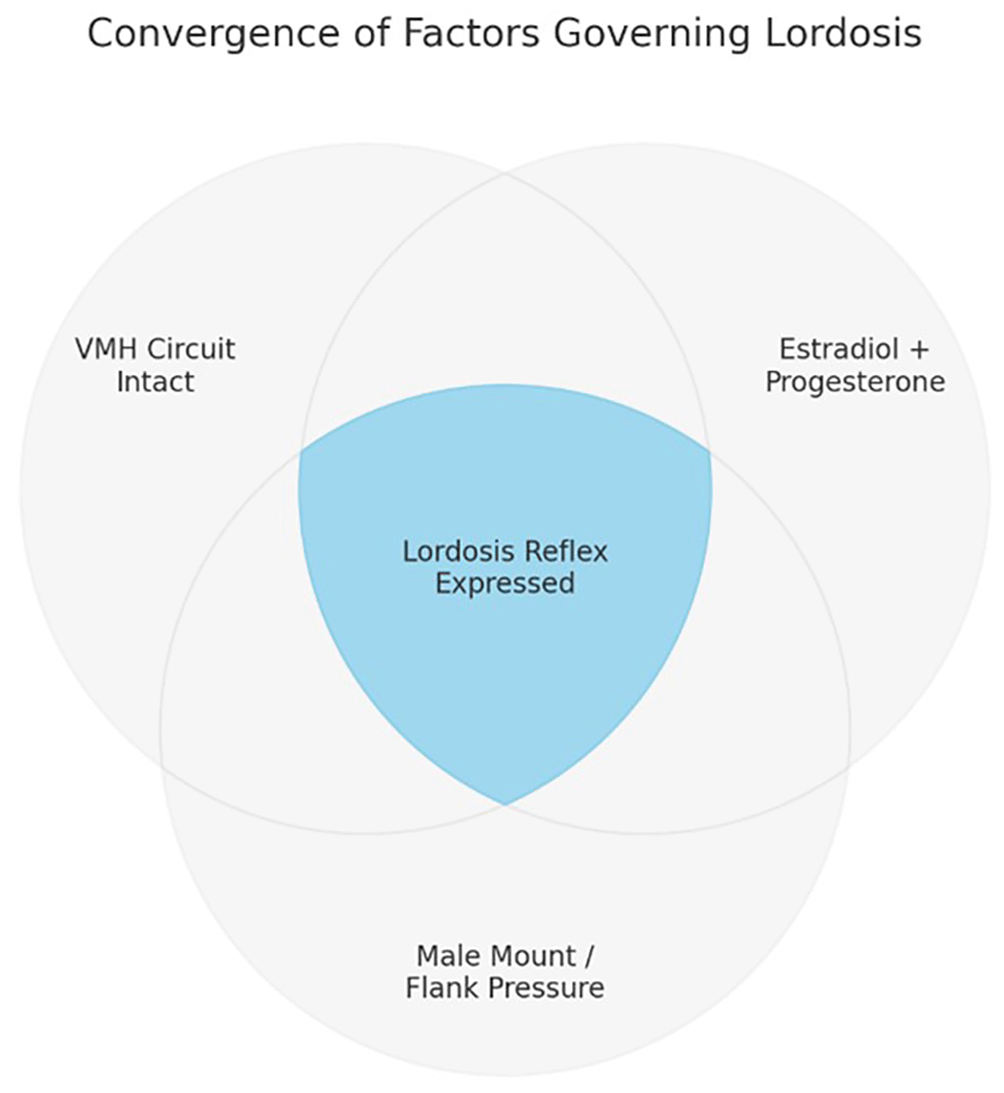
Figure 1. Convergence of factors governing lordosis in female rats.
The lordosis reflex is expressed only when three conditions converge: an intact ventromedial hypothalamic (VMH) circuit, estradiol–progesterone priming, and tactile flank stimulation provided by a male mount. Absence of any one factor abolishes the behavior, consistent with a candidate law of convergence.
1.4 Research questions
This paper explores whether a law of convergence might govern the expression of lordosis in female rats—that is, whether behavior is abolished when any one requisite factor is absent, even if the others are present. To examine this possibility, three classical necessity tests will be analyzed:
• Neural archetype (A): Is an intact ventromedial hypothalamic (VMH) circuit required, such that VMH lesions abolish the reflex even under permissive hormonal and contextual conditions?
• Hormonal drive (D): Is sequential estradiol priming followed by progesterone indispensable, such that ovariectomy without replacement eliminates the reflex despite intact neural and contextual factors?
• Contextual input (C): Is immediate tactile stimulation of the flanks and hindquarters essential, such that its absence precludes lordosis even when neural and hormonal conditions are optimal?
1.5 Hypothesis
Lordosis in the female rat is expressed only when the product A × D × C surpasses a critical threshold, with baseline arousal (Φ) held constant. If any single component is absent or reduced to near zero, behavioral expression should fail, regardless of the state of the other factors.
2.1 Overview of the model
The ARCH × Φ equation conceptualizes behavior as a conjunctive, multiplicative product, defined by the relationship:
In the present analysis, the threshold field (Φ)—representing baseline arousal—is held constant to isolate and evaluate the necessity of Archetype (A), Drive (D), and Context (C) for lordosis expression in female rats. The following subsections outline how each factor is operationalized, drawing on extensive foundational work in behavioral neuroendocrinology.4–7
2.2 Archetype (A): Neural substrate
• Definition: An intact ventromedial hypothalamic (VMH) circuit, particularly the ventrolateral subdivision (VMHvl), is required for lordosis.
• Operationalization:
○ A ≈ 1: VMHvl neurons and their projections to the periaqueductal gray (PAG) are functionally intact.
○ A ≈ 0: Bilateral VMH lesions or loss of connectivity abolish lordosis, even under permissive hormonal and contextual conditions.
• Key Evidence: Classic studies demonstrated that lesioning the VMH eliminates lordosis in hormonally primed females, whereas electrical or hormonal stimulation can restore the reflex.7 More recent circuit-level approaches have reinforced the VMHvl’s role as a causal node. Optogenetic manipulations of hypothalamic circuits demonstrate that VMHvl activity is both necessary and sufficient for specific motivated behaviors, including social and sexual responses,8 and reviews of VMHvl circuitry highlight its broader function as a hub for social, aggressive, and reproductive behaviors.9
2.3 Drive (D): Hormonal priming
• Definition: Behavior is gated by circulating ovarian hormones, with estradiol and progesterone playing central roles in sexual receptivity.
• Operationalization:
• D ≈ 1: Ovariectomized females sequentially primed with estradiol and progesterone exhibit robust lordosis quotients.
• D ≈ 0: Ovariectomized females lacking hormone replacement do not express lordosis.
• Nuance: Estradiol alone, administered at sufficiently high doses for ≥6 days, can induce lordosis, illustrating that drive is a threshold-dependent cascade rather than strictly binary.
• Key Evidence: Estradiol primes the VMH, enabling progesterone to activate lordosis circuits.
2.4 Context (C): Somatosensory stimulation
• Definition: Immediate tactile input to the flanks and hindquarters is necessary to release the lordosis reflex.
• Operationalization:
• C ≈ 1: Adequate flank or tail-base pressure provided by a male mount or calibrated manual palpation.
• C ≈ 0: Absence or disruption of somatosensory input (e.g., through denervation) prevents lordosis.
• Key Evidence: Lordosis does not occur spontaneously; it requires flank pressure, and denervation abolishes the behavior even in hormonally and neurally permissive contexts.
2.5 Outcome measure
In experimental studies, lordosis expression is typically quantified using the lordosis quotient (LQ)—the percentage of male mounts that elicit the posture.10 In the present paper, I do not collect new behavioral measures; instead, I will rely on decades of published reports of LQ and related behavioral outcomes as proxies for testing the multiplicative model. Within this framework, each factor—neural archetype (A), hormonal drive (D), and contextual input (C)—is operationalized based on classic lesion, hormone, and tactile stimulation studies. The ARCH × Φ model predicts, consistent with this literature, that LQ approximates zero whenever any single component is absent, even when the other two are present.
3.1 Archetype lesion test (A = 0)
The neural substrate underlying lordosis is located in the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl), which projects to the periaqueductal gray (PAG). Bilateral lesions of the VMH in female rats abolish lordosis, even when hormonal conditions and tactile stimulation are permissive.11 Conversely, electrical stimulation or local estradiol infusions targeting the VMHvl can restore sexual receptivity, underscoring this region as the critical neural archetype for the behavior.12 When A≈0 (VMHvl lesioned), the lordosis quotient (LQ) falls to zero despite intact drive and context.
Conclusion: An intact neural archetype is necessary for lordosis; removal of A extinguishes the behavior (1 × 1 × 0 = 0).
3.2 Hormone deprivation (D = 0)
Adequate hormonal priming with estradiol and progesterone is crucial for the expression of lordosis. Ovariectomized rats exhibit negligible lordosis unless they are sequentially treated with estradiol followed by progesterone.13 Estradiol primes the VMH and enables progesterone to activate lordosis circuits.14 In the absence of these hormones, neither tactile stimulation nor an intact neural substrate suffices to produce the reflex.10 While chronic, high-dose estradiol alone may eventually permit lordosis, this phenomenon reflects a threshold effect, not evidence against hormonal necessity.12
Conclusion: Hormonal drive is necessary; deprivation of D abolishes lordosis (1 × 0 × 1 = 0).
3.3 Contextual sensory removal (C = 0)
Lordosis does not occur spontaneously; immediate tactile input to the female’s flanks and hindquarters is required. Pressure applied by a male mount or manual palpation reliably triggers the reflex.7 Disruption of somatosensory input—such as flank or perineal denervation—dramatically reduces or eliminates lordosis, even under optimal hormonal and neural conditions.12 Without contextual sensory stimulation, the posture is not expressed.
Conclusion: Contextual tactile input is necessary; absence of C results in loss of lordosis (1 × 1 × 0 = 0).
3.4 Summary of necessity tests
Across lesion, hormonal deprivation, and sensory removal studies, the evidence is consistent: removing any single factor—archetype (A), drive (D), or context (C)—prevents lordosis, even when the remaining conditions are permissive. These necessity tests support the conjunctive multiplicative model:
Each factor is essential, but none is strictly binary. Instead, A, D, and C vary continuously, and lordosis can be understood as a threshold phenomenon: the product Φ·(A·D·C) must exceed some critical value (θ) for expression. Weak contributions from one factor can sometimes be offset by stronger input from the others or by elevated Φ, but if any term approaches zero, the product remains insufficient. This formulation captures the nonlinear dynamics observed in the literature—for example, the robust lordosis elicited by the standard E2→P sequence versus the delayed or weaker expression following chronic estradiol alone—consistent with a sigmoid, threshold-dependent response.
4.1 Conjunctive necessity in Lordosis
The present synthesis demonstrates that lordosis in the female rat is expressed only when all three components—Archetype (A), Drive (D), and Context (C)—are present above threshold. Empirical literature provides three independent necessity tests:
(1) Lesions of the ventromedial hypothalamus abolish lordosis despite hormones and mounts6;
(2) Ovariectomy without hormonal replacement eliminates lordosis despite intact circuitry and tactile input14
(3) Absence or denervation of flank stimulation prevents lordosis despite permissive hormones and circuitry.7 In each case, the absence of one factor reduces the behavioral output to zero, consistent with the multiplicative model:
This formalizes long-standing observations in behavioral neuroendocrinology into a single conjunctive law of expression.
4.2 Lordosis as reflex versus motivation
A common critique is that lordosis represents a spinally mediated copulatory reflex rather than a measure of sexual motivation.10 Indeed, motivational processes in female rats are more clearly indexed by paced mating or partner preference paradigms.15 Acknowledging this distinction, the present model addresses lordosis as a reflexive posture, rather than as a comprehensive measure of motivation. Nonetheless, motivational states likely interact with the contextual term (C) and the arousal field (Φ) to shape the likelihood of tactile stimulation being received and acted upon. Thus, while lordosis itself is not a direct measure of motivation, its gating provides a valuable proof case for conjunctive behavioral control.
4.3 Species and sex boundaries
The current framework is deliberately constrained to female rats. Much of the foundational work on the neuroendocrinology of lordosis was conducted in rats, with consistent findings across lesion, hormone, and tactile manipulations.12 While mice share many features of lordosis circuitry, they exhibit stronger effects of developmental social isolation and strain differences.16 Similarly, male rodents can show lordosis under atypical hormonal manipulations, but their circuitry differs in organization. Recent circuit-level studies reinforce these distinctions: optogenetic manipulations of the ventrolateral VMH demonstrate its necessity and sufficiency for specific motivated behaviors, including aggression and reproduction, while also revealing sex-specific patterns of connectivity and activation.9 For these reasons, the present synthesis is restricted to female rats as a clean test case, with the expectation that comparative extensions across species and sexes will further test the generality of the model.
4.4 The role of Φ (Arousal, stress, and neuromodulation)
In this analysis, Φ was held constant. However, decades of research demonstrate that arousal systems—including dopaminergic and serotonergic tone—modulate the threshold for lordosis.17,18 Dopaminergic facilitation can permit lordosis with reduced hormonal priming, whereas serotonergic activation can suppress lordosis even under optimal endocrine conditions. Consistent with this, psychostimulant studies show that elevating dopaminergic tone with agents such as amphetamine or cocaine can allow lordosis to occur under otherwise suboptimal hormonal conditions, providing pharmacological support for the idea that Φ functions as a manipulable threshold-scaling factor. Experimental evidence also indicates that lordosis can be strengthened: intravenous progesterone or central infusion of a D1 dopamine agonist rapidly facilitates the reflex in estrogen-primed females, and higher-intensity flank stimulation yields stronger responses.19,20 These findings suggest that Φ, along with C, can shift lordosis probability in a dose-dependent manner.
Stress offers another well-documented influence. Activation of the hypothalamic–pituitary–adrenal (HPA) axis, and particularly elevated corticosterone, reduces sexual receptivity in female rats.21,22 Developmental stressors such as social isolation during puberty can produce long-lasting reductions in lordosis even with appropriate hormonal treatment.23 Acute stressors, including restraint or exposure to predator odor, can likewise suppress lordosis expression.24,25
Puberty itself can also be viewed as a developmental threshold. Only when neural circuits such as the VMH mature, ovarian cycles provide the estradiol–progesterone sequence, social experience calibrates contextual responsiveness, and arousal systems stabilize, does A × D × C, scaled by Φ, surpass the critical value required for lordosis. Before puberty, at least one of these factors is typically absent or subthreshold, preventing expression of the reflex.
Taken together, these findings suggest that Φ is not merely a generalized arousal index but a dynamic state variable that incorporates neuromodulatory, stress-axis, and developmental influences. By scaling the product of A × D × C, Φ shifts the effective threshold for lordosis, accounting for both suppression under stress and facilitation under dopaminergic stimulation.
4.5 Predictions and future directions
The conjunctive model yields several testable predictions:
• Zeroed components veto behavior: If any single factor (A, D, or C) is reduced to near zero, lordosis should fail, regardless of the magnitude of the others.
• Threshold dynamics: Near threshold, small changes in hormone dose, tactile stimulation intensity, or neuromodulator tone should produce nonlinear changes in lordosis quotient (LQ).
• Interaction effects: Elevating Φ (e.g., via dopaminergic facilitation) should reduce the level of D or C required for expression, whereas lowering Φ (e.g., via serotonergic activation or stress) should increase these requirements.
These predictions are amenable to factorial experimental designs, such as dose–response manipulations of estradiol and progesterone, calibrated flank pressure paradigms, and pharmacological modulation of arousal systems.
4.6 Lordosis as a convergence phenomenon
The present analysis suggests that decades of empirical work on lordosis can be reframed within a formal conjunctive model. Where Lewin (1936) described behavior as a function of person and environment,1 the ARCH × Φ framework extends this logic by specifying neural archetype, hormonal drive, contextual input, and arousal threshold.
Each necessity test highlights the role of convergence:
• Loss of A (VMH lesions): Lordosis fails despite optimal hormones and context.
• Loss of D (ovariectomy without replacement): Lordosis fails despite intact circuits and tactile stimulation.
• Loss of C (absence of flank pressure or denervation): Lordosis fails despite permissive neural and hormonal states.
Together, these observations illustrate that lordosis is abolished whenever one factor is absent, even if the others are present. In contrast, full expression emerges reliably only through the convergence of all three conditions, scaled by Φ. This exploratory synthesis raises the possibility that conjunctive multiplicative gating may represent a more general principle of behavioral expression across motivated acts, including aggression, caregiving, and social bonding.
4.7 Strengths and weaknesses of the present study
A key strength of this work is its synthesis of decades of neuroendocrinological and ethological research into a simple, falsifiable framework. By formalizing the idea of convergence as a conjunctive equation, this paper organizes classic findings on lesions, hormone manipulations, and contextual stimulation into a coherent model. The ARCH × Φ framework highlights threshold dynamics that were implicit in earlier work and generates concise predictions for future experiments. The use of well-characterized behaviors such as lordosis, with a long empirical history and robust replication across laboratories, further strengthens the validity of the model as a proof-of-concept.
At the same time, several limitations must be acknowledged. This paper does not present new empirical data; instead, it relies on published findings as proxies for A, D, and C. The formalism is necessarily a simplification, treating complex biological systems as multiplicative variables. In reality, cross-dependencies exist—for example, estradiol influences both D and A through receptor induction, and stress can alter C as well as Φ. Moreover, the model is currently static, describing a threshold at a given time point rather than dynamic changes across cycles or developmental stages. Finally, while lordosis is an excellent test case, it represents a reflexive posture rather than a full measure of sexual motivation, and caution is needed in generalizing beyond this specific behavior.
Taken together, the present study should be viewed as an exploratory step: a proposal to reframe existing findings as a candidate law of convergence. Its strength lies in clarifying logic and generating predictions; its weakness lies in its reliance on secondary data and abstraction. Future work should aim to empirically test the model with factorial designs, parametric manipulations, and cross-species comparisons.
4.8 Future experiments
Although this paper relies on existing studies, several targeted experiments could directly test the convergence model. Factorial designs that systematically vary estradiol and progesterone doses (D) alongside calibrated flank stimulation (C) would reveal whether lordosis expression follows the predicted sigmoid threshold function, with small parametric changes near the boundary producing sharp transitions in lordosis quotient. Manipulations of the ventromedial hypothalamus (A), through partial lesions or reversible inhibition, would test whether reducing archetypal integrity shifts the hormonal and contextual requirements upward. Neuromodulatory manipulations of Φ, such as dopaminergic facilitation with low-dose amphetamine or D1 agonists, or serotonergic activation and corticosterone exposure, could determine whether the arousal term acts as a gain factor that rescales the effective threshold. Developmental studies across puberty, including social isolation paradigms, would help establish whether convergence thresholds emerge only after neural, hormonal, and contextual systems mature together. Finally, closed-loop circuit manipulations during mounts could provide causal evidence that ventrolateral ventromedial hypothalamus (VMHvl) to the periaqueductal gray (PAG) output gates lordosis in a multiplicative, conjunctive manner.
A recent analysis showed that VMHvl population activity during mating follows a line attractor whose state tracks female receptivity.26 Their recurrent dynamical-systems model demonstrates that receptivity emerges only when neural activity crosses a threshold—very similar to the present proposal that lordosis requires the convergence of archetype, drive, and context. In this way, their computational approach provides a circuit-level example of the same thresholded convergence principle formalized here as a multiplicative law.
Although lordosis provides an ideal proof case due to its extensive empirical foundation, the convergence model could also be applied to other ethologically salient behaviors and across species. Maternal caregiving may require the convergence of hypothalamic circuits (archetype), prolactin and oxytocin priming (drive), pup cues (context), and an appropriate arousal state (Φ).27 Predator defense appears to depend on amygdalar and PAG circuits, stress hormones, and immediate threat cues.28 Courtship, aggression, and social bonding may each have analogous conjunctive structures.
Together, such experiments would move the present exploratory synthesis from a restatement of existing knowledge toward a directly testable and potentially generalizable framework for understanding convergence in behavior.
4.9 Falsifiability
A central strength of the convergence model is that it generates falsifiable predictions. The law of convergence holds that lordosis should be absent if any one requisite factor—neural archetype (A), hormonal drive (D), or contextual input (C)—is missing, even if the others are intact. This stands in contrast to an additive model, which would predict that partial contributions from two strong factors could compensate for the absence of a third.29 In the multiplicative framework, by contrast, any zero term vetoes the outcome (1 × 1 × 0 = 0). These computations are summarized in
Table 1.
Table 1. Additive versus multiplicative predictions for lordosis.
| Factors present | Additive prediction |
Multiplicative prediction |
|---|
| A + D (no C) | Partial | No (0) |
| A + C (no D) | Partial | No (0) |
| D + C (no A) | Partial | No (0) |
| A + D + C (all three) | Full | Yes1 |
The model is therefore refutable in specific ways. If future experiments were to show that lordosis can be robustly expressed without an intact VMH circuit, in the complete absence of estradiol and progesterone, or without flank stimulation, the current framework would be falsified. Similarly, if manipulations of Φ, such as dopaminergic facilitation or serotonergic suppression, failed to shift the threshold at which A × D × C yields behavior, the proposed role of Φ as a scaling factor would not be supported. These boundary conditions ensure that the model is not only descriptive but testable, inviting empirical validation or revision.
5. Conclusion
The present analysis suggests that lordosis in the female rat provides a uniquely well-characterized system for examining how behavior emerges only through the convergence of multiple factors. Reframing decades of empirical findings within the ARCH × Φ framework emphasizes that neural circuitry, hormonal drive, and contextual input are each indispensable; the absence of any one abolishes the behavior, while their joint alignment above threshold produces a reliable all-or-none reflex. The addition of Φ as an arousal and modulatory field helps account for how stress, neuromodulators, and developmental transitions shift the effective threshold.
This exploratory synthesis advances a candidate law of convergence: behavior is expressed only when all necessary conditions align above threshold, and it fails with the loss of any one — 1 × 1 × 0 = 0. While developed in the context of lordosis, this principle may extend to other motivated behaviors where convergence defines the boundary between latent potential and expressed action.
In doing so, the framework also returns to Lewin’s vision of a field theory of behavior, inspired by Einstein’s equations in physics. Lewin argued that behavior arises from the interplay between person and environment, rather than from either alone. The present analysis builds on that vision by specifying how neural circuits, hormones, context, and arousal must converge to cross a behavioral threshold.
The value of this proposal lies in making explicit what has long been implicit: behavior is not additive but conjunctive. By formalizing this principle, the model generates falsifiable predictions, invites direct experimental tests, and opens the possibility that similar convergence laws may govern diverse motivated acts. In this sense, the framework aims not only to honor Lewin’s project of a behavioral field theory but also to advance it toward genuine scientific law—transforming descriptions into predictions and fragmented findings into unifying principles.
AI disclosure
Portions of this manuscript, including text drafting, organization, tables, diagrams and language refinement, were assisted by OpenAI’s GPT-5. The AI was used to improve clarity, style, and structure, but all conceptual content, interpretation of findings, and final revisions were developed and approved by the author. The author assumes full responsibility for the accuracy, integrity, and originality of the work.
Data availability
No new data were generated or analyzed in support of this research. Therefore, no data are available.
References
- 1.
Lewin K:
Principles of topological psychology.
New York:
McGraw-Hill;
1936.
- 2.
Tinbergen N:
On aims and methods of ethology.
Z. Tierpsychol.
1963; 20(4): 410–433. Publisher Full Text
- 3.
Rahman T, Meloy JR:
Archetype Killers.
J. Threat Assess. Manag.
2025. (in-press). Publisher Full Text
- 4.
Pfaff D, Montgomery M, Lewis C:
Somatosensory determinants of lordosis in female rats: behavioral definition of the estrogen effect.
J. Comp. Physiol. Psychol.
1977 Feb; 91(1): 134–145. PubMed Abstract
| Publisher Full Text
- 5.
Hardy DF, DeBold JF:
The relationship between levels of exogenous hormones and the display of lordosis by the female rat.
Horm. Behav.
1971 Dec 1; 2(4): 287–297. Publisher Full Text
- 6.
Pfaff DW, Sakuma Y:
Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus.
J. Physiol.
1979 Mar 1; 288(1): 189–202. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Pfaff DW, Kow LM, Loose MD, et al.:
Reverse engineering the lordosis behavior circuit.
Horm. Behav.
2008; 54(3): 347–354.
- 8.
Yang CF, Chiang MC, Gray DC, et al.:
Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males.
Cell.
2013 May 9; 153(4): 896–909. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Hashikawa Y, Hashikawa K, Falkner AL, et al.:
Ventromedial hypothalamus and the generation of aggression.
Front. Syst. Neurosci.
2017 Dec 20; 11: 94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 10.
Beach FA:
Sexual attractivity, proceptivity, and receptivity in female mammals.
Horm. Behav.
1976 Mar 1; 7(1): 105–138.
- 11.
Pfaff DW:
Drive: Neurobiological and Molecular Mechanisms of Sexual Motivation.
Cambridge, MA:MIT Press; 1999. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Pfaff DW:
Estrogens and brain function.
Springer-Verlag;
1979.
- 13.
Mani SK, Blaustein JD, O'malley BW.:
Progesterone receptor function from a behavioral perspective.
Horm. Behav.
1997 Jun 1; 31(3): 244–255. Publisher Full Text
- 14.
MacLusky NJ, McEwen BS:
Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others.
Nature.
1978 Jul 20; 274(5668): 276–278. Publisher Full Text
- 15.
Paredes RG, Vazquez B:
What do female rats like about sex? Paced mating.
Behav. Brain Res.
1999 Nov 1; 105(1): 117–127. PubMed Abstract
| Publisher Full Text
- 16.
LeFevre JA, McClintock MK:
Social modulation of behavioral reproductive senescence in female rats.
Physiol. Behav.
1992 Sep 1; 52(3): 603–608. PubMed Abstract
| Publisher Full Text
- 17.
Hull EM, Du J, Lorrain DS, et al.:
Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation.
J. Neurosci.
1995 Nov 1; 15(11): 7465–7471. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Uphouse L:
Pharmacology of serotonin and female sexual behavior.
Pharmacol. Biochem. Behav.
2014 Jun 1; 121: 31–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Apostolakis EM, Garai J, Fox C, et al.:
Dopaminergic regulation of progesterone receptors: brain D5 dopamine receptors mediate induction of lordosis by D1-like agonists in rats.
J. Neurosci.
1996 Aug 15; 16(16): 4823–4834. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Frye CA, Walf AA, Sumida K:
Progestins' actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors.
Pharmacol. Biochem. Behav.
2004 Jul 1; 78(3): 405–418. PubMed Abstract
| Publisher Full Text
- 21.
Yoon H, Chung WS, Park YY, et al.:
Effects of stress on female rat sexual function.
Int. J. Impot. Res.
2005 Jan; 17(1): 33–38. Publisher Full Text
- 22.
Hanson LA, Gorzalka BB:
The influence of corticosterone on serotonergic stereotypy and sexual behavior in the female rat.
Behav. Brain Res.
1999 Oct 1; 104(1-2): 27–35. PubMed Abstract
| Publisher Full Text
- 23.
Södersten P:
Receptive behavior in developing female rats.
Horm. Behav.
1975; 6(4): 307–317. Publisher Full Text
- 24.
Donadio MV, Kunrath A, Corezola KL, et al.:
Effects of acute stress on the day of proestrus on sexual behavior and ovulation in female rats: participation of the angiotensinergic system.
Physiol. Behav.
2007 Nov 23; 92(4): 591–600. PubMed Abstract
| Publisher Full Text
- 25.
Klyuchnikova MA, Struchkov PV, Kvasha IG:
The effects of predator odors on stress response and reproduction in Norway rats: A review.
Ukrainian Journal of Ecology.
2020; 10(4): 48–55. Publisher Full Text
- 26.
Liu M, Nair A, Coria N, et al.:
Encoding of female mating dynamics by a hypothalamic line attractor.
Nature.
2024 Oct 24; 634(8035): 901–909. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 27.
Numan M:
Motivational systems and the neural circuitry of maternal behavior in the rat.
Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology.
2007 Jan; 49(1): 12–21. PubMed Abstract
| Publisher Full Text
- 28.
Tovote P, Fadok JP, Lüthi A:
Neuronal circuits for fear and anxiety.
Nat. Rev. Neurosci.
2015 Jun; 16(6): 317–331. Publisher Full Text
- 29.
Diaz-Gallo LM, Brynedal B, Westerlind H, et al.:
Understanding interactions between risk factors, and assessing the utility of the additive and multiplicative models through simulations.
PLoS One.
2021 Apr 26; 16(4): e0250282. PubMed Abstract
| Publisher Full Text
| Free Full Text

Comments on this article Comments (0)